Non-Tuberculosis Mycobacterium Periprosthetic Joint Infections Following Total Hip and Knee Arthroplasty: Case Series and Review of the Literature
Zulipikaer Maimaiti and Zhuo Li contributed equally to this article and are considered co-first authors.
Abstract
Objective
Periprosthetic joint infection (PJI) caused by non-tubercular mycobacteria (NTM) is uncommon but catastrophic. However, conclusive clinical data on PJI caused by NTM are lacking. In this case series and systematic review, the clinical manifestations, diagnosis, and management of NTM PJI are summarized and analyzed.
Methods
From 2012 to 2020, we retrospectively analyzed consecutive PJI cases caused by NTM in our institution. A literature review was also conducted from January 2000 to December 2021, utilizing the PubMed, MEDLINE, Cochrane Library, and EMBASE databases to identify all reported NTM-induced PJI cases. The clinical characteristics, demographics, pathogen identification, treatment protocols, and prognosis of NTM PJI were summarized and analyzed.
Results
In this retrospective analysis, seven patients infected with NTM following total joint arthroplasty at our institution were included, including six cases of PJI caused by NTM and one case of septic arthritis (SA) caused by NTM. There were six men and one woman, and their average age was 62.3 years. The average interval between TJA and PJI onset was 4 months. The preoperative serological markers, including the mean ESR (51 mm/h), CRP (4.0 mg/dL), fibrinogen (5.7 g/L), and D-dimer (1.1 g/L), were increased. Six patients underwent staged revision surgery, and one patient with SA received antibiotic-loaded bone cement beads to treat the infection. After an average of 33 months of observation following surgical intervention, none of the patients showed any symptoms of infection recurrence. From 2000 to 2021, 68 patients with NTM PJI were found in 39 studies in the published literature. Reinfections occurred within 1 year after arthroplasty in more than half (53.2%) of the patients. M. fortuitum and M. abscesses were the most prevalent rapidly growing mycobacteria (RGM) in all PJI patients, whereas Mycobacterium avium intracellulare (MAC) was the most prevalent slowly growing mycobacterium (SGM). The corresponding antibiotics were amikacin and ethambutol. The rate of culture-negative without specific clinical symptoms was as high as 36.4% (12/33), while 45% (18/40) utilized additional diagnostic techniques such as NGS. A final clinical follow-up record was available for 59 patients (86.7%; mean follow-up period, 29 months), and 10.1% of patients failed to respond to treatment.
Conclusion
Orthopaedic surgeons should consider NTM in patients with negative routine cultures who are at risk for Mycobacterium infection. Treatment options rely on the accurate result of microbiologic identification and drug sensitivity testing, and to achieve this, it may be necessary to send multiple culture specimens, extend the culture time, and change the culture medium. Every effort should be made to identify NTM and its various subtypes through modern diagnostic tools if necessary.
Introduction
Total joint arthroplasty (TJA) has been one of the most successful orthopaedic surgeries for almost half a century.1 However, periprosthetic joint infection (PJI) is an occasional but devastating complication after TJA, and occurs in approximately 1%–2% of patients after primary TJA and nearly 20% of patients after revision arthroplasty, which results in an increased burden on the patient's health and socioeconomic status.2-6 Most commonly, PJI is due to Gram-positive cocci, Staphylococcus aureus (S. aureus) and Coagulase-negative staphylococci (CNS), which are responsible for approximately 60% of cases, while Enterococci and Streptococci are responsible for approximately 15% of cases.7 However, almost 7%–15% of patients with PJI cannot obtain positive culture results because of the presence of atypical microorganisms.8
Non-tubercular mycobacteria (NTM) are environmental microorganisms that are frequently discovered in animal samples, as well as in soil and water systems.9, 10 Based on the respective growth rates of NTMs, NTM species are typically divided into two categories: rapid-growing mycobacteria (RGM) and slow-growing mycobacteria (SGM).11 It has been determined that Mycobacterium. abscessus, Mycobacterium chelonae, and Mycobacterium fortuitum belong to the category of RGM, while Mycobacterium avium intracellulare (MAC), Mycobacterium kansasii, and Mycobacterium gordonae belong to the category of SGM.10 NTM are usually considered harmless, and immunocompromised patients are the only ones who should be concerned about their potential for harm. Nevertheless, some studies have found that individuals with a healthy immune system could be infected by NTM.12 Several studies also noted that NTM species can colonize prostheses to form biofilms.13, 14 Even though NTM, which are atypical pathogens, are becoming more prevalent around the world, there is still a lack of studies related to NTM as a causative pathogen of postoperative TJA infections.15
Pulmonary infections are the most common manifestation of NTM infections, and the treatment of pulmonary NTM infections frequently involves the administration of antibiotics for an extended period. However, the clinical outcomes highly depend on the patient's physical condition, including any comorbidities and their immunological function.16, 17 Due to their natural resistance to a wide variety of antimicrobial and anti-tuberculosis medications, NTM infections are notoriously difficult to treat. The majority of extrapulmonary NTM infections manifest in the skin and soft tissues, and most patients with NTM infections have impaired immune function, rendering them more susceptible to persistent infection.10 To date, few studies have provided sufficient information for clinicians to decide how to treat musculoskeletal infections caused by NTM species.9, 18
Despite the growing awareness of the importance of treating PJI, there is still a dearth of well-established protocols, and without clear guidelines, the prevention and clinical management of NTM PJI may be subpar. Because NTM is able to easily invade immunocompromised or immunosuppressed patients, the presence of NTM makes it more difficult to control infections. Consequently, it is necessary to collect additional clinical data that might be used to guide therapeutic interventions for patients with NTM infections after TJA.
The objectives of this research were to (i) report the clinical management and treatment outcomes of NTM-related PJI cases diagnosed in our hospital; (ii) review the literature, including case reports, case series, and clinical articles over the last 20 years, and summarize additional clinical data to characterize the risk factors, clinical symptoms, diagnosis, treatment approaches, and prognosis of patients with NTM PJI; and (iii) provide supportive advice on therapeutic interventions for patients with severe PJI caused by NTM.
Materials and Methods
Case Series
Patient Information
After Institutional Review Board approval (No. S2021-015-01), we retrieved clinical and microbiological data from our joint replacement database from January 2012 to December 2020. Six NTM PJI patients and one NTM-caused septic arthritis (SA) patient were identified. These patients were available for clinical and imaging evaluation for at least 1 year of follow-up. The diagnosis of PJI was based on 2014 MSIS criteria, and infection was confirmed when one of the major or three out of the five minor criteria existed. Positive cultures from synovial fluid, periprosthetic tissue, or purulence in at least one specimen can confirm NTM as a pathogenic organism.4, 19 Synovial fluid or tissue samples were obtained preoperatively or intraoperatively and sent to the microbiology department for routine culture (aerobic, anaerobic, fungal, and acid-resistant bacilli; PCR and NGS).
Literature Review
Inclusion and Exclusion Criteria
This review includes papers that met the following inclusion criteria: (i) total knee/hip arthroplasty; (ii) diagnosis of nontuberculous mycobacterium infection after TJA; and (iii) NTM infection. The exclusion criteria were as follows: (i) diagnosis of joint (knee or hip) mycobacterium prior to knee arthroplasty; (ii) reviews, meta-analyses, or meetings; (iii) NTM infections in other body parts; (iv) research not published in English; and (v) animal research. Two researchers independently collected data from each report.
Search Strategies
The following keywords were used in the search strategies: (periprosthetic/prosthetic joint infection or PJI), (infection), (rapidly/slowly growing), (non-tuberculosis), (mycobacteri*), (knee or hip), (arthroplasty or replacement), and language (English), and the search was restricted to studies of humans. All publications (clinical research, systematic reviews, or case reports) of PJI caused by NTM between January 2000 and December 2021 were found using the following databases: PubMed, MEDLINE, the Cochrane Library, and EMBASE. Two researchers manually performed a secondary search, scanning reference lists of the crucial identified articles for relevant publications that were missed by the original investigation. We collected the following data from the literature: author, publication year, patients' age and sex, underlying medical condition, preoperative diagnosis, clinical symptoms, time from TJA to PJI diagnosis, classification of NTM (RGM or SGM), surgical intervention, antibiotic-loaded spacer and systematic antibiotic treatment, duration of follow-up and outcome, bacterial identification method, and sample types.
Results
Case Series
Case Information
This study included seven patients (six males and one female) with a mean age of 62.3 years and a body mass index (BMI) of 24.84 kg/m2 (Table 1). One patient had both hypertension and diabetes, one had diabetes, and one had both hypertension and a history of cardiovascular disease. Six patients (three hips and three knees) with osteoarthritis received TJA. The mean time from primary TJA to the diagnosis of PJI was 4.9 months (range, 1–10 months). Three patients underwent primary TJA at different hospitals. Prior to primary TJA, none of the patients had a history of bacterial infection or intra-articular injections. The mean follow-up duration was 3 years (12–85 months).
| NO. | Age/sex | Underlying medical conditions | joint | Pre-op diagnosis | Clinical symptoms | Time from arthroplasty to infection (months) | Organism (s) | Surgical intervention | Antibiotic treatment | Duration of Follow-up (m) & Outcome | Spacer (loaded with antibiotics) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 70/F | HTN | Hip | DJD | Pain | 5 | M.abscessus |
|
Linezolid → Imipenem, Amikacin, Azithromycin | Cured with spacer left in place 18 m of follow-up | Vancomycin (6 g) + imipenem (1 g) |
| 2a | 64/M | Knee | SA | Swelling, Effusion, Fever, Pain | 10 | RGM |
|
Linezolid, Ceftriaxone → Vancomycin, Meropenem | 36 m infection free | Vancomycin (6 g) + Meropenem (1 g) | |
| 3 | 60/M | Hip | DJD | Effusion | 2 | RGMb (S. epidermidis, streptococcus) |
|
Linezolid, Cefuroxime | 48m infection free | Vancomycin (6 g) + cefuroxime (2.25 g) + Meropenem (0.5 g) | |
| 4 | 49/M | Hip | DJD | Poor Wound Healing, Effusion | 9 | RGM |
|
Linezolid, Ceftriaxone, Amikacin→ Vancomycin, Amikacin | 24 m infection free | Vancomycin (6 g) + Meropenem (3 g) | |
| 5 | 63/M | DM, HTN | Knee | DJD | Pain | 1 | RGM |
|
Vancomycin, Levofloxacin (Amikacin) → Azithromycin, Ciprofloxacin, Amikacin → Penicillin, Meropenem → Azithromycin | 12 m infection free | Meropenem, Azithromycin, Amikacin |
| 6 | 65/M | Knee | DJD | Swelling, Effusion, Fever, Pain | 6 | M.abscessus |
|
Cefoperazone/Sulbactam, Vancomycin→ Amikacin, Imipenem, Clarithromycin | 12 m infection free | Vancomycin (4 g) + Meropenem (2 g) | |
| 7 | 65/M | DM, HTN, CAD | Knee | DJD | Pain, Sinus tract | 1 | MACb (S. warneri) |
|
Ciprofloxacin, Amikacin, Azithromycin | 85 m infection free with decreased ROM | Vancomycin (1.5 g) + Meropenem (1.5 g) |
- Abbreviations: CAD, Coronary artery disease; DJD, degenerative joint disease; DM, Diabetic mellitus; F, Female; HTN, hypertension; M, male; m, months; RGM, rapidly growing mycobacterium; ROM, range of motion; SA, septic arthritis.
- a Case 2 septic arthritis without TKA, and managed with antibiotic loaded cement beads
- b MAC:M. avium complex.
Clinical Symptoms and Laboratory Indications
Patients with NTM infection presented with pain (four patients), effusion (four patients), fever (two patients), swelling (two patients), and fever (two patients) or sinus tracts (one patient). The patients' inflammatory condition was revealed by preoperative serological results: mean ESR 51 mm/h (range, 27–81 mm/h), CRP 4.0 mg/dL (range, 1.8–11.5 mg/dL), and five patients had increased fibrinogen 5.7 g/L (range, 3.2–8.1 g/L) and D-dimer 1.1 g/mL (range, 0.5–1.5 g/mL). Three patients had a synovial fluid analysis: the mean white blood cell count (WBC) was 13,300 cells/mL (range: 5450–17,440 cells/mL), and the PMN% was 91.7% (range: 89%–94%). Frozen sections of five patients had >10 polymorphonuclear neutrophils (PMNs) per high-power field (HPF), and histopathological examination showed the presence of inflammation in five patients. RGM was isolated in six cases, whereas SGM was identified in one. Bacteria coexisted in two cases, one with S. warneri and the other with S. epidermidis and Streptococcus. Three patients' initial culture results were negative, and then NTM was identified; subsequently, joint aspiration or intraoperative tissue culture was repeated with special detection methods (PCR or NGS).
Treatment Strategy
Debridement, antibiotics, and implant retention (DAIR) were the first considered treatment options for acute infection. Patients with chronic infection were planned for two-stage exchange arthroplasty, including removal of the implanted components, vigorous debridement and irrigation, and insertion of an antibiotic-loaded cement spacer. Reimplantation was performed when the patient's preoperative inflammatory indicators normalized, and the culture results were negative. Five patients underwent delayed reimplantation at a mean of 6 months (range 3–18 months) after first-stage exchange; one patient still had not received reimplantation at the final follow-up. Three patients repeated the first-stage revision due to recurrent NTM infection. One patient had a spacer implanted at an outside institution and underwent a second-stage revision at our institution. However, he then underwent another two-stage revision due to reinfection with MRSA. One patient received a “1.5 stage revision” (placing an articulating spacer) for the bilateral knee.20 One patient received antibiotic-loaded cement bead insertion for NTM infection of the native knee (X-ray results detailed in Supplemental Figure S1, S2). For antibiotic administration, we initially used antibiotics empirically and adjusted them based on the results following microbial susceptibility reports. Details of the antibiotic use, procedure details, and antibiotic-cemented spacer information for all of the patients are shown in Table 1.
Literature Review
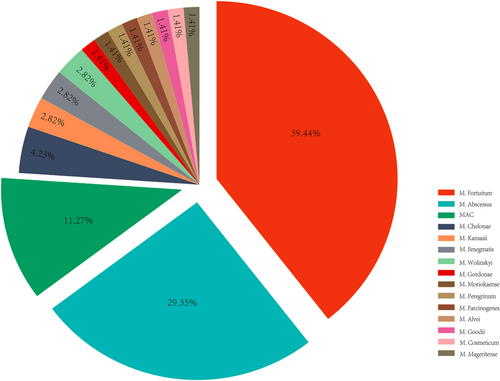
We reviewed 39 articles, including 68 patients with PJI caused by NTM [18, 21-58]. Subsequently, all 68 patients were analyzed in detail. Based on the major subtypes, we compared the 58 RGM-caused PJIs with the 10 SGM-caused PJIs (Table 2, Figure 1). The mean age of the patients was 65.9 years (IQR, 59–74.5 years), with a slightly higher age in the SGM group than in the RGM group (68.2 years (IQR 61.5–78 years) vs. 65.5 years (IQR, 59.3–72 years)). There were 35 women and 33 men, with a male-to-female ratio close to 1 to 1, while SGM infections were more common in men (seven men and three women). NTM infections were more likely to occur in the knee (72%) than in the hip (28%). The patients underwent primary TJA surgery for a diagnosis of osteoarthritis (64.4%), rheumatoid arthritis (11%), and fracture (8.9%).
| Characteristic | All Patients | RGM | SGM |
|---|---|---|---|
| Cases | 68 | 58 | 10 |
| Age (IQR) | 65.9 (59–74.5) | 65.5 (59.3–72) | 68.2 (61.5–78) |
| Gender | |||
| Female | 35 | 32 | 3 |
| Male | 33 | 26 | 7 |
| Joint | |||
| Hip | 19 | 17 | 2 |
| Knee | 49 | 41 | 8 |
| Preoperative diagnosis | |||
| Totala | N = 45 | N = 39 | N = 6 |
| OA | 29 | 27 | 2 |
| RA | 5 | 2 | 3 |
| Fracture | 4 | 4 | 0 |
| Avascular necrosis | 3 | 3 | 0 |
| Other diagnosisb | 4 | 3 | 1 |
| Risk factor | |||
| Totalc | N = 38 | N = 30 | N = 8 |
| DM | 9 | 8 | 1 |
| HTN | 14 | 13 | 1 |
| CAD | 2 | 1 | 1 |
| Steroid injection history | 7 | 4 | 3 |
| Preoperative surgery history | 4 | 3 | 1 |
| Malnutrition | 2 | 1 | 1 |
| Multiple comorbiditiesf | 16 | 13 | 3 |
| Coexist other bacteria | 7/68 | 6/58 | 1/10 |
| Outcomed | |||
| Clinical infection free | 53/59 (89.8%) | 46/49 (93.8%) | 7/10 (70%) |
| Failuree | 6/59 (10.1%) | 3/49 (6.1%) | 3/10 (30%) |
| NTM-related death | 2 | 2 | |
| Additional surgery | 1 | 1 | |
| Reinfection | 3 | 2 | 1 |
- Abbreviations: CAD, coronary artery disease; DM, diabetes mellitus; HTN, hypertension; NTM, non-tuberculous mycobacteria; RGM, Rapidly growing mycobacteria; SGM, Slowly growing mycobacteria.
- a Preoperative diagnosis was recorded in 45 patients.
- b Other diagnosis details see in Supplementary Table S1.
- c Risk factors were recorded in 38 patients.
- d Clinical outcome was recorded in 62 patients, three patients lost follow-up.
- e Failure defined as: functional lost, died, additional surgery, infection recurrence.
- f Defined as patients with multiple comorbidities (≥2) at the same time.
Clinical Manifestations and Comorbidities
The mean time from TJA to the onset of symptoms of infection was 33 months (range, 0.5–300 months). Infection occurred within 1 year after TJA in 53.2% (33/62) of patients. The most common clinical manifestations were pain, swelling, elevated skin temperature, and sinus drainage. Comorbidities were present in 79% (30/38) of the patients. Diabetes mellitus (n = 9) and hypertension (n = 14) were common comorbidities, and 16 patients had multiple comorbidities (Table 2). In addition, two patients were considered malnourished. Seven patients had a history of preoperative steroid injection, and four had a prior history of multiple surgeries.
Culture Results
Preoperative joint aspiration and intraoperative tissue cultures were used to detect microorganisms. The specimen types were available in 33 patients, including joint aspiration fluid (n = 16), intraoperative periprosthetic tissue (n = 13), or both (n = 4). The initial culture negativity rate was up to 36.4% in these 33 patients, and the tissue culture negativity rate (61.5%) was higher than that of joint fluid (25%) (Table 3). Routine microbiological cultures and acid-fast bacilli staining were the standard methods for NTM detection. At the same time, PCR and/or NGS (45%, 18/40) were reliable methods for confirming the diagnosis in those with multiple negative cultures. M. fortuitum, M. abscessus, M. chelonae, M. wolinskyi, M. moriokaense, M. Smegmatis, M. peregrinum, M. farcinogenes, M. alvei, M. goodii, M. cosmeticum, and M. mageritense were detected as causative RGMs for PJI. In addition, MAC, M. gordonae, and M. kansasii were PJI-causative SGMs (Figure 1, Supplementary Table S2).
| Routine culturea | Total | Positive | Negative |
|---|---|---|---|
| NTM type | 33 | 21 | 12 |
| RGM | 28 | 18 | 10 |
| SGM | 5 | 3 | 2 |
| Specimen type | |||
| Synovial Fluid | 16/33 | 12/16 | 4/16 |
| Tissue | 13/33 | 5/13 | 8/13 |
| Both | 4/33 | 4/4 | – |
- Abbreviations: RGM, Rapidly growing mycobacteria; SGM, Slowly growing mycobacteria.
- a Specimen types and culture results were recorded in 33 patients.
Anti-Nontuberculous Mycobacteria Drugs and Treatment Outcome
The most commonly used agents for treating SGM were ethambutol, azithromycin, rifampin, and amikacin, while for RGM they were amikacin, clarithromycin, ciprofloxacin, and cefoxitin, as detailed in Table 4. Two-stage revision is still the primary surgical approach for treating NTM PJI (Supplementary Table S1). Overall, 59 (86.7%) patients had clinical outcomes reported at the final follow-up; the mean follow-up time was 29 months (ranging from 2 months to 8 years). Fifty-three (89.8%) cases were cured. However, 38.2% (26/68) of patients underwent multiple surgeries before achieving complete infection control. Treatment failure occurred in six patients, including three patients with recurrent infections, two patients who died, and one who experienced multiple surgeries.
| Rank | Total | RGM | SGM |
|---|---|---|---|
| 1st | Amikacin (41/68,60.2%) |
Amikacin (38/58,65.5%) |
Ethambutol (9/10,90%) |
| 2nd | Clarithromycin (37/68,54.4%) |
Clarithromycin (34/58,58.6%) |
Azithromycin (7/10,70%) |
| 3rd | Ciprofloxacin (30/68,44.1%) |
Ciprofloxacin (29/58,50%) |
Rifampin (5/10,50%) |
| 4th | Cefoxitin (23/68,33.8%) |
Cefoxitin (23/58,39.6%) |
Amikacin (3/10,30%) |
- Abbreviations: RGM, Rapidly growing mycobacteria; SGM, Slowly growing mycobacteria.
Discussion
NTM are infrequently reported as pathogenic bacteria in PJI compared to S. aureus, CNS, and Streptococcus.7 With the advancement of DNA testing technology and the global rise in NTM infection rates, more NTM species have been identified from clinical specimens.59, 60 Although NTM can cause PJI, the number of studies on NTM-induced PJI is still relatively low. The clinician's limited knowledge of recognizing NTM often leads to difficulty and a delay in diagnosis. For treatment, patients with PJI caused by NTM often deal with the adverse side effects of long-term usage of various antibiotics and the high cost of revision surgery.6, 12 However, empirical treatment outcomes are often unsatisfactory due to the lack of clinical guidelines for managing NTM PJI.
NTM and Common Related Diseases
NTMs are ubiquitous in the environment and are found in natural waters, potting soils, plants, and animals; municipal water systems; sewage systems; residential plumbing, swimming pools, bathtubs, showers, and washing machines; hospital cooling systems; and improperly sterilized surgical equipment.61 Human infections induced by NTM are primarily spread via aerosols in polluted surroundings.62 Buser GL reported several NTM PJIs in the operating room that were caused by instrument vendors.63 Recent research indicates that NTM can be transmitted from person to person. However, the exact mode of transmission requires further study.15
According to its growth rate, NTM can be divided into RGM and SGM. Under optimal conditions, it takes more than 7 days for SGM to establish mature colonies on solid media, but RGM requires a shorter development period.10 Nevertheless, the treatment techniques for SGM and RGM are distinct.16, 17 NTM is the cause of numerous ailments, the most prevalent of which is lung infection. Additionally, NTM can induce cutaneous and soft tissue infection, musculoskeletal infection, lymphangitis, and disseminated infection.14, 15, 64 In a retrospective study of patients with musculoskeletal infections caused by NTM, the most common microorganisms found to cause infections were MAC, M. fortuitum, and M. abscessus.55 Our study found that various NTM species were also causative pathogens of PJI.
NTM Infection in PJI
According to previous studies, MAC was the most commonly identified mycobacterium in patients with pulmonary NTM disease (80.1%), followed by M. chelonae and M. abscessus (12.1%), M. fortuitum (5.6%), and M. kansasii (5.6%).65 Furthermore, recurrences are common in NTM infections, and the recurrence rate of MAC infection can reach 50%.66, 67 Only one PJI case was caused by SGM (MAC) in our hospital. In the reviewed literature, 10 SGM PJIs have been described, with MAC (n = 7) being the most prevalent pathogen, followed by M. kansasii and M. gordonae. There were 58 RGM infections recorded, with M. fortuitum (n = 28) and M. abscessus (n = 18) being the most common. In a retrospective study, M. fortuitum was also found to be a common causative RGM after TJA.54 In this study, the average age of patients with NTM infection was 65.9 years, and these patients also had diabetes, hypertension, or cardiovascular disease. Winthrop et al.68 showed that pulmonary NTM was more prevalent in elderly men (mean age 66 years). A similar study showed that diabetes, immunological weakness, malignancy, and alcohol abuse are risk factors for NTM infection.69
Causes of Missed or Misdiagnosed NTM in PJI
As an unusual bacterium, NTMs are frequently neglected throughout the diagnostic process for a number of reasons. First, NTM infection has no specific clinical symptoms. As with the majority of PJI patients, serological markers such as CRP and ESR, fibrinogen, and D-dimer are elevated. Second, routine NTM cultures are generally negative, necessitating special media and extended culture durations. The literature, including extensive culture data, revealed that the negative rate of routine cultures was as high as 36.3% and even as high as 61.5% when only tissue specimens were sent for culture. Therefore, it is necessary to send several samples of joint fluid and periprosthetic tissue for culture in patients at risk for Mycobacterium infection who have negative routine cultures. In places with a high prevalence of tuberculosis, NTM are sometimes misdiagnosed as Mycobacterium tuberculosis.15 Mycobacterium tuberculosis and nontuberculous mycobacteria are both positive for traditional smear antacid staining.15 The differential diagnosis of Mycobacterium tuberculosis and NTM is critical because NTM infection does not respond to anti-tuberculosis drugs.70 According to one investigation,71 Mycobacterium is primarily responsible for up to 43% of culture-negative cases of PJI. In culture-negative PJI, surgeons must focus on atypical microorganisms, specifically nontuberculous mycobacteria. PCR dsRNA analysis and next-generation sequencing (NGS) are the most effective differential diagnosis methods for culture-negative PJI.
Treatment Strategies of NTM Infection after TJA
NTM can form biofilms. Biofilms shield NTM from external influences and promote colonization.10 Therefore, NTM can withstand high temperatures and develop in low oxygen, nutrient-poor, disinfectant, and antibiotic-rich settings.72 Eid et al. demonstrated that infection control was achieved in all PJI patients after the removal of the infected prosthesis and completion of antibiotic therapy; in contrast, patients with remaining prosthetic components experienced recurrence in the absence of efficient antibiotic treatment.30 Reinfection also occurred in this study, with three patients reporting failed DAIR followed by effective infection control with a two-stage repair operation. A case study demonstrated that NTM infection could be successfully treated with a single-stage revision.26 Nevertheless, the majority of studies indicated that two-stage revision is the surgeon's preferred treatment for NTM PJI.35-45
Variations in Antibiotic Use among NTM Subgroups
In our literature review, the most frequently used antibiotics for SGM, especially MAC-induced PJI, were ethambutol, azithromycin, rifampin, and amikacin, consistent with the recommended antibiotics for the treatment of MAC infection in a previous study.17 For RGM, amikacin, clarithromycin, ciprofloxacin, and cefoxitin were the most commonly used antibiotics. The in vitro sensitivity of RGM to amikacin, clarithromycin, cefoxitin, imipenem, fluoroquinolones, and sulforaphane has been widely studied.73 Hence, it is vital to accurately distinguish between RGM and SGM for successful treatment of NTM disease. Early determination of whether the causative organism is an RGM or SGM helps select appropriate antibiotics, as an antibiotic regimen effective for RGM may not be effective for SGM, and vice versa.74 In addition, drug rash, gastrointestinal symptoms, syncope, and even seizures due to antibiotic toxicity during treatment have been reported.28, 40, 50, 52 When treating NTM with multiple antibiotics, one should pay attention to drug toxicity and drug–drug interactions.
Previous studies recommended 6 months of antibiotics to treat bone diseases caused by NTM.30 Some studies have also suggested 6 weeks of intravenous antimicrobial therapy followed by 3 to 6 months of oral treatment with delayed reimplantation for PJI.61 The antibiotic regimen of Hwang et al. was intravenous cefoxitin and amikacin for at least 6 weeks to eradicate the infection before reimplantation, followed by intravenous amikacin for 6 weeks and oral clarithromycin for 3 months.54 Our study indicated that patients underwent reimplantation after a mean of 5.5 months following spacer implantation. In our cohort, delayed reimplantation was performed after an average of 6 months, and no complications were associated with delayed implantation. Since NTM are special pathogenic organisms, it is essential to exclude infection before reimplantation. In addition to the disappearance of infection symptoms, it is important to pay attention to the normalization of ESR, CRP, and other blood inflammation indicators and to send multiple cultures, along with NGS tests, to confirm the absence of pathogenic bacteria by preoperative joint aspiration. Therefore, we recommend extended antibiotics, repeated confirmation of infection eradication, and appropriate delay of reimplantation to improve the two-stage exchange's success rate.
Strengths and Limitations
This study has several strengths. To our knowledge, this study represents one of the largest cohorts of patients with NTM-PJI reported to date. This article documented clinical data from almost all NTM PJI case reports or case series studies published in the last 20 years. We summarized the patients' clinical symptoms, time of onset, type of specimen, microbial species, culture type, antibiotic administration, and treatment strategy. Additionally, this article provides informative suggestions that are directly applicable in clinical practice.
There are also limitations of this study. Due to the rarity of NTM-PJI, our case series study has a limited sample size and is therefore susceptible to bias; thus, our results need to be verified in a broader population. Second, in four cases of RGM, a specific subtype was not recorded, but complete and accurate clinical data were available for all patients. Third, this analysis only carefully analyzed publications published during the last two decades; older studies were excluded because the diagnostic criteria and methodologies have been updated and improved. Fourth, the literature review section consists of case reports and case series studies with inherent publication bias. Therefore, the surgical and antibiotic regimens should be individualized. Nonetheless, all NTM PJI studies published in the last two decades were included in our analysis; thus, we are confident that our findings provide helpful information for the proper management of PJI due to NTM.
Conclusion
In conclusion, infections caused by NTM will be encountered from time to time in clinical practice. In contrast, PJI caused by NTM is rarely reported and easily overlooked or misdiagnosed. The diagnosis and management of NTM PJI are challenging, and surgeons should consider the possibility of NTM microorganisms in culture-negative PJI patients, especially those who have undergone multiple surgeries, have poor health conditions or multiple comorbidities, or are immunocompromised. Prolonging the culture time, selecting the appropriate culture medium, and sending multiple specimens for cultivation can increase the positive culture rate. An accurate diagnosis may require molecular microbiological techniques to identify NTM and its subspecies. A combination of multiple antibiotics should be considered based on drug sensitivity testing and surgical treatment. Currently, two-stage revision surgery is the first choice for most surgeons in treating NTM PJI; however, delayed implantation should be considered when necessary. More animal and in vitro experiments are needed in the future to determine the pathogenic mechanism of PJI caused by NTM and provide evidence for clinical treatment.
Acknowledgments
We would like to express our sincere gratitude to the study participants.
Author Contributions
Z. M. drafted the manuscript and responsible for data collection. Z. M., Z. L., C. X., J. F., L-B. H., J-Y. C., X. L., and W. C. participated in the design of the study, performed data interpretation, and participated in coordination. X. L. and W. C. revised and approved the final version of the manuscript. All authors reviewed and approved the final manuscript.
Conflict of Interest
All authors declare that they have no competing interests.
Ethics Statement
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRB) of our hospital approved this study.
Informed Consent
Informed consent was obtained from all individual participants included in the study.




