Pathological neutrophil migration predicts adverse outcomes in hospitalized patients with liver cirrhosis
Matthias Gunzer and Christian M. Lange contributed equally to this study.
Handling Editor: Luca Valenti
Abstract
Background and Aims
Given the early response of neutrophil granulocytes to infections, detection of pathological neutrophil migration might help in predicting adverse events in patients with liver cirrhosis.
Methods
Migration of blood neutrophils in hospitalized patients with cirrhosis was characterized by a novel standardized migration assay. Pathological neutrophil migration patterns were associated with a composite endpoint of ACLF, sepsis or death within 7 or 30 days.
Results
Overall, 125 patients were included, of whom 11 (8.8%) had compensated cirrhosis, 84 (67.2%) had acute decompensation (AD) and 30 (24%) had acute-on-chronic liver failure (ACLF). The migration response of neutrophils from patients with AD or ACLF to stimulation with the chemotactic formylpeptide f-Met-Leu-Phe (fMLP) was significantly impaired, while the response to chemokine (C-X-C motif)-ligand 8 (CXCL8) was affected less pronouncedly. In contrast, no relevant differences in response to CXCL1 were observed. Of note, neutrophils of a number of patients with AD and ACLF were largely immotile at resting and stimulated conditions. Patients with non-migrating neutrophils at unstimulated conditions were at high risk to develop the composite endpoint of ACLF, sepsis or death. Moreover, expression of chemokine receptors CXCR1 and CXCR2 was significantly decreased in patients with ACLF. Interestingly, the expression of chemokine receptors did not correlate with neutrophil migration patterns, but—based on the increased expression of the cell surface markers CD66b and CD177—neutrophils of patients with AD and ACLF were strongly pre-activated.
Conclusion
Pathological neutrophil migration in patients with cirrhosis indicates a high risk of developing adverse outcomes.
Lay Summary
Neutrophil granulocytes are important components of very early immune responses against infections. We have observed neutrophil migration patterns in hospitalized patients with liver cirrhosis, that strongly differ from healthy controls and are associated with a high risk of developing adverse outcomes like sepsis or death. These migration patterns can be monitored with a novel, standardized migration assay.
1 INTRODUCTION
Patients with unstable acute decompensation (AD) of liver cirrhosis or with acute-on-chronic liver failure (ACLF, i.e. development of defined organ failures in patients with AD) are burdened with significant short-term mortality.1-3 On a pathophysiological basis, increasing data show that AD and in particular ACLF are associated with high levels of systemic inflammation and co-existence of immunosuppression.1-3 As a consequence, AD and ACLF are characterized by a high risk of developing infections, which further fuel immune dysfunction and contribute to the high mortality of these patients.1-3 Many patients with unstable AD and in particular with ACLF require early liver transplantation to improve survival. Yet, liver transplantation can be difficult or impossible because of delayed diagnosis of infections or development of ACLF with multiple organ failures.4, 5 Hence, early diagnosis of infections and prediction of development of ACLF remain relevant unmet medical needs in the management of patients with liver cirrhosis.
Neutrophils are important effectors in the first-line defence against bacterial and fungal infections, and impaired neutrophil function is considered a major determinant of susceptibility to cirrhosis-associated infections.6 Given the very early response of neutrophils to infections and autonomous cell migration being a key determinant of infection defence, detection of pathological neutrophil migration might be useful for early prediction of infections and ACLF in patients with cirrhosis. Indeed, we have shown before that defective neutrophil motility can be a highly specific marker for disease conditions.7, 8 In this study, we therefore aimed to characterize migration of blood neutrophils in patients throughout the entire spectrum of liver cirrhosis using a novel standardized, reliable migration assay.8
2 PATIENTS AND METHODS
2.1 Patients
Consecutive patients who were hospitalized with liver cirrhosis to the Department of Gastroenterology and Hepatology of the University Hospital Essen between January 2020 and April 2021 were included in this study. Patients were excluded if they did not agree in the participation of the study, if they were younger than 18 years old, if hepatocellular carcinoma (HCC) beyond Milan was diagnosed, if they were infected with human immunodeficiency virus or in case of therapy with immunosuppressive agents. In addition, patients with end-stage cardiovascular disease, strongly impaired renal function not because of hepatorenal syndrome, cancer other than HCC or severe neurological disease were excluded. This study was approved by the local ethical committee (18-8291BO). In addition, neutrophils of 24 healthy individuals were analysed as control.
2.2 Quantification of neutrophil migration
Neutrophil migration was quantified as described previously.8 In brief, neutrophils were isolated from EDTA blood at baseline of admission by negative isolation using magnetic beads (MACSxpress® Whole Blood Neutrophil Isolation Kit, human [Miltenyi Biotec]), resulting in a purity of living neutrophils of 94 ± 8% (mean ± SD) as assessed by CD15 and CD16 expression by flow cytometric analysis. An age- and sex-matched cohort of healthy volunteers was measured in parallel. The average yield of neutrophil isolation was 10.0 ± 0.6 × 106 cells from 5 ml blood. Within less than 6 h after isolation, neutrophils were seeded in 96-well flat-bottom plates in X-Vivo 10 Medium (Lonza), supplemented with human serum replacement 3 (Sigma-Aldrich) at a density of 8250 cells per well and stimulated with or without 10 nM N-Formylmethionyl-leucyl-phenylalanine (fMLP) (Sigma-Aldrich), 100 ng/ml C-X-C motif chemokine ligand (CXC-L)1 (PeproTech) or 100 ng/ml CXCL8 (PeproTech). Dose–response curves had been established previously8 in order to identify optimal concentrations of chemoattractants and migration conditions, since too low doses of chemoattractants may result in suboptimal migration while too high doses may result in downregulation of migration.
Neutrophil migration was then quantified by imaging with a Leica DMI6000 B (Leica Microsystems) system coupled to a workstation running Leica Application Suite X (LASX, Leica Microsystems) with a motorized stage with a HC PL FLUOTAR L 20×/0.40 DRY objective (Cat. No.: 11506243, Leica Microsystems) at an imaging rate of one frame every 8 s for 60 minutes at 37°C, without CO2. The generated movies were exported as *.mov files. These files were analysed with the Automated Cellular Analysis System (ACAS, Metavi-Harmony software, MetaVi Labs). The evaluation interval was set to 30 s, the minimum track duration to 60 s, the movement threshold to 8 μm and the microscopy resolution to 0.458716 pixel/μm. A total quantification time of 60 min has been shown to be optimal in the establishment of our assay.8
2.3 Flow cytometry
For flow cytometry, 100 000 neutrophils were seeded in 96-well plates and washed once with PBS at 300 g for 5 min. Cells were stained with antibodies targeting neutrophil lineage markers (CD15 [VioBlue; Miltenyi Biotec] and CD16 [FITC; Miltenyi Biotec]), and chemokine receptors CXCR1 (PE; Miltenyi Biotec), CXCR2 (PE Vio770; Miltenyi Biotec) and formyl peptide receptor 1 (FPR1) receptor (AF647; BD). Additionally, activation markers CD11b (PE; Miltenyi Biotec), CD66b (PE; Miltenyi Biotec) and CD177 (PE; Miltenyi Biotec) were stained. After incubation for 15 min at 4°C, 100 μl PBS were added before measurement on a MACS Quant VYB (Miltenyi Biotec) running the corresponding software MACS Quantify (version 2.11).
2.4 Statistical analysis
Patients were prospectively followed and associations between neutrophil migration and a composite endpoint of developing sepsis, ACLF or death within 7 and 30 days were assessed. Statistical analyses were performed using Graphpad Prism version 9.3.1 (GraphPad Software Inc.). Statistical analysis of patient characteristics was assessed by means of χ2 contingency tables or one-way anova or Kruskal–Wallis test, as appropriate. Experimental group differences were assessed by one-way anova or Kruskal–Wallis test as appropriate after normality Test. Associations of outcomes with continuous or dichotomic variables were assessed in linear and logistic regression models respectively.
3 RESULTS
3.1 Baseline characteristics of included patients
Overall, 125 patients who fulfilled the described selection criteria were included in our study. Of those, 11 (8.8%) patients had compensated cirrhosis, 84 (67.2%) patients had AD of cirrhosis without acute-on-chronic liver failure (ACLF) at admission and 30 (24%) patients had ACLF at baseline. During 30 days of follow-up, 0 (0%), 6 (7.14%) and 6 (20%) of patients with compensated cirrhosis, AD and ACLF died; and 0 (0%), 3 (3.57%) and 6 (20%) of patients with compensated cirrhosis, AD and ACLF developed sepsis within 30 days. Detailed baseline characteristics are shown in Table 1. In addition, neutrophil migration was quantified in 24 healthy individuals (eight females [33%] and 16 males [67%]), with a mean age of 45.1 ± 14.6 years.
| Comp. (N = 11) | AD (N = 84) | ACLF (N = 30) | p-value (Comp. vs AD) | p-value (Comp. vs ACLF) | p-value (AD vs ACLF) | |
|---|---|---|---|---|---|---|
| General characteristics | ||||||
| Age (years), mean (SD) | 53.18 (15.54) | 54.87 (11.80) | 56.70 (10.99) | .79 | .79 | .79 |
| Male gender, N (%) | 6 (54.55) | 46 (54.76) | 20 (66.67) | .99 | .5 | .3 |
| Child–Pugh score, mean (SD) | 5.27 (0.47) | 8.54 (1.50) | 9.37 (1.69) | <.0001 | <.0001 | .08 |
| CLIF OF score, mean (SD) | 6.73 (1.79) | 8.03 (2.36) | 10.0 (1.73) | .06 | <.0001 | <.0001 |
| CLIF C AD/ACLF, mean (SD) | 32.75 (10.17) | 38.51 (9.58) | 47.71 (8.63) | .06 | <.0001 | <.0001 |
| MELD score, mean (SD) | 10.00 (2.92) | 18.13 (7.55) | 23.92 (8.50) | .02 | .0002 | .0092 |
| Aetiology of liver cirrhosis | ||||||
| Viral, N (%) | 4 (36.36) | 7 (8.33) | 1 (3.33) | .006 | .004 | .4 |
| NASH, N (%) | 3 (27.27) | 8 (9.52) | 3 (10.00) | .08 | .2 | .9 |
| Alcoholic, N (%) | 1 (9.09) | 45 (53.57) | 23 (76.67) | .006 | <.0001 | .03 |
| Cholestatic, N (%) | 1 (9.09) | 5 (5.95) | 1 (3.33) | .7 | .4 | .6 |
| Others, N (%) | 2 (18.18) | 19 (22.62) | 1 (3.33) | .7 | .1 | .02 |
| Clinical biochemistry | ||||||
| Leukocytes (per nl), mean (SD) | 5.07 (2.61) | 7.27 (5.23) | 9.22 (5.99) | .7 | .08 | .2 |
| Neutrophils (per nl), mean (SD) | 4.90 (2.15) | 6.19 (4.50) | 9.55 (6.41) | .99 | .7 | .5 |
| Monocytes (per nl), mean (SD) | 0.51 (0.34) | 0.72 (0.40) | 1.10 (0.57) | .4 | .1 | .1 |
| Lymphocytes (per nl), mean (SD) | 0.88 (0.50) | 1.40 (1.28) | 1.27 (0.89) | .99 | .99 | .99 |
| Haemoglobin (g/dl), mean (SD) | 12.09 (1.51) | 9.15 (2.30) | 8.29 (1.55) | .0006 | <.0001 | .1 |
| Platelets (per nl), mean (SD) | 125.9 (82.65) | 129.6 (103.7) | 97.20 (58.28) | >.99 | >.99 | .8 |
| CRP (mg/dl), mean (SD) | 1.40 (2.17) | 2.72 (2.76) | 5.20 (4.76) | .2 | .0003 | .0029 |
| Sodium (mmol/L), mean (SD) | 138.6 (3.04) | 136.0 (4.93) | 135.9 (5.80) | .4 | .4 | >.99 |
| Creatinine (mg/dl), mean (SD) | 0.98 (0.20) | 1.08 (0.41) | 2.56 (1.24) | >.99 | .0001 | <.0001 |
| Bilirubin (mg/dl), mean (SD) | 1.15 (0.47) | 5.06 (6.03) | 9.93 (11.09) | .0037 | .0001 | .1 |
| AST (U/L), mean (SD) | 49.55 (36.66) | 70.48 (78.51) | 73.20 (80.63) | .5 | >.99 | >.99 |
| ALT (U/L), mean (SD) | 61.73 (74.34) | 48.82 (104.2) | 37.10 (40.18) | >.99 | .96 | .4 |
| GGT (U/L), mean (SD) | 151.5 (178.7) | 158.7 (167.2) | 146.4 (205.9) | >.99 | >.99 | .8 |
| AP (U/L), mean (SD) | 173.1 (153.0) | 155.2 (94.49) | 145.3 (102.5) | >.99 | >0.99 | >0.99 |
| INR, mean (SD) | 1.13 (0.17) | 1.51 (0.49) | 1.78 (0.95) | .001 | .0002 | .5 |
| Albumin (g/dl), mean (SD) | 3.55 (0.21) | 3.00 (0.60) | 3.04 (0.72) | .5 | .5 | .8 |
| ACLF grade | ||||||
| Grade 1, N (%) | – | – | 13 (43.33) | – | – | – |
| Grade 2, N (%) | – | – | 15 (50.00) | – | – | – |
| Grade 3, N (%) | – | – | 2 (6.67) | – | – | – |
| Complications of liver cirrhosis | ||||||
| Hepatic encephalopathy | ||||||
| Grade 0, N (%) | 11 (100.00) | 33 (19.88) | 15 (50.00) | <.0001 | .003 | .0003 |
| Grade 1, N (%) | 0 (0.00) | 7 (8.33) | 9 (30.00) | .3 | .04 | .003 |
| Grade 2, N (%) | 0 (0.00) | 3 (3.57) | 4 (13.33) | .5 | .2 | .06 |
| Grade 3, N (%) | 0 (0.00) | 2 (2.38) | 2 (6.67) | .6 | .4 | .3 |
| Gastrointestinal bleeding | ||||||
| N (%) | 0 (0.00) | 8 (9.76) | 5 (16.67) | .3 | .1 | .3 |
| Infections | ||||||
| N (%) | 0 (0.00) | 26 (30.95) | 19 (63.33) | .4 | .01 | .002 |
| Ascites | ||||||
| No ascites, N (%) | 10 (90.91) | 24 (28.57) | 15 (50.00) | <.0001 | <.0001 | .2 |
| Moderate, N (%) | 0 (0.00) | 7 (8.33) | 4 (13.33) | .3 | .2 | .4 |
| Massive, N (%) | 1 (09.09) | 53 (63.10) | 21 (70.00) | .0007 | .0005 | .5 |
| Oesophageal varices | ||||||
| Grade 0, N (%) | 5 (45.45) | 29 (35.52) | 13 (45.45) | .5 | .9 | .4 |
| Grade 1, N (%) | 3 (27.27) | 29 (34.52) | 5 (16.67) | .6 | .4 | .1 |
| Grade 2, N (%) | 1 (9.09) | 24 (23.08) | 12 (40.00) | .3 | .06 | .07 |
| Grade 3, N (%) | 2 (18.18) | 5 (5.95) | 0 (0.00) | .1 | .02 | .17 |
| Outcome | ||||||
| Mortality within 30 days, N (%) | 0 (0.00) | 6 (7.14) | 6 (20.00) | >.99 | .4 | .05 |
| ACLF development within 30 days, N (%) | 0 (0.00) | 5 (5.95) | – | .4 | – | – |
| Sepsis within 30 days, N (%) | 0 (0.00) | 3 (3.57) | 6 (20.00) | .5 | .1 | .004 |
| Composite outcome (ACLF development, sepsis, death) within 30 days, N (%) | 0 (0.00) | 11 (13.10) | 10 (33.33) | .2 | .03 | .01 |
3.2 Pathological neutrophil migration predicts adverse outcomes of patients with liver cirrhosis
The average proportion of unstimulated moving neutrophils was numerically lower (but statistically not different) in patients with AD or ACLF compared to healthy controls, while no differences in the average speed of unstimulated neutrophils were observed (Figure 1A,B). Interestingly, however, the migration response of neutrophils from patients with AD or ACLF to stimulation with fMLP was significantly impaired compared to neutrophils of patients with compensated cirrhosis or healthy controls (Figure 1A,B). The reduction in the proportion of moving neutrophils in patients with ACLF and reduced average speed of neutrophils in patients with AD and ACLF after stimulation with CXCL8 was less pronounced (Figure 1A,B). In contrast, no significant differences in the proportion of moving cells and their average speed were observed between patient groups after stimulation of neutrophils with CXCL1 (Figure 1A,B). Neutrophil migration patterns according to sub-stratification of AD in stable AD, unstable AD and pre-ACLF1-3 are shown in SI Figure S1 and suggest a progressive impairment of neutrophil migration with the severity of AD. As shown in SI Figure S2, the precipitating event (alcoholic hepatitis, bacterial infection or others) of AD or ACLF, as well as the number of organ failures, had no significant impact on the average proportion of moving neutrophils and average speed of moving neutrophils.
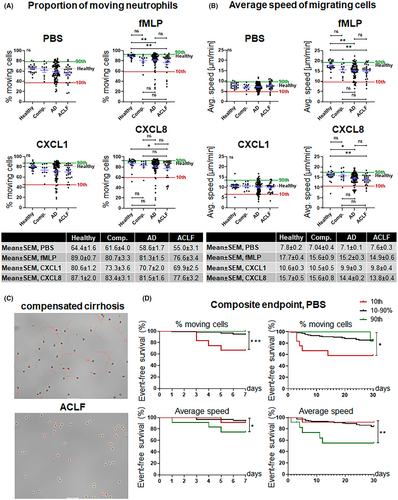
Of note, neutrophils of a number of patients with AD and in particular with ACLF were largely immotile (<40%–50% moving cells and average speed <5–10 μm/min with or without stimulation with chemoattractants). Representative examples of immotile neutrophils are shown in Figure 1C and in Video S1. Since pathological neutrophil migration might be an early event in the development of infections and inflammatory complications, we calculated percentile ranks (10/90%) of the proportion of moving neutrophils and their average speed and assessed the association of these values with the short-term outcome. Of note, significantly more patients with a proportion of migrating neutrophils at unstimulated conditions below the 10th percentile developed a composite endpoint of ACLF, sepsis or death within 7 or 30 days compared to patients with higher proportions of migrating neutrophils (Figure 1D). As shown in SI Figure S3, the proportion of neutrophil migrating at unstimulated conditions increased after recovery from ACLF or sepsis in those patients who survived. Furthermore, patients with very high proportions of moving neutrophils at unstimulated conditions were largely protected from developing the composite endpoint. A different pattern was observed for the average speed of moving neutrophils. Here, fast-moving neutrophils at unstimulated conditions were predictive for the development of the composite endpoint (Figure 1D). The proportion of moving neutrophils and average speed of moving neutrophils after stimulation with chemoattractants were less predictive for the development of complications (SI Figure S4).
Since both, a low proportion of migrating neutrophils and a high average speed of neutrophils at unstimulated conditions are predictive for adverse outcomes in patients with liver cirrhosis, we assessed the relationship between these neutrophil migration parameters in more detail. As shown in Figure 2A, proportion of moving neutrophils and their speed correlate weakly under unstimulated conditions, while stronger correlations between these parameters were observed after stimulation with fMLP. The combination of the migration parameters' proportion of moving neutrophils and their speed showed that a low proportion of migrating neutrophils and average speed of neutrophils are rather independent than synergistic predictors of short-term outcome (Figure 2B).
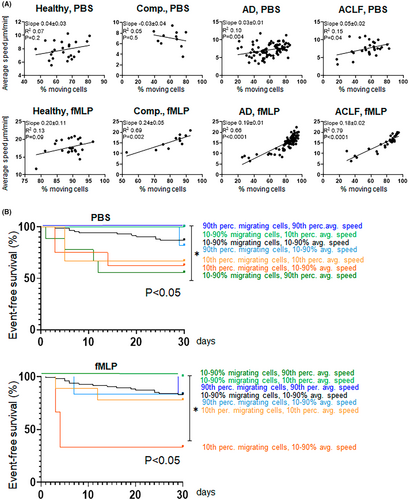
Next, we tested which clinical variables were associated with low proportions of migrating neutrophils and a high average speed of the cells at unstimulated conditions. As shown in SI Table S1, the neutrophil count in peripheral blood did not correlate with migration patterns. In univariate logistic regression analyses, the CLIF OF score was associated with a low proportion of migrating neutrophils at unstimulated conditions (p = .04, SI Table S2), which remained significant in multivariate analysis, together with CRP (SI Table S2). The CLIF OF score and CRP were also associated with a high average speed of neutrophils in univariate logistic regression analyses (p = .04 and p = .04), but not in multivariate analysis (SI Table S2).
3.3 Association between neutrophil migration and expression of chemokine receptors and activation markers on neutrophils
We next assessed cell surface expression of chemoattractant receptors and neutrophil activation markers by flow cytometry. Expression of FPR1, CXCR1 and CXCR2 in patients with compensated cirrhosis was largely comparable to our previously published expression levels on neutrophils of healthy individuals.8 Furthermore, no significant differences in the expression of the formyl peptide receptor 1 (FPR1) were observed between patients with compensated cirrhosis, AD or ACLF (Figure 3A). In contrast, the expression of CXCR1 was lower in AD and ACLF compared to compensated cirrhosis (Figure 3A), while the expression of CXCR2 was significantly decreased in patients with ACLF compared to compensated cirrhosis (Figure 3A).
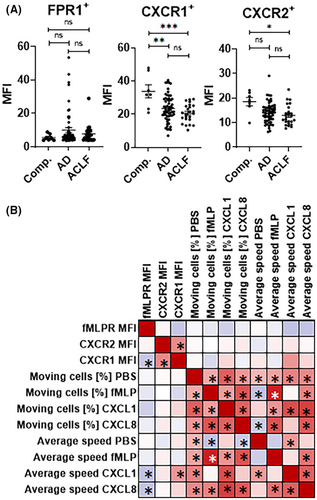
Overall, expression levels of neutrophil activation markers were largely comparable between healthy controls and patients with compensated liver cirrhosis (Figure 4A). In contrast, the expression of the activation marker CD66b was significantly higher on neutrophils of patients with AD versus healthy controls (and numerically higher in ACLF), while the expression of the activation marker CD177 was significantly higher in neutrophils of patients with ACLF versus compensated cirrhosis (Figure 4A).
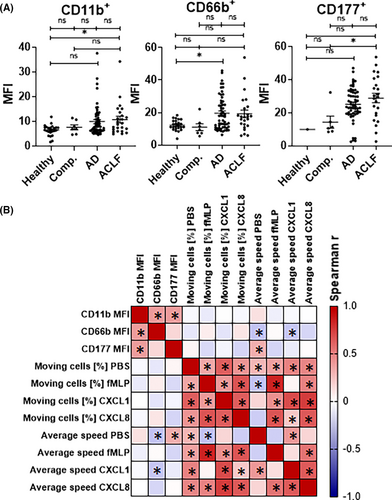
Yet, the expression of these molecules correlated poorly with the proportion of migrating neutrophils and average speed of migrating neutrophils (Figures 3B and 4B), suggesting downstream mechanisms within the signalling cascade to chemoattractants as a basis of neutrophil migration defects in patients with liver cirrhosis.
4 DISCUSSION
In this study, we used a novel, standardized migration assay to identify pathological neutrophil migration patterns (namely a low proportion and high average speed of migrating neutrophils) in hospitalized patients with liver cirrhosis, which were associated with adverse short-term outcomes.
Impaired neutrophil function in patients with liver cirrhosis has been described in previous studies. For example, neutrophils of patients with decompensated alcoholic cirrhosis were shown to exhibit decreased antibacterial activity, which was mediated by defective AKT/p38-MAPK signalling and myeloperoxidase release.9 In another study, neutrophils of patients with ACLF were characterized by downregulation of cell migration and cell-cycle genes, despite neutrophilia being typical for ACLF.10 Artru et al11 have reported defective neutrophil migration in patients with severe alcoholic hepatitis, which was mechanistically explained by a defective IL-33/ST2 pathway in these patients. Although ACLF appears to be associated with typical neutrophil signatures,10 it is important to note that impaired neutrophil function was observed already in early (stable) stages of cirrhosis, suggesting that this is an early event in the pathogenesis of complications of cirrhosis.12 Our study adds knowledge to these previous observations by revealing pathological migration patterns of neutrophils in a subgroup of patients with cirrhosis that are at a high risk of developing adverse outcomes. In particular, a large proportion of immotile neutrophils and a high average speed of moving neutrophils are characteristic for patients with high risk of developing ACLF, sepsis or death in the short term after the identification of these changed motility parameters. These patterns are in line to the systemic inflammation hypothesis of AD and ACLF, since a cell-intrinsic stop of neutrophil migration in an environment of very high levels of chemoattractants has been shown in a recent milestone study.13 Of note, a low proportion of immotile neutrophils and a high average speed of moving neutrophils are no synergistic but rather independent predictors of adverse outcome in patients with cirrhosis, probably because both pathological migration parameters reflect different phases during immune responses (exhaustion/overstimulation vs acute activation). In fact, the proportion of migrating neutrophils and their average speed correlated (weakly) with each other (Figure 2A), indicating that a large proportion of immotile neutrophils and high average speed of migrating neutrophils usually do not co-exist at a given time point.
In general, pathological neutrophil migration in hospitalized patients with liver cirrhosis was particularly pronounced during unstimulated conditions (reflecting an unaltered ex vivo activation status) and in neutrophils stimulated with fMLP, while—for currently unknown reasons—the responses to CXCL1 and CXCL8 were less predictive for adverse outcomes.
From a clinical point of view, our findings might help to manage patients with AD of liver cirrhosis or ACLF before liver transplantation. Systemic inflammation and infections are key drivers of organ failures in these patients, which indicate high short-term mortality and need for early liver transplantation. However, patient selection in particular of patients with ACLF remains challenging, given the high incidence of infections as a trigger and complication of ACLF.4, 5 In this regard, pathological neutrophil migration might, for example, help to identify patients on the waiting list for transplantation who require immediate work-up (and treatment) for infections, an important measure to ensure translatability of patients with unstable AD or ACLF.
Our finding that cell surface expression of chemoattractant receptors does not correlate with pathological neutrophil migration is important from both, a diagnostic and mechanistic point of view. Since flow cytometric analyses of chemoattractant receptors alone are not able to predict the migration of neutrophils in cirrhosis, defective neutrophil migration has to be analysed directly by migration assays like the assay proposed in this study. Furthermore, the molecular basis of the here observed pathological migration patterns must be downstream of the chemoattractant receptors, although we can currently only speculate about the detailed underlying mechanisms.
Our proposed assay is particularly useful to detect defects in spontaneous migration (although the spontaneous migration speed is relatively low) as well as migration in response to strong chemoattractants like fMLP, whereas defective responses to weaker chemoattractants like CXCL1 are not well detected. Furthermore, because of its setup our assay specifically measures defects in chemokinesis but not in chemotaxis, which might behave differently. Finally, the technique should be further developed to enable easy analysis of multiple samples in parallel, which would more accurately reflect the need of a broadly used diagnostic assay.
In conclusion, this study reveals pathological migration patterns of neutrophils in a subgroup of patients with cirrhosis that are at high risk of developing adverse outcomes. Since monitoring of neutrophil migration with the assay proposed here is relatively cost efficient, easy to perform and to standardize between centers,8 the detection of pathological neutrophil migration may serve as an early warning signal of upcoming adverse events in the future.
FUNDING INFORMATION
This study was supported by the Deutsche Forschungsgemeinschaft (LA 2806/7-1 and INST 20876/390-1 to CML) and CRC TRR332 (project C6 to MG).
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
ETHICS APPROVAL STATEMENT.
The study was approved by the local ethical committee (18-8291BO).
PATIENT CONSENT STATEMENT.
Patients gave written informed consent to the study.