Case–Control Investigation of Association of Clinician-Determined Variables With Progressive Myelomalacia After Acute Thoracolumbar Disc Extrusion in Dogs
Funding: The authors received no specific funding for this work.
ABSTRACT
Background
Reliable identification of clinician-controlled factors that increase the risk of progressive myelomalacia after acute thoracolumbar intervertebral disc extrusion could aid in decreasing its occurrence.
Hypothesis/Objectives
Examination of possible risk factors for the development of progressive myelomalacia in a susceptible sample population of adequate size to achieve sufficient power to re-evaluate previously reported associations.
Animals
A total of 244 dogs with acute thoracolumbar intervertebral disc extrusion presented to a single neurology clinic with absent pain perception in the hindquarters.
Methods
Case–control study analyzing the association of exposure to putative risk factors with the development of progressive myelomalacia within 14 days of decompressive surgery.
Results
Progressive myelomalacia was not associated with any of the clinician-controlled factors examined, including surgical timing and duration, peri-operative anti-inflammatory medications, or variations in intra-operative blood pressure. Some evidence supported previous associations of progressive myelomalacia with disc extrusion in the lumbar intumescence and with French bulldog breed, but study power was insufficient to confirm these associations. Unneutered dogs, notably males, had increased incidence in this sample, but this finding might be unreliable because of unknown, likely low, study power.
Conclusions and Clinical Importance
Progressive myelomalacia was not associated with factors that can be controlled by clinicians and that were investigated in our study. It will be necessary to develop new therapeutic approaches to decrease the occurrence of progressive myelomalacia. The possibility that unneutered dogs are at higher risk requires repeated investigation in another sample population.
Abbreviations
-
- BP
-
- blood pressure
-
- CI
-
- confidence interval
-
- CT
-
- computed tomography
-
- Dachs
-
- dachshund
-
- FB
-
- French bulldog
-
- FI
-
- female intact
-
- FS
-
- female spayed
-
- h
-
- hours
-
- IQR
-
- interquartile range
-
- lasso
-
- least absolute shrinkage and selection operator
-
- LS
-
- lumbosacral
-
- MC
-
- male castrated
-
- MI
-
- male intact
-
- MRI
-
- magnetic resonance imaging
-
- NSAIDs
-
- nonsteroidal anti-inflammatory drugs
-
- OR
-
- odds ratio
-
- PMM
-
- progressive myelomalacia
-
- SD
-
- standard deviation
-
- vs.
-
- versus
1 Introduction
Progressive myelomalacia (PMM) is an uncommon but often fatal complication of acute thoracolumbar intervertebral disc extrusion and is defined as a liquid, hemorrhagic necrosis of the spinal cord that can ascend and descend from the initial site of injury [1-3]. The underlying mechanism that generates this phenomenon is not entirely understood but is generally considered to result from tissue destruction and cytokine release caused by the disc extrusion, combined with increased intraparenchymal pressure resulting from extradural compression and intraparenchymal edema [2, 4]. Progressive myelomalacia is reported to occur in approximately 10%–15% of dogs that are paraplegic with absent pain perception although the reported proportion varies considerably from one report to another [3, 5, 6], with some reports suggesting a higher frequency in specific breeds (notably the French bulldog) [7]. It appears that conventional decompressive surgery does not prevent the development of PMM, but timing of surgery could be important (see below) [8].
Currently, uncertainty exists regarding factors that might predispose specific individuals to develop progressive myelomalacia. Knowledge of variables that influence its induction might aid in development of effective therapeutic approaches and, of these, the most important to discover are factors under clinician control. Previous publications have suggested age, intervals between development of specific clinical signs and surgery and administration of corticosteroids might influence the risk of PMM [3, 6-8]. However, because of the difficulties in analysis of retrospective data and because replication is a key aspect of the scientific process [9], there is a need for repeated examination of these relationships.
Our primary aim was to investigate the association of progressive myelomalacia with factors under veterinary control. As a secondary aim, we also examined the association of PMM with patient characteristics. Case–control studies aiming to identify disproportionate exposure to putative causal factors in cases inevitably must rely on retrospective analysis, but there is also a need to ensure appropriate study power to avoid spurious conclusions that cannot be replicated and may be misleading [10]. In our investigation, testing of previously generated hypotheses regarding the effect of various risk variables was based on pre-investigation sample size calculation. The power of each analysis was calculated based on previously reported effect sizes. This manuscript was constructed with reference to the STROBE guidelines for reporting observational studies in veterinary medicine [11].
2 Material and Methods
Ours was a case–control study on dogs with absent pain perception in the hindquarters that were presented to a single veterinary school neurology clinic from January 2015 to December 2024. Cases were defined as dogs that developed PMM within the first 14 days after surgery and controls were those that did not.
Dogs appropriate for inclusion in the study were identified through a medical record database search of the Texas A&M University Veterinary Teaching Hospital from January 2015 to December 2024 for dogs that underwent surgical decompression for acute (i.e., up to 21 days between onset of inability to walk after first onset of any related clinical sign, including back pain) thoracolumbar (i.e., T9-L6 discs) intervertebral disc extrusion (Figure 1). This period was selected to acquire an adequate number of dogs as determined by sample size calculation based on previous publications (see below). Included dogs were identified by searching for invoice fees for hemilaminectomy, corpectomy, or pediculectomy and selected for inclusion or exclusion in the analysis by manual search.
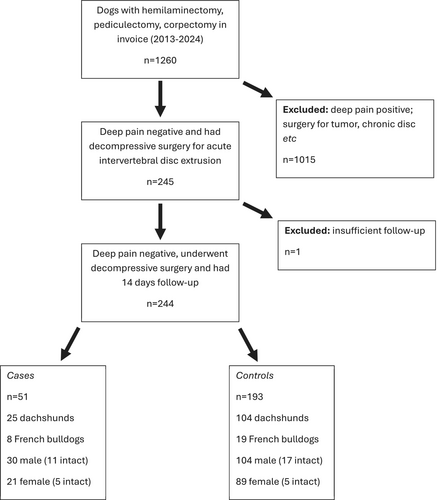
Dogs that were presented to the hospital with evidence of consistent conscious response to noxious stimulation anywhere on the hindquarters were excluded, even if such a response was subtle (e.g., behavioral responses such as turning the head to the stimulus site). Perception of noxious stimulus was tested by compressing at least one distal phalanx of each pelvic limb and the base of the tail with metal instruments. Dogs that underwent surgical decompression for reasons other than acute intervertebral disc extrusion (e.g., tumor cytoreduction) also were excluded. Diagnosis of intervertebral disc extrusion was confirmed by cross-sectional imaging (magnetic resonance imaging [MRI] or computed tomography [CT]) in each dog before surgery.
Development of PMM in each dog was recorded during the first 14 days after presentation to the clinic [12] and necropsy confirmation was recorded when available. Observation of clinical signs consistent with PMM (see below) was facilitated because deep pain-negative dogs are routinely kept in our hospital for at least 3–4 days after decompressive surgery because they are recognized to be at risk of developing PMM. After dogs left the hospital, follow-up was obtained from referring veterinarians and return clinic visits. Presumptive diagnosis of PMM was based on the development of any or all of the following: decreased muscle tone and reflexes in the pelvic limbs (if the dog presented without these deficits) with progression to flaccid paraplegia (complete loss of pelvic limb muscle tone), cranial migration of the cutaneous trunci muscle reflex cut-off by at least two vertebral lengths, generalized loss of abdominal muscle tone, loss of thoracic limb reflexes, respiratory distress (including paradoxical breathing) or loss of ability to vocalize. Although decrease in bladder and anal sphincter tone was noted in some individuals, this information was not systematically recorded in the medical records and did not contribute to the diagnosis of PMM in our study. Diagnosis of PMM required at least two consecutive examinations at least 12 h apart showing progression of clinical signs. Dogs that developed neurologic deficits consistent with ascending or descending myelomalacia (as defined above) but were not euthanized because progression halted were included in the PMM group.
Neurologic examination findings in deep pain-negative dogs at presentation that could be early signs of PMM were noted (e.g., cutaneous trunci reflex cut-off cranial to T9 vertebra or decreased pelvic limb reflexes together with a cutaneous trunci reflex cut-off cranial to the skin level with L5 vertebra). However, given the possibility of spinal shock as an explanation in at least some cases [13], possible errors in defining the cutaneous trunci reflex cut-off, and because some individual dogs exhibit recovery of more caudal cutaneous trunci reflex cut-off over time [14], these dogs only were designated as PMM cases if subsequent examinations showed progressive changes as defined above.
Several categories of information were extracted from the medical records, including demographics (age also was dichotomized into categories of < and > 5.8 years for comparison with a previous report) [3], details of clinical signs, peri-operative medications, and intra-operative measurements. Because of its high importance, and scope for clinician control, we extracted many details about surgical timing: the interval from onset of clinical signs to becoming non-ambulatory (“Interval 1”), the duration of nonambulatory status before presentation (“Interval 2”; importantly, it is not possible to know for how long dogs have lost “deep pain” sensation before presentation), and the interval from presentation to the hospital and completion of the surgical procedure (“Interval 3”). These intervals were analyzed for their association with PMM and also used to calculate the interval between becoming non-ambulatory and commencement of surgery (“Interval 4”) that was examined in a previous publication [8].
We recorded the duration of surgery from the time of the first incision to the end of the procedure, as recorded in anesthetic records. We selected surgery duration rather than the duration of general anesthesia mainly because it facilitated comparison with a previous report [15]. There are also difficulties in determining how best to incorporate data from dogs that undergo sedated imaging before induction of general anesthesia for decompressive surgery.
We also recorded the location of the disc extrusion (which was dichotomized into thoracolumbar junction region [T9-L3 discs] or lumbosacral intumescence [L4-L6 discs]), number of vertebral interspaces that underwent decompression, episodes of high (defined as systolic pressure > 160 mmHg at ≥ 2 consecutive readings at 5-min intervals) or low (mean arterial pressure < 60 mmHg at ≥ 2 consecutive recordings) blood pressure (BP) and if durotomy was performed. In our study, durotomy was undertaken only with intent to improve functional outcome and minimize the development of PMM (as opposed to aiming to diagnose or mitigate pre-existing PMM) and almost exclusively as part of a randomized controlled trial [16]. The durotomy procedure included here was centered on the site of disc extrusion and extended for 2 vertebral bodies cranially and caudally. Peri-operative administration of nonsteroidal anti-inflammatory drugs (NSAIDs) and glucocorticoids was recorded dichotomously because dosing and schedules varied markedly. Post-operative anti-inflammatory medication was determined by the medication that had been given before surgery because of the need to avoid close temporal administration of glucocorticoids and NSAIDs.
2.1 Statistical Analysis
Various putative causal factors were examined for their association with the development of PMM using univariable and multivariable logistic regression. Many of the variables analyzed here have been reported previously to be associated with PMM, permitting the calculation of appropriate power for our current study to replicate previous findings. We initially determined the appropriate sample size for the study by reference to previous evidence on the timing of surgery, because it is the most salient aspect of current treatment under clinician control that might have an impact on the outcome. A previous study reported an odds ratio of 3.4 for the development of PMM between dogs in which surgery commenced at > 12 h after the onset of non-ambulatory status (versus those undergoing surgery at ≤ 12 h) [8]. Based on a typical 12% expected incidence in the reference group, this indicates that 168 deep pain-negative dogs in total would be required to achieve a power of 0.8 (a typical minimum power for clinical trials). Our clinic carries out approximately 200 hemilaminectomies per year for acute thoracolumbar disc extrusion, of which we estimated approximately 10% would be deep pain-negative. Therefore, we estimated the need to acquire data over at least 10 years to allow for some records being incomplete or otherwise requiring exclusion while still ensuring appropriate power.
After data collection and collation, demographic variables were plotted on histograms; normally-distributed continuous data were summarized using mean and SD and non-normally distributed data were summarized by median and interquartile range (IQR). Association of the various possible risk factors with development of PMM was analyzed using univariable logistic regression and summarized as odds ratios (ORs) with 95% confidence intervals (CIs). Increased or decreased risk of PMM was indicated with OR point estimates of > or < 1.0, respectively, but variables with OR CIs including the null value (1.0) were assumed not to be reliably associated with PMM. Our ability to detect previously reported associations of variables with PMM was investigated by power analyses (assuming a power of at least 0.8 to indicate reliability). Assessment of the value of multivariable models was made using the least absolute shrinkage and selection operator (lasso), with model selection based on minimizing the Bayesian information criterion, applied to the full set of variables under clinician control and the set of demographic variables that cannot be controlled. All statistical analyses were carried out using Stata 18 (StataCorp, College Station, TX).
3 Results
In total, 244 deep pain-negative dogs were included, and PMM was diagnosed according to our clinical definition in 51 (21%). Of these 51 PMM cases, 46 dogs were euthanized within 14 days of surgery (at a median of 3 [IQR: 1–5] days after presentation to our clinic), 1 dog died at home (7 days after surgery) and 4 survived long-term (months to years) with persistent deficits associated with ascending (n = 2) or descending (n = 2) myelomalacia that was not fatal. The diagnosis of PMM was confirmed in 9 euthanized dogs at necropsy examination. Demographic variables are summarized below (and in Figure 1).
3.1 Clinician-Controlled Variables
Fifty-nine (24%) dogs received peri-operative glucocorticoids, and 71 (29%) received peri-operative NSAIDs. Seventy-eight (32%) dogs underwent 4-vertebral length durotomy, and surgery was commenced at ≤ 12 h after dogs became non-ambulatory in 55 (23%) dogs. During surgery, high BP events were identified in 150 dogs and low BP events in 70 dogs. The length of the surgical site varied from 2 vertebrae (i.e., a single site hemilaminectomy) to involvement of 7 vertebral bodies, with a median of 3 (IQR, 2–4). Surgery duration varied from 1 to 5.5 h, with a mean of 2.3 ± 0.9 hours.
On univariable analysis, none of the investigated factors under veterinary control, including peri-operative administration of glucocorticoids or NSAIDs, interval from onset of paralysis to completion of surgery, recording of high or low BP events during anesthesia, 4-vertebral length durotomy, surgery time and extent of decompressive surgery were associated with progressive myelomalacia (see Table 1). Lasso did not identify any of these variables as sufficiently impactful to be included in a multivariable model (i.e., there also was no evidence of meaningful interaction among these variables).
| Variable | Variable exposure | Odds ratio | 95% CI | |
|---|---|---|---|---|
| case | control | |||
| NSAID | 0.90 | 0.45–1.80 | ||
| Yes | 14 | 57 | ||
| No | 37 | 136 | ||
| Glucocorticoid | 0.72 | 0.33–1.54 | ||
| Yes | 10 | 49 | ||
| No | 41 | 144 | ||
| Surgery size (vertebral lengths) | 51 | 193 | 0.96 | 0.74–1.23 |
| Durotomy | 0.76 | 0.38–1.51 | ||
| Yes | 14 | 64 | ||
| No | 37 | 129 | ||
| Low BP (events) | 0.53 | 0.25–1.14 | ||
| Yes | 10 | 60 | ||
| No | 40 | 128 | ||
| High BP (events) | 1.18 | 0.61–2.27 | ||
| Yes | 33 | 117 | ||
| No | 17 | 71 | ||
| Interval 3 (h) | 51 | 193 | 1.01 | 0.97–1.07 |
| Surgery time (h) | 51 | 193 | 0.85 | 0.59–1.23 |
| Interval 4 > 12 ha | 1.07 | 0.51–2.27 | ||
| Yes | 40 | 149 | ||
| No | 11 | 44 | ||
- Abbreviations: BP, blood pressure (as “events”—see text); h, hours; Interval 3, interval from presentation to the clinic and completion of surgery; NSAID, nonsteroidal anti-inflammatory drug.
- a In this table Interval 4 is the interval between loss of ambulation and commencement of surgery. It was dichotomized into categories defined as 12 h or less, or > 12 h for comparison with previous reports.
There was a lower percentage of dogs that developed PMM among those that had received glucocorticoids (17%) than in those that had not (22%). However, this difference in PMM proportion was much smaller than previously reported [8] and the power of our study to detect the previously reported effect (OR = 3.1) was 95%. One individual dog received both glucocorticoids and NSAIDs and that did not develop PMM was included in the reporting for both anti-inflammatory medications. Interval 4 (i.e., time to surgery) within ≤ 12 h was associated with a PMM frequency of 20.9%, whereas dogs that went to surgery after that time had a frequency of 21.1%. This difference is much smaller than previously reported (OR = 3.4) [8] and our study had a power of 96% to detect an effect of that magnitude.
The durotomy used in our study was shorter than that described in previous studies [17, 18] as being helpful in decreasing the incidence of PMM, but the 4-vertebral length durotomy carried out in our clinic [19] does not appear beneficial (study power 0.999) in decreasing the incidence of PMM to the same extent as suggested in those previous reports. To evaluate an exploratory question about extremely early surgery and its possible effect on development of PMM, we also examined whether surgery undertaken < 7 h of becoming unable to walk was beneficial. The proportion of dogs developing PMM was marginally higher in those dogs operated on that quickly (24% vs. 21%, Table 2).
| Time to surgery < 7 h | Time to surgery ≥ 7 h | |
|---|---|---|
| PMM | 4 | 47 |
| No PMM | 13 | 180 |
| Proportion PMM | 0.24 | 0.21 |
- Abbreviations: PMM, progressive myelomalacia; Time to surgery, interval between loss of ambulation and commencement of surgery.
3.2 Variables Not Under Clinician Control
In our study sample, 27 (11%) of the dogs were French bulldogs, 129 (53%) were dachshunds, and 88 (36%) were of other breeds (including mixed dachshunds). There were 134 males (28 intact) and 110 females (18 intact). Median weight was 7.5 kg (IQR: 5.9–10) and mean age was 5.2 ± 2.5 years. Time intervals from onset of clinical signs to onset of paraplegia (Interval 1) and from onset of paraplegia to presentation to the clinic (Interval 2) are summarized in Table 3. Interval 2 also was incorporated into the analysis in the section above on clinician-controlled variables to enable direct comparison with a previous study.
| Median | Interquartile range | |
|---|---|---|
| Interval 1 (hr) | 12 | 1–48 |
| Interval 2 (hr) | 12 | 5–24 |
| Interval 3 (hr) | 6 | 4.5–15 |
- Abbreviations: hr., hours; Interval 1, time between onset of clinical signs and loss of ambulation; Interval 2, time between loss of ambulation and presentation to the clinic; Interval 3, time between presentation to the clinic and completion of surgery.
Interval 1 was categorized for comparison with a previous report [3] suggesting that the duration of clinical signs for < 24 h was associated with a higher incidence of PMM; 168 (69%) dogs in our study had exhibited clinical signs for ≤ 24 h and 76 had clinical signs for > 24 h before becoming unable to walk. Similarly, age was categorized to make comparison with a previous suggestion [3] that younger dogs had a higher incidence of PMM. In our study, 170 (69%) dogs were ≤ 5.8 years old, and 74 (31%) were older.
Most of these demographic variables were not associated with the development of PMM in our univariable analysis; exceptions were lesion site (i.e., lumbar intumescence versus thoracolumbar junction), as previously suggested [3, 8], and sex (Table 4). Of 15 dogs with lesions in the lumbar area, 47% progressed to PMM (similar to a previous report) [8] as compared with 19% for lesions affecting the thoracolumbar junction region (OR: 3.68; 95% CI: 1.27–10.69). However, the number of dogs with lumbar lesions was relatively small (n = 15), which limits the power of this analysis to confirm the previous finding (power = 0.40 to detect the reported OR of 3.0). In our dataset, it appears that intact dogs, especially males (11/28 [39%] intact males developed PMM), were at higher risk of PMM than other sex categories, although the power of this analysis is unknown. Lasso did not identify any of the factors outside clinician control for inclusion in an optimized multivariable model.
| Variable | Variable exposure | Odds ratio | 95% CI | |
|---|---|---|---|---|
| case | control | |||
| Age (years) | 51 | 193 | 1.00 | 0.88–1.13 |
| Sex: MI | 11 | 17 | Base | |
| MC versus MI | 19 | 87 | 0.34 | 0.14–0.84 |
| FI versus MI | 5 | 13 | 0.59 |
0.17–2.14 |
| FS | 16 | 76 | 0.33 | 0.13–0.83 |
| Weight (kg) | 51 | 193 | 1.02 | 0.97–1.07 |
| Breed: Dachs | 25 | 104 | Base | |
| FB versus Dachs | 8 | 19 | 1.75 | 0.69–4.46 |
| Other versus Dachs | 18 | 70 | 1.07 | 0.54–2.11 |
| Interval 1 (hr) | 51 | 193 | 1.00 | 1.00–1.00 |
| Interval 2 (hr) | 51 | 193 | 0.99 | 0.97–1.00 |
| Site: T9-L3 | 42 | 168 | Base | |
| Intumescence (L4-L7) | 7 | 8 | 3.5 | 1.20–10.20 |
| Both | 2 | 17 | 0.47 | 0.10–2.12 |
| Interval 1a | ||||
| < 24 h | 15 | 61 | Base | |
| > 24 h | 36 | 130 | 0.89 | 0.45–1.74 |
| Age categoryb | ||||
| > 5.8 years | 13 | 61 | Base | |
| < 5.8 years | 38 | 132 | 0.74 | 0.37–1.49 |
| “Mismatched reflexes” | 118 | |||
| No | 25 | Base | ||
| Yes | 24 | 74 | 1.53 | 0.81–2.88 |
- Note: “Mismatched reflexes” refers to dogs in which there are depressed reflexes in the pelvic limbs but a cutaneous trunci reflex cut-off cranial to the wings of the ilia.
- Abbreviations: Dachs, dachshund; FB, French bulldog; FI, female intact; FS, female spayed; hr., hour; Interval 1, time between onset of clinical signs and loss of ambulation; Interval 2, time between loss of ambulation and presentation to the clinic; kg, kilogram; LS, lumbosacral intumescence; MC, male castrated; MI, male intact; vs., versus.
- a Interval 1 dichotomized into groups with interval of > 24 h or ≤ 24 h after first clinical signs of disc herniation.
- b Age dichotomized into groups of age > 5.8 years or ≤ 5.8 years.
There was weak support for the notion that French bulldogs are more susceptible to PMM than dachshunds, as previously suggested [7]. Eight of a total of 27 French bulldogs developed PMM compared with 25 of 129 dachshunds (OR: 1.75). However, the 95% CI (0.69–4.46) included the null value and, overall, the power of our study (0.73) was insufficient to reliably confirm or refute this previously suggested association. In our data, neither age group (OR: 0.77; 95% CI: 0.38–1.53) nor duration of signs to become non-ambulatory from first development of clinical signs (dichotomized as ≤ or > 24 h; OR: 0.91; 95% CI: 0.46–1.78) were associated with the development of PMM. Both of these analyses had high power (99%) to detect effects of the magnitudes previously reported.
The prevalence of disc extrusion between the thoracolumbar junction region and the lumbosacral intumescence was different between breeds. In French bulldogs 3 of 27 (11%) had disc extrusion affecting the lumbosacral intumescence (none of which developed PMM) compared with 5 of 129 (4%) dachshunds.
Decreased pelvic limb reflexes were identified in 98 dogs in which the cutaneous trunci muscle reflex cut-off was cranial to L5. Of 51 Cases that developed PMM, 24 (49%) had this mismatch, whereas in Controls it was detected in 74 of 192 (39%), associated with an OR of 1.53 (95% CI: 0.81–2.88). The power of this analysis is unknown. In 3 dogs, the cutaneous trunci muscle reflex had a cut-off cranial to T9; two of these dogs developed PMM and one did not.
3.3 Power Needed to Re-Examine Our Findings
The calculated point estimate of effect size for administration of corticosteroid (OR: 0.72) and a baseline proportion of 20% in our reference (untreated) group suggests the need for 937 dogs per group to have sufficient power (0.8) to reliably re-examine this finding. The difference in PMM frequency between dogs operated within 12 h of becoming non-ambulatory and those operated later is so small that many thousands of dogs (approximately 651 000) would be needed to verify this difference. To re-examine the possibly detrimental effect of very early surgery (i.e., < 7 h) would require 120 animals in each group. In this dataset, intact dogs had a higher frequency of PMM than those that had been neutered (35% vs. 18%). To re-examine this variable with a power of 0.8 would require 105 dogs per group.
4 Discussion
Our data provide little evidence to support the previously reported association of the development of PMM with many clinician-controllable risk factors in dogs that have absent pain perception after thoracolumbar intervertebral disc extrusion. Although there are some minor differences in the proportion of PMM among the different categories of investigated exposure factors, these possible effects are, in general, insufficiently large to have meaningful clinical implications, or are associated with wide CIs that include the null value (i.e., OR of 1.0). Most pertinently, this assessment includes the timing of surgery and factors that might affect spinal cord perfusion during anesthesia such as surgery duration and episodes of BP change. In this dataset, we detected minimal differences in the frequency of PMM between animals operated on < 12 h after becoming unable to walk (20.9%) and those operated on after this time (21.1%), together with OR CIs for Interval 3 that were close to the null value, and also between animals receiving peri-operative corticosteroids (17%) and those that did not (22%). The previously reported ORs for the effects of these two variables (time to surgery and glucocorticoid administration) are not supported by our dataset, and the high study power in our analysis suggests that it is probable that each of these clinician-controlled variables is less influential over outcome than previously suggested [8]. Comparison with the previous report does not suggest a difference in the study populations as a plausible explanation for the contrasting results. A more probable reason for the disparity between our results and previous studies is that the relatively small sample size in both analyses allows an outsize role for the effect of chance, making it difficult to be sure of the real effects of many of these variables. Nevertheless, many of our analyses have high power to detect the previously reported effect sizes, implying that the real effects are likely to be considerably smaller than previously reported.
Although timing of surgery can be considered only partially under clinician control, it is nevertheless important for veterinary neurologists to consider and can be divided into various sub-components (as designated by various intervals in our study) that vary in their amenability to neurologist control. In this dataset, time to surgery, howevedefined, does not appear to influence development of PMM. There are three possible explanations: (i) surgery has no effect on the development of PMM regardless of when it is undertaken (i.e., surgery does not prevent PMM); (ii) surgery does have an impact but either: (a) all dogs were operated within the critical period needed for it to be effective, or (b) none of the dogs were operated within the critical period. There is a suspicion in human medicine, although not proven, that earlier surgery provides benefit in terms of neurologic recovery [20], albeit not a decrease in PMM, which would be consistent with the possibility that surgery itself is beneficial. Our overall analysis of surgical timing, and that for very early surgery (< 7 h after loss of ambulatory ability), does not support that conclusion (at least in terms of development of PMM). Together our data then suggest that it is more likely either that surgery has no beneficial effect in preventing PMM or that the timing of the surgery can be considerably delayed from the onset of paralysis and still be effective.
Despite the lack of proven benefit from glucocorticoid dosing, it remains possible that an alternative method of delivery, such as longer duration or more local application (such as that previously reported) [21] might be more efficacious and could be investigated. Our analysis suggests a PMM proportion of approximately 22% in our baseline (non-treated group) implying that if glucocorticoids might decrease this to a proportion of 15%, then a sample size of 482 deep pain-negative dogs in each group would be required. If a larger effect could be elicited (e.g., decrease of PMM to 10%) then only 146 cases per group would be required to attain a power of 0.8.
Although longer intra-operative time has been associated with worse outcome for dogs that are presented with loss of deep pain sensation [15], in this dataset increasing surgery time was not associated with a higher frequency of PMM. Indeed, longer surgery time had a point estimate effect suggesting an association with a decreased risk of PMM, but with wide CIs, including the null value (Table 1). The difference in results between our study and the previous study most likely is a consequence of relatively low study power, allowing a larger role for the effect of chance. Although reasons why longer surgery could be associated with worse ambulatory outcome have not been determined, one of the suggestions has been changes in BP during anesthesia. Our dataset provides no reason to suspect that episodes of low BP are associated with development of PMM (OR < 1.00), similar to previous reports on lack of association of low intra-operative BP with poor outcome in thoracolumbar disc surgery [22]. One of the difficulties in exploring the possibly detrimental effects of excessively high or low BP is the selection of criteria to designate pressures that will be detrimental. In our exploration of abnormal BP as a possible factor in promoting PMM, we defined low or high BP by criteria that would prompt a response from our anesthesia team. However, many other criteria that could be considered, including magnitude of BP change and time over which it occurs, or combinations of the two factors. Without a clear understating of the putative pathologic mechanism that links abnormal BP with development of PMM, many possible options could be explored in future studies.
Although our data do not confirm the previously reported high susceptibility of French bulldogs to develop PMM [7], our point estimate of effect does provide some relatively weak support for the hypothesis that French bulldogs might be more at risk for developing PMM than other breeds, notably dachshunds. However, the 95% CI for our estimate of effect also includes the null value. Based on the proportion of dogs developing PMM in each group, it appears that lesions affecting the lumbosacral intumescence also might carry a higher risk than lesions affecting the thoracolumbar region, which supports previous reports [3, 8]. However, our analysis has relatively low power because few dogs had disc herniation between L4 and L6 and we cannot confirm or refute this apparent susceptibility.
Sex category was associated with varied frequency of PMM in our dataset, and an association of female sex with superior recovery after experimental spinal cord injury in rats has been reported previously [23]. However, our finding may be spurious and associated with low study power, because some categories contain relatively few individuals and an association between PMM and sex category was not apparent in previous reports [3, 8]. Nevertheless, it would be useful to re-examine this possible association in another dataset because if there is a true effect of sex, it might aid in elucidating mechanisms that underlie PMM.
At present, the most promising, albeit controversial, currently available treatment to mitigate PMM is extensive durotomy [17, 18], or that provided by the so-called cranial limited extended durotomy [24]. In comparison, our dataset implies that the relatively short (4-vertebral body) durotomy performed in deep pain-negative dogs at our clinic and centered on the site of disc extrusion does not have similar beneficial effects in preventing the development of PMM in dogs presented without clearly defined clinical signs of the condition. Although more extensive durotomy apparently is associated with superior outcomes in preventing the progression of PMM in dogs that already have clinical and MRI features consistent with the condition, the procedure has not been evaluated by a randomized controlled trial and thus it is difficult to fully define efficacy. Furthermore, although dogs might avoid PMM after the extended durotomy procedure, they do not recover to walk again (or regain continence) and thus this outcome may only be acceptable to a small proportion of owners.
In common with all retrospective studies, our dataset may be subject to bias resulting from clinician decisions. This influence might be apparent in the dimensions of the decompressive hemilaminectomy but could also possibly affect the timing of surgery according to breed or weight. However, although these biases are somewhat inevitable, there is little reason to consider that they will be highly influential on the results we have reported, largely because of the inability of clinicians to reliably identify dogs (of the stage or severity of clinical signs of individuals included here) that will predictably progress to PMM. Almost all of the durotomy cases included here were allocated to that procedure through randomization rather than clinician decision. Overall, the ability to investigate causal factors for PMM is hampered by its relatively infrequent occurrence and the consequent low power of many investigations. Even in our clinic where numerous dogs undergo thoracolumbar decompressive surgery for disc extrusions, we accumulated only approximately 50 cases of myelomalacia during a 10-year period. To investigate all of the possible risk factors in more detail, especially those under clinician control, would require multicenter collaboration.
We conclude that the clinician-controllable factors we examined did not have reliable or major effects on development of PMM in deep pain-negative dogs that underwent decompressive surgery for acute thoracolumbar intervertebral disc herniation. This observation should stimulate investigation of other approaches that might prevent development of this devastating condition.
Disclosure
Authors declare no off-label use of antimicrobials.
Ethics Statement
Authors declare no institutional animal care and use committee or other.
Conflicts of Interest
The authors declare no conflicts of interest.




