Adenine-induced chronic kidney disease in rats
ABSTRACT
Many animal models have been developed to study the causes and treatments of chronic kidney disease (CKD) in humans, an insidious disease resulting from kidney injury and characterized by persistent functional decline for more than 3 months, with or without evidence of structural deficit. The eventual outcome of CKD may be end-stage kidney disease (ESKD), where patients need dialysis or transplantation to survive. Cardiovascular disease is accelerated in patients with CKD and contributes to increased mortality, with the relationship between CKD and cardiovascular disease being bi-directional. Most animal models do not mimic the complexity of the human disease as many do not develop CKD-associated cardiovascular disease. The adenine diet model of CKD in rodents is an exception. The original adenine diet model produced rapid-onset kidney disease with extensive tubulointerstitial fibrosis, tubular atrophy, crystal formation and marked vessel calcification. Since then, lower adenine intake in rats has been found to induce slowly progressive kidney damage and cardiovascular disease. These chronic adenine diet models allow the characterization of relatively stable kidney and cardiovascular disease, similar to CKD in humans. In addition, interventions for reversal can be tested. Here the key features of the adenine diet model of CKD are noted, along with some limitations of other available models. In summary, the data presented here support the use of chronic low-dose adenine diet in rats as an easy and effective model for understanding human CKD, especially the links with cardiovascular disease, and developing potential therapeutic interventions.
Chronic kidney disease (CKD) is a worldwide health problem, usually in adults, that is increasing in both developed and developing countries.1 The disease is insidious over many years, and may result in end-stage kidney disease (ESKD) needing enhancement of kidney function by dialysis or transplantation, with poor patient outcomes.2 An important cause of the increased morbidity and mortality in patients with CKD is cardiovascular disease,1 but the links between CKD and cardiovascular disease are understudied. The determinants of progression of CKD, per se, and of CKD to ESKD, are poorly understood. Thus, rodent models of CKD are often used to investigate the causes and progression of the disease, the links with cardiovascular disease, and to test potential interventions.3 Choosing the appropriate model to address specific hypotheses is important. This review addresses the benefits and limitations of the use of rodent models of CKD, and discusses the adenine model of CKD in rats.
ESTABLISHED RODENT MODELS OF CKD
There are many rodent models of CKD (Table S1). The 5/6 nephrectomy model is well established and often used.4, 5 Functionally, this model mimics human CKD with decreased GFR and creatinine clearance, increased blood urea nitrogen (BUN) and decreased BUN clearance, decreased urine osmolality and body weight, and increased blood pressure.4, 6 The characteristic structural changes of CKD are present including tubular atrophy, tubulointerstitial fibrosis, glomerulosclerosis and focal hypertrophy. The major disadvantage of this model is the requirement for complex surgery with mortality of around 40%.5 There is also variability in responses, tubular atrophy, glomerular injury and proteinuria, with two different surgical procedures to achieve 5/6 nephrectomy. In the first procedure, with surgical removal of one kidney and two poles of the remaining kidney, there is only 15–20% remaining renal mass with significant tubular atrophy and minimal glomerular injury and proteinuria. However, in the second procedure, where one kidney is removed and the second is ablated with two-thirds infarction, there is patchy kidney atrophy and hypertrophy, functional decline, but a significant increase in kidney weight of the remaining kidney.7 This suggests an inconsistency of this model.
Other rodent models of CKD include one-kidney one-clip,8 two-kidney one-clip,9 streptozotocin-induced diabetes,10 unilateral ureteral obstruction,11 genetic models of diabetes and diabetic nephropathy such as the diabetic obese db/db mouse, the obese ob/ob mouse, the hypoinsulinaemic non-obese diabetic mouse, the KKAy mouse and the New Zealand obese mouse.12, 13 Two-kidney one-clip and one-kidney one-clip models also require surgery, with the one-kidney one-clip model requiring unilateral nephrectomy. Some models show different pathophysiological responses to humans with CKD: for example, streptozotocin has been used alone or in combination with other chemicals, or with dietary manipulations, for induction of Type 1 or Type 2 diabetes. The chronic renal changes are driven by loss of pancreatic β cells and hyperglycaemia, and structural changes and kidney failure are reported to be mild.14 The unilateral ureteral obstruction model is useful to examine mechanisms of tubulointerstitial fibrosis, but does not show all the features of CKD11 and to our knowledge, there have not been any cardiovascular changes reported in this model.
Addition of 0.75% adenine to the diet of rats for 4 weeks15 gained general acceptance as a model to study kidney damage as this intervention mimicked most of the structural and functional changes seen in human CKD, and did not require surgery or genetic manipulation. Adenine increased serum urea nitrate (or BUN), serum creatinine concentrations and excretion of uric acid in urine, produced proteinuria, and induced kidney atrophy and fibrosis,15 which parallel CKD in humans.16 Further, the model produced a marked decrease in free amino acids and calcium concentration, hypoalbuminaemia, increased albuminuria, hyperlipidaemia and vascular calcification, characteristics that are sometimes seen in human CKD.15 The kidneys were grossly enlarged with interstitial inflammatory infiltrates and crystalline tubulointerstitial deposits,17 and showed oxidative stress,18 mimicking a mechanistic pathway of human CKD.19 However, dietary 0.75% adenine produced rapid onset kidney damage, increasing BUN and serum creatinine concentrations within 6 days, producing apoptotic lesions in 70–80% of kidney tissue within 4 weeks20 and enlarged fibrotic kidneys, with a granular appearance. The rapidity of onset and the structural changes were more similar to a nephrotoxic disease in humans than CKD.15, 20 Increased mortality is not reported in most publications using 0.75% adenine, but when this model was tested in a group of male adult Wistar rats, all rats had to be euthanized by 6 weeks due to marked body weight loss and morbidity in the animals.21 The parallels between this model and human CKD were, however, encouraging. From this, we developed a model that more closely mimicked the slow progression of human CKD as well as showing functional and structural damage in the cardiovascular system to demonstrate the close relationship between cardiovascular disease and CKD in humans.
OPTIMISING THE ADENINE MODEL OF CKD
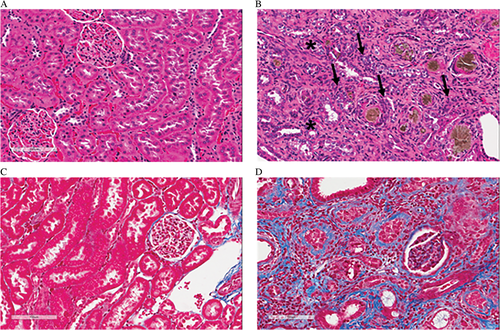
The original 0.75% adenine diet model of CKD was modified after testing different dietary concentrations of adenine (0.075%, 0.25%, 0.5% and 0.75%).21 Dietary adenine was reduced to 0.25% and rats were treated with adenine for up to 16 weeks. This approximates 8 years in humans22 and so mimics the typical slow development of CKD in humans. The 0.25% adenine diet induced continuous progressive kidney damage with increasing plasma creatinine (PCr), BUN, potassium concentration and lactate dehydrogenase (LDH) activity, decreasing urine creatinine (UCr) and urine urea nitrogen (UUN) leading to decreased clearance of creatinine and BUN, and proteinuria, but rats continued to gain weight.21 Structurally, the kidneys from 0.25% adenine-fed rats showed tubular atrophy, erosion of proximal tubular brush borders with flattening of the epithelium, focal tubular epithelial hypertrophy, and glomerular damage in the form of glomerulosclerosis and hypertrophy. Some of these characteristics are demonstrated in Figure 1. There were fewer deposits of crystals in the tubulointerstitium than with 0.75% adenine diet. These deposits may be 2,8-dihydroxyadenine which is a poorly-soluble metabolite of adenine in rats forming crystals in the tubules leading to kidney injury.20 There was podocyte injury in glomeruli with increased expression of desmin and pro-fibrotic transforming growth factor-beta (TGF-β), and collagen deposition in adenine-fed rats. Increased apoptosis contributed to atrophy in the tubulointerstitium and glomeruli, with increased protein expression of pro-apoptotic caspase-3 in kidney tissue. The expression of heme-oxygenase-1 (HO-1), a biomarker of oxidative stress, was increased. Protein expression of tumour necrosis factor-α (TNF-α) and interleukin-6 (IL-6), biomarkers of inflammation, was increased, as were inflammatory cell infiltrates (monocytes, macrophages, myofibroblasts). Endothelial nitric oxide synthase (eNOS) expression was decreased, and expression of inducible nitric oxide synthase (iNOS) was increased. No mortality was observed in rats fed 0.25% adenine for 16 weeks. The CKD outcomes of the different dietary concentrations of adenine (0.075%, 0.25%, 0.5% and 0.75%) are described in Table 1.21
| Parameters/adenine concentration | 0% | 0.075% | 0.25% | 0.5% | 0.75% |
|---|---|---|---|---|---|
| Calculated adenine dose | 0 mg/kg per day | 50.2 ± 0.7 mg/kg per day | 155.4 ± 2.6 mg/kg per day | 334 ± 12 mg/kg per day | 490 ± 32 mg/kg per day |
| Functional changes: | None | None | |||
|
✓ | ✓ | ✓ | ||
|
✓ | ✓ | ✓ | ||
|
✓ | ✓ | ✓ | ||
|
✓ | ✓ | ✓ | ||
| Food intake | No change until the end | No change until the end | Gradually decreased | Rapidly decreased | Rapidly decreased |
| Water intake | No change until the end | No change until the end | Gradually increased until the end | Rapidly increased and then decreased | Rapidly increased and then decreased |
| Crystalline deposition | None | None | ✓ | ✓ | ✓ |
| Intensity and progression of changes | None | None | Moderate and gradual | Rapid and severe | Rapid and severe |
| Tubular damage | None | None | ✓ | ✓ | ✓ |
| Glomerular damage | None | None | ✓ | ✓ | ✓ |
| Interstitial fibrosis | None | None | ✓ | ✓ | ✓ |
| Inflammation | None | None | ✓ | ✓ | ✓ |
The 0.25% adenine diet has now been used by other researchers to study the pathogenesis of CKD and benefits of new therapies. The flavonoid chrysin and gum acacia were investigated as potential CKD treatments for 35 days with 0.25% adenine diet in rats. This treatment prevented functional impairment and structural injuries in the kidney,23, 24 but no cardiovascular parameters were described. The 0.25% adenine diet was also used as a model for CKD in mice either for 10 days25, 26 or for 4 weeks.27, 28 Another study demonstrated similar CKD responses, however, with a higher (0.5%) dose of adenine for 3 weeks29 which approximates to only 1.5 years in humans.22 Kidney disease was established with 0.2% adenine for 4 weeks in C57 BL6 mice.30 Using different doses of adenine in mice and rats, this study suggests that mice are more sensitive to adenine than rats. Similar changes were shown with 0.25% adenine for 10 days in the kidneys of C57 BL6 mice.25
CARDIOVASCULAR DISEASE PROGRESSION WITH 0.25% ADENINE
Cardiovascular disease is accelerated in patients with CKD,31 with many CKD patients dying of cardiac disorders before they reach ESKD.32 Cardiovascular mortality is 10–30 times higher in kidney disease patients compared with age-matched controls with normal kidney function.32 The prevalence of hypertension and left ventricular hypertrophy is 87–90% in CKD patients.33 Most of the CKD models do not report cardiovascular complications. However, 0.25% adenine diet for 16 weeks showed most of the cardiovascular changes expected in humans with CKD.21 In these rats, systolic blood pressure increased from 4 weeks. At 16 weeks, systolic blood pressure was markedly increased with left ventricular hypertrophy, together with decreases in left ventricular internal diameter in systole and diastole, end-systolic and end-diastolic volumes and stroke volume, and increases in interstitial and perivascular inflammation and fibrosis leading to increased left ventricular stiffness. Further, isolated thoracic aortic rings showed impaired contraction with noradrenaline and relaxation with sodium nitroprusside and acetylcholine.21
Adenine is a purine base and purines are metabolized to uric acid by xanthine oxidase,34 increasing plasma uric acid concentrations.35 Uric acid is a risk factor in both cardiovascular disease36 and kidney disease.37 Plasma uric acid increased in adenine-fed rats,21 and this may be the mechanism for the development of both kidney and cardiovascular diseases. Treatment of adenine rats with allopurinol, a xanthine oxidase inhibitor, decreased plasma uric acid concentrations, collagen deposition, tubular injury, apoptosis, inflammation and glomerulopathy, improved kidney function and reduced expression of biomarkers for oxidative stress (HO-1) and fibrosis (TGF-β), decreased left ventricular inflammation, fibrosis and hypertrophy, and improved aortic contractility.21 Thus, increased uric acid production contributes to both CKD and cardiovascular disease in adenine-fed rats, shown by the responses to allopurinol. A recent clinical trial showed that chronic allopurinol treatment may slow the rate of progression of kidney disease and reduce cardiovascular risk.38
GENDER DIFFERENCES IN CKD
Most rodent models for CKD study only one gender, usually males. Although more males develop CKD than females, and males with CKD are more likely to develop ESKD,39 CKD occurs in both genders. In rats fed 0.25% adenine for 16 weeks, both genders developed CKD and cardiovascular disease.35 However, male adenine-fed rats showed greater kidney injury than female adenine-fed rats including more damage to tubules, brush borders and glomeruli, and a greater decline in kidney function.35 Adenine-induced kidney damage may be increased in males due to the suppression of oestrogen receptor-alpha expression.35
BONE AND MINERAL DISORDERS IN 0.25% ADENINE-INDUCED CKD MODEL
Vascular calcification and bone pathologies are two complications of CKD that have a major impact on mortality. Neven et al.40 studied markers of CKD in the 0.75% adenine diet model, showing excessive vascular calcification with chaotic and marked bone mineralisation due to excessive bone turnover. In comparison, the 0.25% adenine-induced CKD model demonstrated only moderate vascular calcification, and significant bone pathology after 8 weeks of adenine treatment. This report was the first to demonstrate these major complications in the adenine model of induced CKD.40
GONADAL FUNCTION IN ADENINE-INDUCED CKD
Male patients with CKD may develop gonadal abnormalities that lead to impotence, reduced libido, decreased testicular size, impaired spermatogenesis and gynaecomastia.41 High dose adenine (150 mg/day per 300 g rat) decreased testicular function of male rats, with reduced serum concentration of testosterone and increased concentrations of 17 α-hydroxyprogesterone and androstenedione at day 30, suggesting that adenine suppresses the activity of 17 β-hydroxysteroid oxidoreductase which converts androstenedione to testosterone.42
IS THE 0.25% ADENINE-INDUCED CKD MODEL BETTER THAN OTHER MODELS?
Adenine 0.25% produced pathophysiological changes that mimicked structural and functional changes of human CKD. In contrast, the other models listed in Table S1 show some limitations. 0.25% adenine-induced CKD showed increased kidney and cardiovascular dysfunction: renal inflammatory, fibrotic and oxidant markers were increased, along with increased tubulointerstitial disease, tubular epithelial apoptosis and podocyte injury. Further, plasma uric acid concentrations were also increased and there were impaired vascular responses together with hypertension and increased left ventricular stiffness and mass.21 Thus, this may be the only model showing most of the pathological changes in kidney and heart structure and function occurring in human CKD. We have discussed the limitations of other available models in Table S1. In contrast, the only limitation of the adenine model is the deposition of adenine or a metabolite as crystals in the tubules (Table 1).
CAN THIS ADENINE MODEL BE USED TO TEST THERAPEUTIC INTERVENTIONS FOR CKD?
Glibenclamide is a sulphonylurea commonly used for the treatment of type 2 diabetes.43 This KATP channel blocker reduced inflammatory cell infiltration and inhibited NOD-like receptor protein-3 (NLRP-3) during ischaemia/reperfusion injury in the kidney.44 NLRP-3 is a pro-inflammatory intracellular protein involved in cell proliferation, differentiation and apoptosis. Increased NLRP-3 expression was associated with progressive kidney disease.45 Uric acid may contribute to the pathogenesis of inflammatory diseases46 by inducing NLRP-3.47 Glibenclamide (10 mg/kg per day) was administered as a therapy to reverse 0.25% adenine-induced CKD and cardiovascular disease in rats for the last 8 weeks of the 16 week model.44 Glibenclamide decreased BUN, PCr, proteinuria, chronic inflammatory cell infiltration, fibrosis, tubular damage and expression of TNF-α, HO-1 and NLRP-3, demonstrating improved kidney structure and function.48 Glibenclamide also decreased systolic blood pressure and improved vascular responses although cardiac fibrosis, left ventricular stiffness and hypertrophy were unchanged.
Rutin is a flavonoid present in onions, apples, black tea and red wine that demonstrated anti-oxidant and anti-inflammatory properties in rats, and preserved heart and liver function in high carbohydrate, high fat diet-induced metabolic syndrome.49 Rutin inhibited phospholipase A2 (PLA2) in rats (membrane-associated PLA2 in lungs) and humans (synovial fluid-derived and non-pancreatic PLA2).50, 51 PLA2 activates pro-inflammatory cytokines involved in inflammation and progression of disease in CKD patients on dialysis.52 PLA2 produces cytokines that can cause tubular injury53 and induce reactive oxygen species that provoke inflammation.6, 54 Uraemia may increase PLA2 activity.55 The PLA2 inhibitor, curcumin,56 reduced proteinuria, glomerulosclerosis, tubulointerstitial injury and improved kidney function in rats.57 We have recently tested rutin as a treatment in the 0.25% adenine model of CKD, adding rutin for the last 8 weeks of the 16 week protocol.58 Rutin treatment (approximately 100 mg/kg per day) improved renal function (decreased BUN, PCr, plasma uric acid and proteinuria), decreased tubular damage, and decreased expression of HO-1 (oxidative stress) and PLA2 (inflammation).58 Rutin also decreased systolic blood pressure and cardiac hypertrophy, demonstrating cardiovascular protection. In adenine 0.25% rats, rutin decreased plasma uric acid concentrations and protein expression of PLA2 in kidneys,58 characteristics known to play important roles in the development of both CKD and cardiovascular disease.21 These results demonstrate the usefulness and reproducibility of the 0.25% adenine model of CKD over 16 weeks.
CONCLUSIONS
A diet of 0.25% adenine for 16 weeks produces progressive CKD with cardiovascular changes, mimicking more closely the pathophysiology of human CKD and its links with cardiovascular disease than other rodent models of CKD. This model can also be used to test therapies that may reverse or prevent progression of CKD to ESKD.




