Revisiting the relevance of Hirano bodies in neurodegenerative diseases
Abstract
Aims
Hirano bodies (HBs) are eosinophilic pathological structures with two morphological phenotypes commonly found in the hippocampal CA1 region in Alzheimer's disease (AD). This study evaluated the prevalence and distribution of HBs in AD and other neurodegenerative diseases.
Methods
This cross-sectional study systematically evaluated HBs in a cohort of 193 cases with major neurodegenerative diseases, including AD (n = 91), Lewy body disease (LBD, n = 87), progressive supranuclear palsy (PSP, n = 36), multiple system atrophy (MSA, n = 14) and controls (n = 26). The prevalence, number and morphology of HBs in the stratum lacunosum (HBL) and CA1 pyramidal cell layer were examined. In addition, we investigated the presence of HBs in five additional hippocampal subregions.
Results
The morphological types of HBs in CA1 were divided into three, including a newly discovered type, and were evaluated separately, with their morphology confirmed in three dimensions: (1) classic rod-shaped HB (CHB), (2) balloon-shaped HB (BHB) and the newly described (3) string-shaped HB (SHB). The prevalence of each HB type differed between disease groups: Compared with controls, for CHB in AD, AD + LBD, PSP and corticobasal degeneration, for BHB in AD + LBD and PSP, and SHB in AD + LBD and PSP were significantly increased. Regression analysis showed that CHBs were independently associated with higher Braak NFT stage, BHBs with LBD and TDP-43 pathology, SHBs with higher Braak NFT stage, PSP and argyrophilic grain disease and HBLs with MSA.
Conclusions
This study demonstrates that HBs are associated with diverse neurodegenerative diseases and shows that morphological types appear distinctively in various conditions.
Key Points
- Hirano bodies can be categorised into three distinct morphological phenotypes.
- Each phenotype is associated with different background pathologies.
- Age does not seem to be a direct risk factor for the development of Hirano bodies.
- Pathological staging of Hirano bodies in the posterior hippocampus could offer a simplified assessment method.
- Hirano bodies may disappear before the loss of hippocampal pyramidal cells due to the progression of background pathology.
INTRODUCTION
A Hirano body (HB) is a rod-shaped, eosinophilic pathological structure initially described by Hirano et al. in 1966, localised in the CA1 region of the hippocampus in patients with Guam ALS-Parkinson disease [1, 2]. Comprising a variety of proteins including actin [3], actin-associated proteins [4, 5], tau [6], coenzyme Q [7] and others, these structures garnered significant neuropathological attention until the early 2000s [1]. However, subsequent decades saw a dearth of research on post-mortem brain tissue, resulting in an uncertain understanding of the prevalence and significance of this pathology. HBs have been identified in Alzheimer's disease (AD) [8, 9], Pick's disease [10], ageing individuals and prion diseases such as Creutzfeldt–Jakob disease (CJD) [11, 12] and Kuru [13]. HBs have also been reported in neoplastic conditions, including cerebellar haemangioblastoma [14] and cerebellar astrocytoma [15], as well as in progressive multifocal leukoencephalopathy [16] and Wilson's disease [17]. Notably absent, however, are systematic evaluations of HBs in several major neurodegenerative disorders including progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), multiple system atrophy (MSA), argyrophilic grain disease (AGD) and Lewy body disease (LBD). A single study of six cases of dementia with DLB reported five cases with moderate or severe AD pathology and HBs in the hippocampal CA1 region [18].
Since the 1990s, many staging systems have emerged to gauge the severity and extent of neurodegenerative diseases, such as the Braak neurofibrillary change (NFT) stage for AD [19]. In addition to established disease-specific proteins like tau in tauopathies (e.g., AD and PSP) [20], amyloid-β in AD and Down's syndrome (DS) [21] and α-synuclein in LBD and MSA [22], the significance of TDP-43 has been recognised in frontotemporal lobar degeneration (FTLD) and amyotrophic lateral sclerosis [23, 24]. The hippocampus frequently serves as the primary site for the deposition of these abnormal proteins in many neurodegenerative diseases [19, 25-29]. Despite this, the interplay between these pathological stages, the accumulation of abnormal proteins and the presence of HBs remains unexplored.
The three-dimensional structure of HBs and their morphological diversity has not been examined, and it has not been determined if the typical rod-shaped HBs are truly rod-shaped in three dimensions. A 2012 report noted the predominance of relatively large round HBs, as opposed to the more common rod-shaped HBs, in CJD [12], indicating that HB morphology may be more diverse and potentially disease-specific. Nevertheless, subsequent investigations into the spectrum of HB morphology and whether morphological types of HB differ between neurodegenerative diseases are limited.
Thus, our study delved into the pathological implications of neurodegenerative conditions (including AD, LBD, PSP and MSA), as well as the abnormal protein aggregates characteristic of these disorders, within hippocampal HBs using post-mortem brain specimens. Moreover, we aimed to investigate disease-specific variations in the morphological diversity of HBs by classifying them into distinct types.
MATERIALS AND METHODS
Cases and tissue collection
We conducted a retrospective review of 256 neuropathological autopsies obtained from the University Health Network Neurodegenerative Brain Collection (UHN-NBC). All brain specimens were procured through post-mortem examinations, following the approval of the local ethics committee and adherence to appropriate consent protocols.
AD-related pathology was assessed through the Braak neurofibrillary tangle (NFT) stage [19], Thal phase [26] and the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) criteria for Aβ deposition [30]. The ABC score, as defined in the National Institute on Aging - Alzheimer's Association (NIAAA) guidelines [31], was employed to assess Alzheimer's disease neuropathologic change (ADNC) level. Assessment of the severity of Lewy disease-related lesions adhered to the Lewy disease consensus criteria using α-synuclein immunohistochemistry [29]. Cases with Intermediate and High AD status, along with Limbic and Neocortical types, were classified as AD + LBD. PSP [32], CBD [33] and chronic traumatic encephalopathy (CTE) [34] were diagnosed following published criteria. AGD was classified based on the Saito stage [28], and TDP-43 pathology was categorised according to the limbic-predominant age-related TDP-43 encephalopathy neuropathologic change (LATE-NC) stage [27] and FTLD-TDP classification system [35-37]. Controls were defined as cases with Not or Low ADNC levels and no other major degenerative diseases.
From our initial cohort of 256 cases, we included 193 cases in the study, comprising 31 with AD, 38 with AD + LBD, 16 with LBD, 36 with PSP, five with CBD, 14 with MSA, 11 with DS-related AD neuropathologic change, five with CTE, 11 with FTLD-TDP, and 26 control cases. This selection process involved excluding cases with inherited diseases such as familial AD and Niemann–Pick disease, as well as cases for which no sections of the posterior hippocampus were available. All 11 FTLD-TDP cases were Type A or Type A + B, with one Type C case excluded. Amygdala-only Lewy pathology was found in two cases, both of which were classified as AD due to high ADNC levels.
Pathological evaluation
Formalin-fixed, 4.5-μm paraffin-embedded tissue sections of the posterior hippocampus from all 193 cases were stained with haematoxylin, eosin and Luxol fast blue (HE/LFB). The hippocampus and its subregions were identified according to established anatomical criteria [38, 39], with annotations performed by K. Y and reviewed by T. K and G.G.K. Subsequently, the number of HBs in the pyramidal cell layer of CA1 and the stratum lacunosum of CA1 (HBL) were manually counted.
Evaluation of HBs extended to the CA1, CA2, CA3, CA4, subiculum and stratum lacunosum within hippocampal subregions as well as in the entorhinal cortex. The number of pyramidal cells in the CA1 region was assessed as follows: pyramidal cells in the distal, central and proximal portions of CA1 were each randomly captured under a 100× field of view, and the count of pyramidal cells with discernible nuclei was undertaken manually. The total number of pyramidal cells within the CA1 region was estimated by multiplying the average density from the three views by the overall
CA1 area (Figure S1A).
For the assessment of vascular pathology, arterial wall thickening was evaluated at two locations in the frontal and basal ganglia regions using a 4-point scale (0–3). This evaluation followed the methodology outlined in the Vascular Cognitive Impairment Neuropathology Guidelines (VCING) system [40]; however, due to the availability of the frontal but not the occipital cortex in all cases, we focused on that region. Subsequently, the scores from both locations were summed to derive the vascular injury score (0–6).
In evaluating mixed pathology, various immunostains including p-tau (clone AT8, 1:1000, Invitrogen/ThermoFisher, Carlsbad, USA), Aβ (Clone 6F/3D, 1:50, Dako, Glostrup, Denmark), alpha-synuclein (clone 5G4, 1:4000, Analytikjena, Jena, Germany) [41] and phosphorylated TDP-43 (clone 11-9, 1:2000, CosmoBio, Tokyo, Japan) were employed based on established protocols in our laboratory [42, 43]. The pan-actin antibody clone C4 (Millipore Sigma, 1:300000) was used as the primary antibody against HBs. Immunostained slides depicting posterior hippocampal p-tau, Aβ, α-synuclein and TDP-43 were digitally scanned at 40× magnification using a TissueScope LE120 (Huron Digital Pathology, St. Jacobs, Ontario, Canada) and stored as comprehensive whole-slide images. Subsequently, these images underwent analysis via the Area Quantification module within the HALO software (Indica Labs, Albuquerque, New Mexico, USA) to determine the percentage of positive area of each immunostain within the CA1 region and the stratum lacunosum for all 193 cases (722 sections) (Figure S1B,C).
Three-dimensional image analysis
To analyse the three-dimensional structure of different morphological forms of HBs comprehensively, 20-μm-thick sections from the posterior hippocampus of each of the HB-positive cases were subjected to staining with a 200× dilution of clone C4. The secondary antibody used was Alexa Fluor 488 donkey anti-mouse IgG (Invitrogen/ThermoFisher, 1:500), and sections were treated with Sudan Black B and sodium borohydride to suppress autofluorescence. Nuclei were counterstained with spectral DAPI (Akoya Bioscience). Utilising a Nikon confocal microscope (Eclipse Ti2, Nikon, Tokyo, Japan), ~40 images were captured at 0.5-μm intervals at five locations for each section, generating Z Stack images. These images were subsequently subjected to denoising using NIS-Elements (version 5.30.04, Nikon, Tokyo, Japan) image analysis software. Following the denoising process, the images were reconstructed into three-dimensional representations using the same software.
Statistical analysis
The variation in characteristics among each case group was assessed using Kruskal–Wallis and c2-tests. Continuous variables were subjected to comparison between groups utilising the Steel multiple comparison test or Mann–Whitney U test with the Bonferroni correction, with the control or pathological negative group serving as the reference. Binomial variables underwent comparison using Fisher's exact test with the Bonferroni correction applied. Spearman's rank correlation coefficient was utilised to assess correlations among the various continuous or ordinal variables. The individual impacts of background pathology, age and sex on the quantity of different HB types in CA1, as well as the quantity of HBLs, were evaluated through analysis of covariance (ANCOVA). A two-group t test with Bonferroni correction was used to assess post hoc power. The significance level was set at p < 0.05. Statistical analyses were conducted using SPSS Statistics version 26 (IBM Corporation, Armonk, NY, USA), JMP 14.3 (JMP Statistical Discovery LLC, Cary, North Carolina, USA) and GraphPad Prism version 9.5.1 (GraphPad Software, San Diego, California, USA).
RESULTS
Demographics and characterisation of examined cases
Table 1 summarises the characteristics of each case group, including mean age, sex distribution and the representative pathological stage. Significant variations were observed between groups for all variables except sex. Therefore, each group underwent a comparison to the control group in a post hoc analysis to evaluate these differences. The AD and PSP groups had a significantly higher mean age of death compared with the control group. The number of CA1 pyramidal cells was significantly smaller in the AD, FTLD-TDP and DS groups, and there were four cases of severe hippocampal sclerosis with no pyramidal cells in the CA1 region (two cases of FTLD-TDP, one case of AD + LATE, and one case of AD + LATE). Vascular injury score was significantly higher in the MSA and PSP groups. We substituted the ABC score and ADNC level with values ranging from 0 to 3, subsequently comparing their means to those of the control group. The outcomes indicated significantly higher values in the AD and AD + LBD groups, as well as in the DS group. Additionally, only the B score was significantly elevated in the LBD and PSP groups.
| Control (n = 26) | AD (n = 31) | AD + LBD (n = 38) | LBD (n = 16) | MSA (n = 14) | PSP (n = 36) | CBD (n = 5) | FTLD-TDP (n = 11) | DS (n = 11) | CTE (n = 5) | p value* | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, mean ± SD, years | 65.8 ± 14.3 | 77.9 ± 10.3# | 75.3 ± 9.4 | 76.7 ± 8.5 | 66.3 ± 8.1 | 75.7 ± 7.1 | 67.0 ± 6.4 | 69.3 ± 13.7 | 54.7 ± 9.0 | 76.3 ± 2.9 | <0.0001 |
| Sex (male/female) | 17/9 | 9/18 | 17/18 | 11/4 | 6/6 | 19/17 | 3/1 | 4/6 | 5/6 | 3/0 | 0.2444 |
| Number of pyramidal cells, mean ± SD | 897.8 ± 318.1 | 489.2 ± 331.6### | 596.6 ± 443.7# | 941.1 ± 281.2 | 868.8 ± 240.9 | 822.3 ± 459.3 | 1176.3 ± 414.7 | 296.3 ± 371.7## | 295.4 ± 301.7## | 526.3 ± 441.4 | <0.0001 |
| Vessel injury score | 2.7 ± 1.7 | 3.5 ± 1.6 | 3.7 ± 1.5 | 4.0 ± 1.2 | 4.3 ± 1.3# | 4.7 ± 1.0#### | 3.8 ± 1.3 | 3.4 ± 1.6 | 3.8 ± 0.8 | 4.0 ± 1.9 | 0.0003 |
| ADNC level, mean ± SD | 0.6 ± 0.5 | 2.9 ± 0.3#### | 2.5 ± 0.5#### | 0.8 ± 0.4 | 0.4 ± 0.5 | 0.9 ± 0.8 | 0.8 ± 0.8 | 0.5 ± 0.7 | 2.7 ± 0.6#### | 0.8 ± 1.3 | <0.0001 |
| A score, mean ± SD | 0.8 ± 0.8 | 3.0 ± 0.0#### | 2.6 ± 0.5#### | 1.2 ± 0.9 | 0.6 ± 0.9 | 1.1 ± 1.1 | 1.0 ± 1.0 | 0.5 ± 0.5 | 2.9 ± 0.3#### | 0.8 ± 1.3 | <0.0001 |
| B score, mean ± SD | 0.7 ± 0.5 | 2.9 ± 0.3#### | 2.7 ± 0.5#### | 1.2 ± 0.4# | 0.9 ± 0.5 | 1.4 ± 0.6### | 1.0 ± 0.3 | 1.2 ± 0.4 | 2.7 ± 0.6#### | 1.6 ± 1.1 | <0.0001 |
| C score, mean ± SD | 0.7 ± 0.9 | 2.9 ± 0.3#### | 2.6 ± 0.6#### | 0.9 ± 0.8 | 0.3 ± 0.6 | 0.9 ± 1.0 | 0.8 ± 0.8 | 0.7 ± 1.0 | 2.8 ± 0.6### | 0.6 ± 0.9 | <0.0001 |
| LBD positive, n (%) | 0 (0.0) | 13 (43.3) | 38 (100.0) | 16 (100.0) | 0 (0.0) | 11 (30.6) | 2 (40.0) | 1 (9.1) | 4 (36.4) | 2 (40.0) | <0.0001 |
| TDP-43 proteinopathy positive, n (%) | 0 (0.0) | 9 (29.0) | 20 (52.6) | 3 (18.8) | 1 (7.1) | 6 (16.7) | 1 (20.0) | 11 (100.0) | 2 (18.2) | 2 (40.0) | <0.0001 |
| AGD positive, n (%) | 1 (4.0) | 5 (16.1) | 6 (15.8) | 8 (50.0) | 3 (21.4) | 17 (47.2) | 2 (40.0) | 1 (9.1) | 1 (9.1) | 3 (60.0) | 0.0003 |
- Note: Statistically significant items are in bold.
- Abbreviations: AD, Alzheimer's disease; ADNC, Alzheimer's disease neuropathological change; AGD, argyrophilic grain disease; CBD, corticobasal degeneration; CTE, chronic traumatic encephalopathy; DS, Down syndrome; FTLD-TDP, frontotemporal lobar degeneration with TPD-43-immunoreactive pathology; LBD, Lewy body disease; MSA, multiple system atrophy; PSP, progressive supranuclear palsy; TDP-43, TAR DNA binding protein 43.
- * Kruskal–Wallis or c2-test. #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001 with post hoc Steel multiple comparison test vs controls.
Distribution of Hirano bodies in the posterior hippocampus
The prevalence of HB differed between hippocampal subregions. Among all subregions evaluated, HB were found in 154 (79.8%) cases in the CA1, 45 (23.4%) in CA2, none (0.0%) in CA3 and CA4, 35 (18.1%) in the subiculum, 42 (21.8%) in the stratum lacunosum and six (3.1%) in the entorhinal cortex. All cases with HBs in CA2, subiculum or entorhinal cortex also contained HBs in the CA1. However, out of the cases with HBLs, six (14.3%) did not contain HBs in the CA1.
Morphological diversity of Hirano bodies
We performed manual counts of HBs in the CA1 subregion, which displayed the highest prevalence among the evaluated subregions, and in the stratum lacunosum, which seemed to be independently affected. In addition to the CHBs originally described by Hirano et al. [1, 2], and the balloon-shaped HBs (BHBs) identified as “bizarre” HBs by Martinez et al. [12], we identified novel, string-shaped HBs (SHBs) in the CA1 subregion, which we counted separately. Specifically, we defined these types of HBs as follows: CHBs appeared as convex lens-shaped HBs with varying diameter between the central and end components when viewed in a planar manner (Figure 1A,D,G), BHBs appeared circular or oval-shaped (Figure 1B,E,H), and SHBs had elongated string-like structures with a thin, uniform diameter along their length (Figure 1C,F,I).
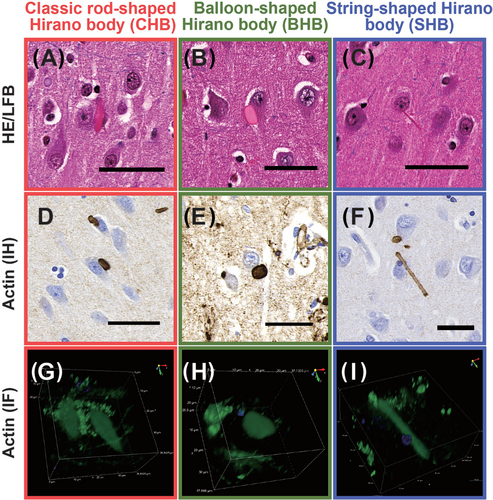
The 3D structures of these HBs were confirmed via confocal microscopy, exhibiting the following characteristics: CHBs displayed elongated rugby ball or teardrop-like structures (Figure 1G), BHBs appeared as sphere-like shapes (Figure 1H), and SHBs exhibited elongated rod-like forms with smooth ends (Figure 1I). These 3D visualisations are available in Movies S1, S2 and S3.
Out of the 193 cases, a total of 160 cases (82.9%) exhibited the presence of HB in either the CA1 subregion or the stratum lacunosum. Specifically, 151 cases (78.2%) had CHB, 42 cases (21.8%) had BHB, 38 cases (19.7%) had SHB, and 42 cases (21.8%) had HBL. Among cases showing HB types other than CHB, a considerable majority also displayed CHB: 39 cases (92.9%) with BHB, 37 cases (97.4%) with SHB and 35 cases (83.3%) with HBL. The number of cases exclusively containing each type of HB was as follows: 66 cases (34.2%) with CHB, one case (0.5%) with BHB, zero cases (0.0%) with SHB and five cases (2.6%) with HBL. The predominant type of HB in each case was CHB, observed in 140 cases (72.5%), followed by BHB in three cases (1.6%), no SHB cases (0.0%) and HBL in 17 cases (8.8%).
Pathological stage of Hirano body in posterior hippocampus
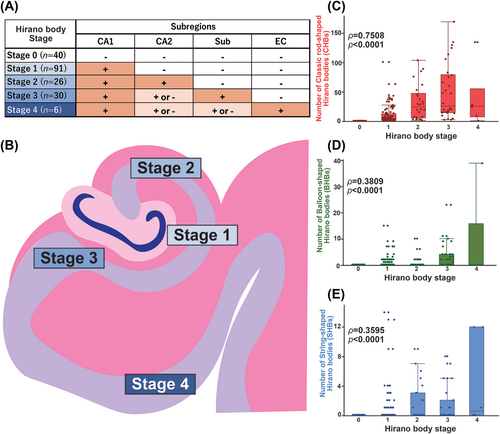
Given our findings indicating that HBs in the CA2 subregion, subiculum and entorhinal cortex always coexist with HBs in the CA1 region, we established a hierarchical pathological staging system for HBs within the posterior hippocampus. This proposed staging scheme commenced with CA1, followed by CA2 as the most common sites and subsequent stages encompassed the subiculum and entorhinal cortex, with a progressive decrease in frequency. The order of prevalence continued with CA1 followed by CA2, then the subicular cortex and finally the entorhinal cortex. Consequently, cases exhibiting HBs solely in CA1 were categorised as Stage 1, while those with HBs present in both CA1 and CA2 were classified as Stage 2. Cases with HBs extending into the subiculum were designated as Stage 3, and those with HBs reaching the entorhinal cortex were assigned to Stage 4 (Figure 2A,B). Notably, this staging framework displayed a statistically significant correlation with the number of HBs of all types (CHB, BHB, SHB) within CA1, and the correlation was particularly pronounced for CHBs (ρ = 0.7508, p < 0.0001) (Figure 2C–E).
Number of Hirano bodies in each neurodegenerative disease
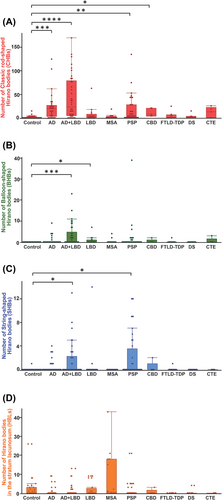
Figure 3 demonstrates the number of HBs in each disease group. The mean number of CHB in each group was as follows: control 2.2 ± 5.4, AD 23.3 ± 4.9, AD + LBD 43.4 ± 4.4, LBD 7.8 ± 6.8, MSA 6.1 ± 7.3, PSP 17.6 ± 4.6, CBD 11.8 ± 12.2, FTLD-TDP 3.9 ± 8.3, DS 2.3 ± 5.4 and CTE 11.8 ± 12.2. Significantly higher numbers of HB were found in AD (p < 0.0001), AD + LBD (p < 0.001), PSP (p < 0.01) and CBD (p < 0.05) groups compared with the control group. The mean numbers of BHB were as follows: control 0.0 ± 0.8, AD 0.7 ± 0.7, AD + LBD 3.3 ± 0.6, LBD 0.8 ± 1.0, MSA 0.4 ± 1.0, PSP 1.7 ± 0.6, CBD 0.4 ± 1.7, FTLD-TDP 0.2 ± 1.2, DS 0.0 ± 1.2 and CTE 0.6 ± 1.7. Significantly higher quantities were observed in AD + LBD (p < 0.001) and LBD (p < 0.05) groups compared with the control group. The mean number of SHB was as follows: control 0.0 ± 0.5, AD + LBD 1.6 ± 0.4, LBD 0.9 ± 0.6, MSA 0.0 ± 0.6, PSP 2.0 ± 0.4, CBD 0.4 ± 1.0, FTLD-TDP 0.1 ± 0.7, DS 0.0 ± 0.7 and CTE 0.0 ± 0.7. Significantly higher quantities were observed in AD + LBD (p < 0.05) and PSP (p < 0.05) groups compared with the control group. The mean numbers of HBL were as follows: control 2.3 ± 5.2, AD 0.6 ± 2.0, AD + LBD 0.9 ± 2.6, LBD 1.9 ± 3.4, MSA 8.4 ± 12.9, PSP 1.1 ± 3.7, CBD 0.6 ± 1.3, FTLD-TDP 0.0 ± 0.0, DS 0.4 ± 1.2 and CTE 0.0 ± 0.0, showing a trend towards higher quantities in MSA. Considering the potential impact of cell loss on the results, we endeavoured to control for the effect of pyramidal cell depletion by dividing each HB count by the estimated pyramidal cell count. Nevertheless, the results remained comparable to those obtained before implementing the control measures (Figure S2).
Correlations of Hirano bodies and the amount of protein inclusions
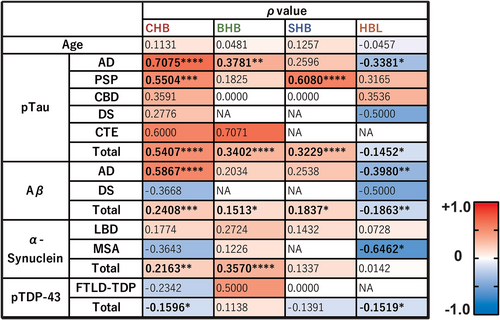
The correlation between each HB and each protein density was examined for each subgroup and the entire case series. The percentage of areas positive for phosphorylated tau staining (AT8) showed a positive correlation with all types of HBs when evaluated across the entire case series, particularly with CHBs in the AD group (ρ = 0.7075, p < 0.0001) and with SHBs in the PSP group (ρ = 0.6080, p < 0.0001). Aβ (6F/3D) also exhibited a relatively strong correlation with CHBs in the AD group (ρ = 0.5867, p < 0.0001), as did phosphorylated tau. α-synuclein (5G4) showed a significant but weak correlation with BHBs in the overall case series (ρ = 0.3570, p < 0.0001); however, this was not significant in the PD or MSA group. Phosphorylated TDP-43 did not show a strong correlation with any HB type but did exhibit a significant weak negative correlation with CHBs across cases (ρ = −0.1596, p = 0.0278). HBL showed a weak negative correlation with phosphorylated tau (ρ = −0.3381, p = 0.0108) and Aβ (ρ = −0.3980, p = 0.0024) in the AD group, and a stronger negative correlation with α-synuclein in MSA (ρ = −0.6462, p = 0.0125) (Figures 4 and S3).
Influence of background pathology, age and sex
To examine the association of background pathology and age with each HB, we first explored whether age correlates with each HB count and each pathological stage within the entire cohort. Our findings revealed that none of the HB types exhibited a significant correlation with age (Figures 4 and S3), whereas all pathological stages evaluated in this study showed a significant correlation with age (Figure S4). The mean number of HBs peaked in the 80s for CHB (21.8 ± 35.9) and in the 90s or older for the other types of HBs: BHB (4.7 ± 12.9), SHB (1.8 ± 2.8) and HBL (2.9 ± 7.0) (Figure S3).
Second, our cohort comprised cases with overlapping background diseases such as AD and LBD, and there was statistically significant variation in age and background pathology among each disease group classified. Also, the number of CHBs exhibited a significant positive correlation with the estimated number of pyramidal cells (ρ = −0.2079, p = 0.0042), suggesting a potential impact of cell loss on this association (Figure S1D–F). To account for these factors, we conducted an ANCOVA to adjust our findings (Table 2). The ANCOVA results indicated that the presence of CHB was independently and significantly influenced by a high Braak NFT stage and the number of preserved pyramidal cells in the CA1 region. BHBs were independently and significantly affected by male sex, the number of pyramidal cells (NPC), LBD, and TDP-43 pathology. SHBs were independently and significantly affected by female sex, high Braak NFT stage, the presence of PSP, and AGD. Finally, HBL was significantly associated with MSA. However, age was not identified as an independent factor influencing the presence or absence of any type of HB.
| Explanatory variable (target side) | Classic rod-shaped Hirano body (CHB) | Balloon-shaped Hirano body (BHB) | String-shaped Hirano body (SHB) | Hirano body in the stratum lacunosum (HBL) | ||||
|---|---|---|---|---|---|---|---|---|
| Estimated value (95% CI) | p value | Estimated value (95% CI) | p value | Estimated value (95% CI) | p value | Estimated value (95% CI) | p value | |
| Age | −0.03 (−0.39, 0.33) | 0.8717 | −0.02 (−0.06, 0.01) | 0.2451 | −0.007 (−0.034, 0.020) | 0.5890 | −0.03 (−0.09, 0.03) | 0.2828 |
| Sex (female) | −0.67 (−4.76, 3.41) | 0.7458 | −0.45 (−0.86, −0.03) | 0.0350 | 0.35 (0.04, 0.66) | 0.0254 | 0.17 (−0.51, 0.84) | 0.6254 |
| NPC in CA1 | 0.021 (0.010, 0.031) | 0.0002 | 0.001 (0.000, 0.002) | 0.0203 | 0.001 (0.000, 0.001) | 0.1563 | ||
| Vessel injury score | 1.00 (−1.79, 3.80) | 0.4805 | −0.02 (−0.31, −0.26) | 0.8629 | −0.08 (−0.29, 0.13) | 0.4570 | −0.17 (−0.64, 0.29) | 0.4633 |
| Braak NFT stage (high) | 12.91 (6.64, 19.18) | <0.0001 | 0.36 (−0.27, 1.00) | 0.2680 | 0.53 (0.06, 1.00) | 0.0286 | −0.24 (−1.25, 0.76) | 0.6342 |
| Thal phase (high) | 3.19 (−3.00, 9.38) | 0.3108 | 0.40 (−0.23, 1.03) | 0.2157 | 0.35 (−0.11, 0.82) | 0.1362 | −0.35 (−1.35, 0.65) | 0.4899 |
| Lewy pathology (positive) | 3.59 (−1.17, 8.35) | 0.1383 | 0.83 (0.35, 1.32) | 0.0008 | 0.13 (−0.22, 0.49) | 0.4615 | 0.34 (−0.44, 1.13) | 0.3896 |
| MSA (positive) | 1.88 (−6.43, 10.19) | 0.6562 | 0.29 (−0.55, 1.13) | 0.4978 | 0.18 (−0.44, 0.81) | 0.5604 | 3.74 (2.34, 5.14) | <0.0001 |
| PSP (positive) | 3.27 (−2.64, 9.17) | 0.2765 | 0.25 (−0.35, 0.85) | 0.4083 | 0.97 (0.52, 1.41) | <0.0001 | −0.27 (−1.24, 0.70) | 0.5828 |
| CBD (positive) | −2.25 (−11.42, 15.92) | 0.7455 | −0.28 (−1.67, 1.11) | 0.6922 | 0.32 (−0.71, 1.35) | 0.5389 | −0.23 (−2.48, 2.01) | 0.8348 |
| AGD (positive) | −3.22 (−8.53, 2.09) | 0.2332 | −0.03 (−0.57, 0.51) | 0.9109 | 0.52 (0.12, 0.92) | 0.0114 | 0.30 (−0.57, 1.17) | 0.4986 |
| TDP-43 proteinopathy (positive) | 0.80 (−4.58, 6.18) | 0.7693 | 0.88 (0.33, 1.42) | 0.0018 | −0.31 (−0.71, 0.10) | 0.1354 | −0.20 (−1.03, 0.63) | 0.6405 |
- Note: Statistically significant items are in bold.
- Abbreviations: AGD, argyrophilic grain disease; ANCOVA, analysis of covariance; CBD, corticobasal degeneration; LBD, Lewy body disease; MSA, multiple system atrophy; NPC, number of pyramidal cells; PSP, progressive supranuclear palsy; TDP-43, TAR DNA binding protein 43.
In the majority of cases with HBs, CHBs were the predominant subtype. Notably, three cases exhibited dominance of BHBs, but these cases were complicated by multiple concurrent pathologies, making it challenging to ascertain which pathology primarily influenced this outcome (Table S1).
Changes in Hirano body counts with disease progression
To investigate the relationship between the number of HBs and disease progression, we analysed the correlation between HB counts and various pathological stages, including ADNC level [31], Lewy pathology consensus criteria [29], LATE-NC stage [28] and AGD Saito stage [27] (Figure S5).
The results revealed that ADNC level and Lewy pathology type exhibited significant positive correlations with the number of all types of HBs. Specifically, ADNC level showed a relatively strong correlation with the number of CHBs (ρ = 0.2784, p < 0.0001), while Lewy pathology type demonstrated a relatively strong correlation with the number of BHBs (ρ = 0.3607, p < 0.0001). The LATE-NC stage displayed a significant positive correlation solely with the number of BHBs (ρ = 0.2559, p = 0.0005), and the AGD stage exhibited a significant positive correlation exclusively with the number of SHBs (ρ = 0.1574, p = 0.0292). Additionally, HBL counts demonstrated a significant weak negative correlation solely with ADNC levels (ρ = −0.1678, p = 0.0197). These correlations remained consistent when each HB count was adjusted for pyramidal cell count, except for the correlation between AGD stage and SHB count (Figure S6).
Looking at the number of HBs in each stage separately, a linear increase in the number of HBs was observed with the progression of the disease in the AGD stage, while a peak was observed in the ADNC level and LATE stage at Intermediate and Stage 1, respectively. This change was particularly observed in the SHB counts of ADNC and LATE stages. The observed change was statistically significant for the ADNC level regarding SHB counts, but not for the decrease from Intermediate to High levels regarding CHBs and BHBs. The post hoc power of these changes was calculated statistically to be 50% for CHBs and 46% for BHBs, suggesting that a total of 229 and 270 cases with ADNC levels of Intermediate and High, respectively, would be required to achieve 80% power.
DISCUSSION
In this study, we conducted a cross-sectional analysis of 193 hippocampi across a range of neurodegenerative diseases to explore the relationship between the quantity and distribution of the different types of HBs and neuropathological variables. Our investigation revealed that HBs are not only present in AD but also in LBD, PSP, MSA, AGD and TDP-43 proteinopathy. Furthermore, we identified a previously undocumented and novel morphological variant of HBs, suggesting potential variations in their morphological appearance based on the underlying pathology. Additionally, we proposed a novel approach for staging HB pathology in the posterior hippocampus using HE-stained sections, offering a convenient means to assess the severity of HB-related changes.
We conducted an extensive analysis of the distribution and prevalence of HBs across seven distinct subregions within the posterior hippocampus. This scrutiny led to the identification of specific patterns, which in turn facilitated the creation of a pathological staging framework. Intriguingly, this staging system exhibited a strong correlation with the quantity of CHBs present in the CA1 region. Consequently, this staging approach provides a convenient means to assess HB severity through a straightforward semiquantitative assessment, obviating the need for direct HB counting within CA1.
While the three-dimensional structure of HBs has been elucidated through electron microscopy in previous studies [44, 45], this current research marks an advance in our morphological understanding of HBs by introducing the first-ever macroscopic three-dimensional visualisation. Our analysis has successfully visualised the three-dimensional morphology of each type of HB, uncovering distinct characteristics such as the rugby ball-like appearance of CHB, the spherical nature of BHB and the elongated rod-like structure of SHB. This three-dimensional insight helps to interpret their planar pathological findings. For example, it can be inferred that it is difficult to distinguish the cut surface shape of CHBs from that of SHBs, and as a result of evaluating SHBs, which were not delineated with a long diameter as were CHBs, the present study may have underestimated SHBs and overestimated CHBs. This could explain why no SHB-dominant cases were detected in this study. In the future, it may be useful to distinguish between CHBs and SHBs in cross-sections of HBs by the length of their short diameters. Such three-dimensional insight has been obtained for several other neuropathological structures [46], and hopefully, more structures can be examined in this fashion.
While previous research has linked HBs to neurological disorders such as AD, Guam ALS-PDC, Pick's disease and CJD [1, 8-12], our study is the first to report and systematically evaluate HBs in association with LBD, PSP, MSA, AGD and TDP-43 proteinopathy. This observation implies that HBs might emerge within the context of various neurodegenerative pathologies, suggesting a common mechanism underlying their formation.
However, there might also be disease-specific aspects regarding the quantity, localisation and morphology of HBs. Notably, our results highlight a particularly strong influence of AD on the prevalence of HBs. Within the spectrum of AD-related pathologies, tau pathology appears to exert a more significant effect on HB formation compared with Aβ pathology. Although prior studies have indicated that Aβ peptides can induce actin polymerisation in hippocampal cells [47], the relationship between tau and HBs warrants further detailed investigation. Additionally, our findings suggest that TDP-43 pathology might lead to the formation of larger HBs, akin to the BHBs described by Martinez et al. in CJD cases [12]. On the other hand, conditions characterised by 4R tau pathologies like PSP and AGD appear to result in elongated HBs, resembling SHBs. In MSA, the presence of HBLs appears to be a distinct feature, providing novel insights into the distinct morphological patterns of HBs associated with different pathologies.
TDP-43, implicated as the major pathological correlate to BHB, serves as the major protein associated with hippocampal sclerosis [48, 49] and preferentially affects CA1 pyramidal cells. Prominent cases of BHB have also been reported in CJD, a prion disease recognised for its association with severe neuronal loss that spares the hippocampus in the most frequent forms [12]. AD is mainly associated with CHB and has been reported to be associated with atrophy and neuronal loss in CA1 [50-52], although it has not been identified as a cause of hippocampal sclerosis. PSP and AGD are associated with SHB and have not been reported to be associated with selective neuronal loss in CA1 but are more likely to affect the CA2 subregion [25]. These observations allow the hypothesis that neurodegenerative diseases highly affecting the CA1 subregion may result in the formation of BHBs, while those associated with less involvement may lead to the formation of SHBs. However, this hypothesis is not entirely supported by the relationship between BHBs and LBD.
In addition to the hippocampus, HBs have been reported in various brain regions. For example, Okamoto et al. observed HBs in three patients with hepatic encephalopathy, affecting regions such as the substantia nigra, dentate nucleus and frontal lobe [53]. Hepatic encephalopathy is a potentially reversible disease, and, intriguingly, HBs are observed in such a condition, suggesting that HBs are pathological findings secondary to various conditions. However, studies of HBs outside the hippocampus in pathologies other than these neurodegenerative diseases are very limited. Therefore, as an initial step, we conducted a comprehensive analysis of the posterior hippocampus in major neurodegenerative diseases. Additionally, there have been instances of HB-like actin polymerisation pathology detected at a microstructural level, which remains unobservable in standard HE-stained sections [1]. This pathology has been documented in anterior horn cells and axons in motor neuron diseases, Purkinje cells in Crow–Fukase syndrome [54], nucleus basalis of Meynert in Parkinson's disease, myelinated axons in progressive multifocal leukoencephalopathy [16] and cerebral white matter axons in Nasu–Hakola disease [55, 56]. The extent to which these structures align with HBs as observed in HE sections, in terms of size and underlying mechanisms, remains uncertain. Nevertheless, these collective findings strongly suggest that the specific sites of HB occurrence may indeed be disease specific. As such, a more systematic investigation into this aspect is warranted.
Distinct patterns emerged among different neurodegenerative pathologies. In cases of AD and LATE-NC, HB counts tended to peak at a specific stage of disease advancement. Notably, AD displayed its peak at the intermediate stage, while LATE-NC exhibited its apex at Stage 1. In contrast, in Lewy pathology, a marked tendency for the number of HBs to increase with disease progression was observed, and this upward trend was also observed between the number of SHBs and the AGD Saito Stage. This may explain why AD + LBD cases exhibited higher HB counts than AD cases. AD cases, characterised by more severe AD pathology, experienced a peak-out phenomenon in HB counts in a greater number of cases. However, it was difficult to show statistical significance in the number of HBs between pathological stages, and the regression analysis could not cover the evaluation of disease duration because the overall number of cases was insufficient. This issue should be resolved in future studies.
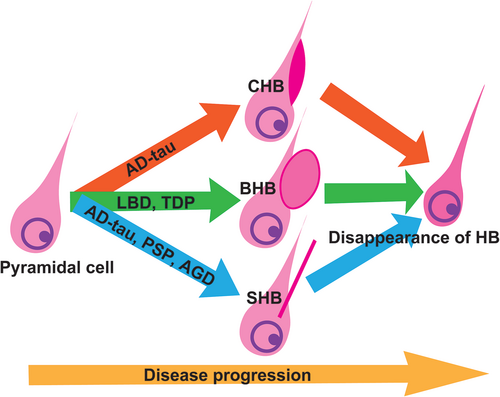
Our study also indicated that HB may represent a transient pathological finding that manifests only during a specific period of disease progression. Considering the neuropathological context of AD and LATE-NC, both conditions are characterised by a propensity for hippocampal neuron depletion, primarily in the CA1 region [49-51, 57-59]. This cell loss may contribute to the observed phenomenon of transient increase of HB numbers in earlier stages. Our analysis indicated significant correlations between the counts of pyramidal cells and CHBs, as well as SHBs. Moreover, while not statistically significant, there was suggestive evidence of a positive correlation between BHB and SHB counts and pyramidal cell counts. Further regression analysis underscored the independent influence of pyramidal cell counts on the appearance of CHBs and BHBs, reinforcing the connection. Given that both AD and LATE-NC are notably linked with the loss of pyramidal cells in the CA1 region, it is logical that LATE-NC, presumed to be more strongly associated, reaches its zenith early on, specifically at Stage 1. However, an additional observation surfaced as the ratio of HBs to pyramidal cell counts exhibited a decline in tandem with the advancement of ADNC levels and LATE-NC stages. This intriguing trend suggests that HBs may diminish even before pyramidal cells undergo substantial loss. In summary, it appears that HBs are transient pathological features that present differently in various pathologies and tend to disappear before significant pyramidal cell loss occurs (Figure 5).
Contrary to the prevailing assumption of an age-related elevation in the number of HBs within CA1 [1, 60], our present findings do not establish a distinct correlation between age and HB counts. However, each of the HBs tended to be more common in older cases, with the average number of HBs peaking in the 80s or older. This phenomenon can likely be attributed to the heightened incidence of various neurodegenerative disorders with advancing age. A multitude of neurodegenerative diseases exhibit an inclination to escalate in both severity and prevalence as age progresses [61]. In our cohort, as illustrated in Figure S3, each pathological stage demonstrated a significant increase with age. As a result, the augmented prevalence of diverse neurodegenerative pathologies with age potentially accounts for the observed rise in HB numbers.
Cerebrovascular damage is indeed known to increase with age [62], and the vulnerability of the CA1 region to ischemic damage is recognised [63-65]. Consequently, it could be assumed that HBs are also impacted by ischaemia. However, our results did not support this assumption. Nonetheless, conducting a comprehensive evaluation of ischaemia poses challenges due to the variety of assessment scales available.
Of particular interest, earlier studies have suggested a phenomenon known as a “peak-out” in HB counts within the stratum lacunosum with ageing, implying a unidirectional increase or decrease solely linked to age [1, 60]. However, our results challenge this notion. Our findings indicate that the presence of HBLs may be influenced by MSA and other underlying pathological conditions, with some reports even indicating an increase in chronic alcohol use disorder [66]. These observations suggest the existence of specific background pathologies that lead to an elevation of HBLs. Moreover, our investigation revealed a negative correlation between the percentage of α-synuclein-positive areas in the stratum lacunosum and the number of HBLs in MSA cases, suggesting a potential increase in HBLs during the early stages of mild MSA. This implies that HBLs may respond quickly to pathological changes caused by MSA and other factors and then decline early.
Our understanding of the symptoms and potential treatments associated with HBs is progressively improving. The hippocampus, a prominent location for HBs, has long been recognised for its role in memory functions [67]. Recent studies conducted in mouse models of HB have unveiled that alterations in synaptic responses within the hippocampus are responsible for impairments in spatial working memory [68, 69]. Furthermore, recent investigations using in vitro and yeast models have demonstrated that specific antimicrobial agents possess the capability to disrupt HB-like actin aggregates [70, 71]. These findings from these mouse and yeast models suggest that HBs are associated with exacerbated cognitive dysfunction, but their formation may be treatable. Our study has further demonstrated that HB occurrence may escalate not only in AD but also in other neurodegenerative diseases such as PSP, AGD and TDP-43 proteinopathy. Since cognitive decline is prevalent in these diseases as well [28, 72-75], and HBs may be associated with cognitive decline in many neurodegenerative diseases, common therapeutic approaches could be effective. However, evidence supporting these findings is currently limited, and further studies are warranted.
A limitation of our study is the scarcity of cases with pure background pathology in our cohort, and many cases presented multiple pathologies, complicating straightforward comparisons of each HB with background pathology. To address this challenge, we endeavoured to elucidate the association between each HB and background pathology as comprehensively as possible through regression analysis and other statistical methods. Additionally, the limited number of cases for CBD and CTE (five each) hindered a thorough evaluation. Our study is largely an exploratory investigation, particularly for these pathologies with a small number of cases.
Our study introduced a new morphological category of hippocampal HBs and visually depicted its three-dimensional structure. Furthermore, we proposed a pathological staging system to easily assess the extent of HB. The study also revealed an increased presence of HBs in the hippocampus across various neurodegenerative diseases, including AD, LBD, PSP, MSA, AGD and TDP-43 proteinopathy, and highlighted how its morphology and effects vary depending on the underlying pathology. This increased presence of HBs in the hippocampus across a variety of neurodegenerative diseases underscores the variations in its morphology and effects associated with different underlying pathologies. Although the pathological examination of HBs has received limited attention in recent years, their prevalence across multiple neurodegenerative diseases underscores the need for more comprehensive pathological elucidation, potentially opening doors to understanding disease pathogenesis. This study serves as an impetus to expedite further research into HBs and highlights that meticulous evaluation strategies of previously underappreciated histological alterations can reveal potential contributing pathogenic aspects.
AUTHOR CONTRIBUTIONS
Koji Yoshida: Study design, data collection and analysis, drafting the manuscript, tables, and figures. Shelley Forrest: Proofreading, antibody testing, contributed to the pathology evaluation, critically reviewed the manuscript. Shojiro Ichimata, Hidetomo Tanaka: contributed to the pathology evaluation, critically reviewed the manuscript. Tomoya Kon: Annotation check, contributed to confocal microscope evaluation, critically reviewed the manuscript. Maria Carmela Tartaglia, Charles H. Tator, Anthony E. Lang: Providing clinical information, critically reviewed the manuscript. Naoki Nishida: Supervision, critically reviewed the manuscript. Gabor G. Kovacs: Supervision, pathology evaluation, data analysis, critically reviewed the manuscript.
ACKNOWLEDGEMENTS
The authors would like to thank the many patients who made this research possible. We would also like to thank Jun Li and Ali M. Karakani for their technical support, and Ain Kim and Eliza King for their assistance with case data collection and slide scanning.
CONFLICT OF INTEREST STATEMENT
AEL has served as an advisor for AbbVie, AFFiRis, Alector, Amylyx, Aprinoia, Biogen, BioAdvance, BlueRock, Biovie, BMS, CoA Therapeutics, Denali, Janssen, Jazz, Lilly, Novartis, Paladin, Pharma 2B, PsychoGenetics, Retrophin, Roche, Sun Pharma and UCB; received honoraria from Sun Pharma, AbbVie and Sunovion; received grants from Brain Canada, Canadian Institutes of Health Research, Edmond J Safra Philanthropic Foundation, Michael J. Fox Foundation, the Ontario Brain Institute, Parkinson Foundation, Parkinson Canada and W. Garfield Weston Foundation; is serving as an expert witness in litigation related to paraquat and Parkinson's disease; received publishing royalties from Elsevier, Saunders, Wiley-Blackwell, Johns Hopkins Press and Cambridge University Press. GGK has served as an advisor for Biogen; received a royalty for 5G4 synuclein antibody and publishing royalties from Wiley, Cambridge University Press and Elsevier; received grants from Edmond J Safra Philanthropic Foundation, Rossy Family Foundation, Michael J. Fox Foundation, Parkinson Canada and Canada Foundation for Innovation. The Editors of Neuropathology and Applied Neurobiology are committed to peer-review integrity and upholding the highest standards of review. As such, this article was peer-reviewed by independent, anonymous expert referees and the authors (GGK) had no role in either the editorial decision or the handling of the paper. KY, SLF, SI, HT, TK, MCT, CHT and NN declare no competing interests.
ETHICS STATEMENT
All brains have been obtained at autopsy through appropriate consenting procedures with Local Ethical Committee approval. This study was approved by the UHN Research Ethics Board (Nr. 20-5258) and the University of Toronto (Nr. 39459) and was performed per the ethical standards established in the 1964 Declaration of Helsinki, updated in 2008.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




