Safety, Efficacy and Doxorubicin Pharmacokinetics During Cannabidiol/Cannabidiolic Acid Rich Hemp Oil Use in Dogs With Lymphoma Undergoing CHOP Chemotherapy
Funding: The authors received no specific funding for this work.
ABSTRACT
Background
The safety of oral cannabidiol (CBD) and cannabidiolic acid (CBDA)-rich hemp oil supplementation in dogs with cancer receiving chemotherapy has not been investigated.
Objectives
To evaluate the safety, tolerability, and influence on doxorubicin exposure over time of oral CBD/CBDA-rich hemp oil supplementation in dogs diagnosed with high grade lymphoma undergoing CHOP chemotherapy.
Animals
Client-owned dogs diagnosed with lymphoma.
Methods
Dogs were enrolled in this prospective, double-blinded, randomized, placebo-controlled clinical trial to receive either CBD/CBDA-rich hemp oil capsules or placebo during one cycle of CHOP chemotherapy. Primary outcomes evaluated included adverse events during chemotherapy, quality of life scores and doxorubicin area under the curve (AUC) over a 5-week period.
Results
Twenty-five dogs were enrolled, with 19 completing the trial. CBD/CBDA supplementation did not significantly affect doxorubicin AUC in the intervention group. The doxorubicin AUC was not different between groups at week 0 [placebo 390.8 nM/h (318.6–479.4); CBD/CBDA 403.4 nM/h (351.9–462.5)] but was different at week 5 [placebo 572.6 (448.3–731.2); CBD/CBDA 406.8 (3.23.2–551.8)]. CBD/CBDA supplementation was well-tolerated, and no serious adverse events were observed. No significant differences between groups were observed in hematological and biochemical variables. The mean (range) quality of life scores for placebo and CBD were 7.56 (0, 35), and 10.75 (0, 56), respectively, with no significant differences.
Conclusion and Clinical Importance
Short-term oral CBD/CBDA-rich hemp supplementation appeared safe and well-tolerated in dogs undergoing CHOP chemotherapy for lymphoma.
Abbreviations
-
- (L)-CHOP
-
- (L-asparaginase), cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy
-
- CBCA
-
- cannabichromenic acid
-
- CBD
-
- cannabidiol
-
- CBDA
-
- cannabidiolic acid
-
- CBG
-
- cannabigerol
-
- CBGA
-
- cannabigerolic acid
-
- CBN
-
- cannabinol
-
- PK
-
- pharmacokinetics
-
- QoL
-
- quality of life
-
- THC
-
- tetrahydrocannabinol
-
- THCA
-
- tetrahydrocannabinolic acid
1 Introduction
The use of hemp supplements derived from Cannabis sativa L., particularly extracts rich in cannabidiol (CBD), is increasing in veterinary medicine [1-3]. The safety and efficacy of CBD-rich hemp extracts have been investigated in dogs with idiopathic epilepsy and osteoarthritis [4-6]. Recent research in human medicine suggests CBD might minimize adverse effects of cancer treatment and, in vitro, might have anticancer effects when used as a whole hemp extract with other minor cannabinoids and terpenes [7]. These potential benefits, coupled with the apparently low risk of severe adverse effects, have made CBD-rich hemp extracts attractive supplements for dogs during cancer treatment [2]. However, no research, to the authors' knowledge, has addressed the safety of CBD in dogs with cancer or the potential drug interactions between chemotherapy and CBD. In dogs, CBD is metabolized in the liver by cytochrome P450 enzymes, with major roles found for CYP1A2 and CYP2C21 in vitro [3, 8-10]. Thus, before recommending CBD administration to dogs receiving chemotherapy, further investigation is necessary.
In this study, we evaluate the safety and tolerability of oral CBD/CBDA-rich hemp administration, describe the health-related quality of life (QoL) assessments, and assess doxorubicin exposure over time in dogs diagnosed with intermediate-to-high grade lymphoma receiving oral CBD/CBDA-rich hemp supplementation compared with placebo while undergoing combination chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP). We hypothesized that administration of a primary CBD/CBDA-rich hemp oil would be safe and would not cause variation in doxorubicin exposure over time in dogs undergoing chemotherapy for lymphoma and would have a beneficial effect on QoL.
2 Materials and Methods
A prospective, double-blinded, randomized, placebo-controlled clinical trial was performed at the University of Florida from 2019 to 2022. All procedures were approved by the University of Florida's Institutional Animal Care and Use Committee (protocol #202110448) and the Hospital Research Review Committee.
Client-owned dogs with intermediate-to-high grade lymphoma were recruited from the presenting population. Dogs were eligible for enrollment after informed client consent if the following criteria were met: cytologic or histologic diagnosis of intermediate or high-grade lymphoma, treatment with L-CHOP (L-asparaginase, cyclophosphamide, doxorubicin, vincristine, and prednisone) or CHOP, body weight at least 15 kg, expected survival of at least 10 weeks, serum bilirubin within reference interval, and no significant co-morbidities that would interfere with the investigators' ability to assess treatment response. Breeds at high-risk of ABCB1-1Δ mutation were required to have genetic testing and be homozygous normal. Concurrent herbal supplements were not allowed, and a one-week washout period before inclusion in the trial was required. Staging tests were recommended but not required, including thoracic radiographs, abdominal ultrasound, urinalysis, immunophenotyping and bone marrow aspirate. Dogs were staged according to the World Health Organization's Clinical Staging System for Lymphoma in Domestic Animals [11].
Dogs receiving L-CHOP or CHOP chemotherapy, summarized in Table 1, were randomized in a serial fashion based on time of presentation by the Pharmacy team to receive either placebo or CBD-rich hemp oil capsules at the time of the first doxorubicin (study week 0). Doses of chemotherapy, timing of administration and body surface areas were collected for each visit. Upon enrollment, the dogs were assessed via physical examination, complete blood count (CBC), chemistry panel, and urinalysis. Dogs were reassessed throughout the 5-week trial period according to Table 1. Clients were asked to fill out the weekly QoL questionnaire as described by Giuffrida et al. [12]. Supportive medications such as gastrointestinal protectants, antibiotics, anti-nausea medications, musculoskeletal supplements (i.e., glucosamine) and parasite preventative medications were permitted.
| L-CHOP or CHOP chemotherapy protocol | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Dose | Week 0 | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 | Week 9 |
| Study Week 0 | Study Week 1 | Study Week 2 | Study Week 3 | Study Week 4 | Study Week 5 | ||||||
| L-asparaginase | 10 000 IU/m2 SC | Optional, only once | |||||||||
| Vincristine | 0.6–0.7 mg/m2 IV | x | x | x | x | ||||||
| Cyclophosphamide | 200–250 mg/m2 PO | x | x | ||||||||
| Doxorubicin | 30 mg/m2 IV | x | x | ||||||||
| Prednisone | Tapering | 30–40 mg/m2 PO q24hrs | 30 mg/m2 PO q24hrs | 20 mg/m2 PO q24hrs | 10 mg/m2 PO q24hrs | ||||||
| CBD or Placebo | 5 mg/kg POQ12hrs | x | x | x | x | x | x | ||||
| Clinical trial procedures | |||||||||||
| Study enrollment | x | ||||||||||
| Quality of life survey | x | x | x | x | x | ||||||
| Complete blood count | x | x | x | x | x | ||||||
| Chemistry panel | x | x | x | ||||||||
| Doxorubicin quantification | x | x | |||||||||
| CBD steady state serum concentration | x | x | |||||||||
- Note: Week 0 (L-asparaginase) was optional. An intended total of 4 cycles was recommended, with the first two cycles weekly (with a one-week break between cycles) and second two cycles treatment administered every other week.
Clients were instructed to begin treatment (placebo or intervention) the evening after the first doxorubicin dose for the 5-week study period. CBD/CBDA-rich hemp derived cannabinoids were dosed at 5 mg kg−1 of total CBD/CBDA from a whole hemp product, to be administered orally with a meal every 12 h. The total CBD/CBDA dose was rounded to the nearest 25 mg based on capsule size availability (25 mg capsules). The oil was an equal mix of CBD and CBDA with minor cannabinoids. Concentrations were verified by an ISO 17,025 certified laboratory analysis (Proverde Inc., Milford, MA) before the trial at 31 mg mL−1, CBDA 33 mg mL−1, Δ9 tetrahydrocannabinol (THC) 1.2 mg mL−1, tetrahydrocannabinolic acid (THCA) 1.3 mg mL−1, and cannabichromene (CBC), cannabichromenic acid (CBCA), cannabigerol (CBG) and cannabigerolic acid (CBGA) each at approximately 0.8–0.9 mg mL−1. Both placebo and CBD-rich hemp extract contained a base sesame oil, and the placebo jars were lined with terpenes to provide similar aroma as the CBD/CBDA-rich extract capsules. The number of placebo capsules matched what would have been prescribed for the intervention group. The Pharmacy team provided all capsules so study investigators and clients remained blinded. One week of capsules was prescribed at each visit, and clients were asked to return the bottle weekly. Missed doses were recorded. Capsules were analyzed to ensure stability at 14 months and were within 5% of initial concentrations, suggesting no product degradation.
Response to treatment at each visit was determined based on physical examination and response evaluation criteria for peripheral nodal lymphoma in dogs [13].
Gastrointestinal toxicoses information was collected weekly and retrospectively graded. Hematological and biochemical toxicoses were assessed through the protocol described in Table 1. Adverse events (AEs) were graded and attributed according to the Veterinary Cooperative Oncology Group Common Terminology Criteria for Adverse Events (VCOG-CTCAE) v2 [14]. Dose reductions and delays were performed at the discretion of the attending clinician. Symptomatic treatment of AEs was permitted and based on the clinician's preference.
In addition, serum CBD concentration and its metabolites (CBDA, THC, THCA, CBG, CBGA, CBC, CBCA and cannabinol (CBN)) were measured at week 0 and 5 when steady-state levels were expected after unmasking of groups. The LC–MS techniques were utilized to measure the cannabinoids and their metabolites [16-18]. Two mL of whole blood was collected via jugular venipuncture into serum separator tubes, blood was centrifuged, and serum was stored at −20°C for batch analysis. Dogs randomized into the placebo group did not undergo CBD serum concentration analysis.
2.1 Statistical Analysis
A priori power analysis indicated that a sample size of 20 dogs per group would be optimal to ensure adequate statistical power for detecting changes in the QOL survey. However, due to temporal constraints, financial limitations, and disruptions associated with the COVID-19 pandemic, the study was terminated before achieving the intended sample size, resulting in unequal group allocations. Furthermore, the sequential randomization procedure without replacement for subjects withdrawing from the study further contributed to group size discrepancies. Continuous data between the groups were tested for normality using Shapiro Wilks testing and examined using students' t test. Nominal demographic data were assessed utilizing Fisher's Exact analysis.
Grades and numbers of laboratory toxicoses were compared between groups using Chi square testing and categorized into no adverse events or grade 1–3 adverse events. All CBC, serum chemistry, and doxorubicin PK data were assessed utilizing an Anderson-Darling normality test. When normality was rejected, data was log-transformed and visually inspected for normal distribution before analysis utilizing repeated measures mixed model analysis on JMP 16 (Wittington House, UK). Different covariance structures were tested for each model and data was then back-log transformed. The model treated age, treatment, time, and the interaction term treatment × time as fixed effect variables. Tukey's post hoc tests were performed for multiple comparison correction of p-values for all pairwise least square means (LSM) comparisons of age, treatment, time, or time × treatment to assess differences observed.
Based on the health-related QoL survey utilized, questions 1–5 and 13–17 were tallied as days in the past week, higher scores would be deemed negative. Questions 6–12 assess dog affection and life enjoyment, with higher scores indicating positive outcomes, but these were converted to negative outcome days per week, ensuring a normalized outcome metric. The sum of negative outcomes, based on the 17 questions, has a maximum of 119 due to treatment. This survey data was treated as continuous data and assessed utilizing Cochran–Mantel–Haenszel and Wilcoxon Scores using SAS 9.4 (North Carolina, USA). The sum of scores/days was analyzed using a mixed model ANOVA with visit as the time variable. For all analyses, statistical significance was set at p ≤ 0.05.
3 Results
3.1 Study cohort
Twenty-five dogs were enrolled; 11 received CBD/CBDA-rich hemp; 14 received placebo. Six dogs (n = 2 intervention group, n = 4 placebo group) were withdrawn from the study before completion due to disease progression (n = 1, placebo group), progression with a change to a different chemotherapy protocol (n = 3, two dogs from placebo and one from intervention group), or euthanasia (n = 2, one from each group). Nineteen dogs completed the trial: 9 in the intervention group, 10 in the placebo group. One dog in the CBD/CBDA-rich hemp group received mitoxantrone instead of doxorubicin at study week 5 due to an increase in serial troponin 1 levels (Triage MeterPro, Quidel) compared with week 0; this dog did not have doxorubicin PK performed and remained in the trial, since the change in protocol was instituted at the last study visit. Nine mixed breeds and 2 German shepherds were represented along with one of each: American Staffordshire terrier, Basset hound, Shi Tzu, Old English sheepdog, miniature Australian shepherd, Labrador, Golden retriever, and Chesapeake Bay retriever. Staging was completed by physical examination with CBC, chemistry panel, and urinalysis in all dogs (n = 19). Additional staging tests included thoracic radiographs (n = 13), abdominal ultrasound (n = 10), and CT scan of the thorax and abdomen (n = 1). All dogs had a partial or complete response to chemotherapy at the time of enrollment and all but one were found in complete remission at trial completion. Table 2 summarizes dog characteristics and therapy response, revealing no significant differences between the two groups.
| Variable | Treatment group | p | |||
|---|---|---|---|---|---|
| CBD | Placebo | ||||
| Mean age (years) | 7.8 (range 2–15) | ||||
| 7.0 | 8.5 | 0.34 | |||
| Least square mean weight (kg; 95% CI) | 33.0 (26.3–39.7) | 27.3 (21–33.6) | 0.21 | ||
| Method of diagnosis (n) | Cytology | 17 | |||
| Histopathology | 2 | ||||
| Location | Multicentric | 16 | |||
| Intraabdominal lymph nodes | 2 | ||||
| Tonsils | 1 | ||||
| Minimum stage (n) | III | 5 | 3 | 0.61 | |
| IV | 2 | 3 | |||
| V | 2 | 4 | |||
| Substage (n) | a | 2 | 6 | 0.20 | |
| b | 7 | 4 | |||
| Immunophenotype (n) | B cell | 6 | 7 | 0.99 | |
| T cell | 1 | 1 | |||
| Unknown | 2 | 2 | |||
| Remission status | Study week 0 | PR | 2 | 7 | 0.07 |
| CR | 7 | 3 | |||
| Study week 2 | PR | 3 | 1 | 0.30 | |
| CR | 6 | 9 | |||
| Study week 3 | PR | 2 | 0 | 0.21 | |
| CR | 7 | 10 | |||
| Study week 4 | PR | 1 | 0 | 0.47 | |
| CR | 8 | 10 | |||
| Study week 5 | PR | 1 | 0 | 0.47 | |
| CR | 8 | 10 | |||
3.2 Chemotherapy Dosing
During the study protocol, 38 vincristine doses were administered (median dosage of 0.59 mg/m2, range 0.68–0.49). Thirty-eight cyclophosphamide doses were administered (median dosage 230.5 mg/m2, range 170.8–271.9). Thirty-seven doses of doxorubicin were administered (median dosage of 29 mg/m2, range 29.8–22.1). One dog received mitoxantrone instead of doxorubicin (dosage 4.85 mg/m2).
Median doxorubicin dosing for the placebo group was 29.1 mg/m2 at week 0 (range 26.1–29.8) and 27.4 mg/m2 at week 5 (range 22.1–29.5). The intervention group median doxorubicin dosing at week 0 was 29.6 mg/m2 (range 22.4–29.6) and 29.1 mg/m2 (range 22.5–29.6) at week 5.
3.3 Complete Blood Count
Table 3 summarizes weekly LSM (95% CI) CBC results during chemotherapy treatments. Our analysis indicated no effects of CBD treatment, treatment over time, or age in any blood variable measured. The LSM (95% CI) hematocrit for the placebo group was 39.2% (36.9–42.3) at week 2% and 41.0% (38.0–44.0) at week 5. The LSM (95% CI) hematocrit for the intervention group was 39.5% (36.7–42.4) at week 2% and 43.1% (40.0–46.2) at week 5. The hematocrit was lower in week 2 compared with week 5 for both placebo and CBD/CBDA groups (p = 0.0013). Similarly, there was a statistical difference over time: red blood cell count was lower at study week 2 compared with study week 5 (p = 0.005); mean corpuscular volume and red cell distribution width were decreased at study weeks 2–5 compared with week 0 (p = 0.001; 0.008; respectively). The total white blood cell and neutrophil counts were increased at study week 2 and were only significantly different compared with week 5 (p = 0.001; 0.005 respectively), and the monocyte count was lower at study week 4 than at baseline, week 2, and week 3, globally across both groups (p = 0.02). An age effect was found for MCHC (p = 0.04), but in no other CBC assessments.
| Value | Treatment group | Study week 0 | Study week 2 | Study week 3 | Study week 4 | Study week 5 | p-value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Tx | Time | Tx × Time | Age | |||||||
| Red blood cell (×103 uL−1) | Placebo | 5.94 | 5.92 | 5.98 | 6.08 | 6.08 | ||||
| (5.36–6.52) | (5.59–6.26) | (5.62–6.24) | (5.67–6.44) | (5.68–6.48) | 0.49 | 0.005 | 0.21 | 0.14 | ||
| CBD | 6.24 | 6.05 | 6.28 | 6.59 | 6.22 | |||||
| (6.23–5.62) | (5.69–6.40) | (5.90–6.66) | (6.21–6.97) | (5.80–6.64) | ||||||
| Hematocrit (%) | Placebo | 40.0 | 39.2 | 39.6 | 40.2 | 41.0 | ||||
| (36.3–43.7) | (36.9–42.3) | (36.9–42.9) | (37.6–42.9) | (38.0–44.0) | 0.54 | 0.0013 | 0.13 | 0.46 | ||
| CBD | 41.5 | 39.5 | 41.0 | 43.2 | 43.1 | |||||
| (37.7–45.4) | (36.7–42.4) | (38.1–43.8) | (40.4–46-1) | (40.0–46.2) | ||||||
| Hemoglobin (g dL−1) | Placebo | 14.6 | 13.8 | 13.7 | 13.8 | 14.0 | ||||
| (13.2–16.0) | (12.9–14-7) | (12.9–14.5) | (12.9–14.6) | (12.-15.1) | 0.8 | 0.2 | 0.2 | 0.11 | ||
| CBD | 14.4 | 13.8 | 14.1 | 14.7 | 14.8 | |||||
| (12.9–15.8) | (12.8–14.7) | (13.3–15.0) | (13.8–15.6) | (13.6–16.1) | ||||||
| Mean corpuscular volume (fL) | Placebo | 67.6 | 66.1 | 66.3 | 66.3 | 66.4 | ||||
| (64.7–70.5) | (63.1–69.1) | (63.5–69.2) | (63.4–69.1) | (63.4–69.3) | 0.76 | 0.008 | 0.98 | 0.81 | ||
| CBD | 66.9 | 65.4 | 65.2 | 65.6 | 65.6 | |||||
| (63.9–70.0) | (62.3–68.6) | (62.3–68.2) | (62.7–68.6) | (62.5–68.7) | ||||||
| Mean corpuscular hemoglobin (pg) | Placebo | 24.8 (22.9–26-8) | 23.2 (22.0–24.3) | 22.9 (21.9–23.9) | 22.7 (21.7–23.6) | 22.6 (21.6–23.5) | 0.23 | 0.15 | 0.76 | 0.38 |
| CBD | 23.1 | 22.8 | 22.6 | 22.4 | 22.6 | |||||
| (21.1–25.2) | (21.6–24.0) | (21.5–23.7) | (21.4–23.3) | (21.7–26.8) | ||||||
| Mean corpuscular hemoglobin concentration (g/dL) | Placebo | 36.7 | 35.1 | 34.5 | 34.2 | 34.0 | 0.22 | 0.15 | 0.41 | 0.04 |
| (24.2–39.2) | (34.2–39.2) | (33.6–25.4) | (33.6–34.7) | (33.1–35.0) | ||||||
| CBD | 34.5 | 34.9 | 34.7 | 34.1 | 34.5 | |||||
| (31.9–37.1) | (33.5–36.3) | (33.7–35.6) | (33.5–34.7) | (33.5–35.5) | ||||||
| Red cell distribution width (%) | Placebo | 16.3 | 17.2 | 17.8 | 18.0 | 18.7 | 0.18 | 0.001 | 0.33 | 0.3 |
| (15.2–17.4) | (15.7–18.8) | (16.5–19.2) | (16.4–19.6) | (16.8–20) | ||||||
| CBD | 15.2 | 17.2 | 18.2 | 17.7 | 18.3 | |||||
| (14.0–16.4) | (15.5–18.8) | (16.8–19.7) | (16.0–19.4) | (16.2–20.3) | ||||||
| White blood cell (×103 uL−1) | Placebo | 7.55 | 7.92 | 6.54 | 6.43 | 4.46 | 0.48 | 0.01 | 0.33 | 0.41 |
| (4.65–10.46) | (5.28–10.55) | (4.70–8.39) | (4.27–8.58) | (3.64–5.29) | ||||||
| CBD | 5.66 | 8.07 | 6.14 | 6.25 | 5.62 | |||||
| (2.59–8.72) | (5.29–10.84) | (4.19–8.08) | (3.98–8.53) | (4.76–6.49) | ||||||
| Neutrophil (×103 uL−1) | Placebo | 5.63 | 5.73 | 4.42 | 4.49 | 2.67 | 0.18 | 0.005 | 0.29 | 0.63 |
| (3.21–8.06) | (3.52–7.93) | (2.93–5.91) | (2.77–6.21) | (2.03–3.31) | ||||||
| CBD | 3.23 (0.67–5.79) | 5.36 (3.03–7.68) | 3.47 (1.90–5.04) | 3.98 (2.17–5.79) | 3.23 (2.56–3.90) | |||||
| Lymphocyte (×103 uL−1) | Placebo | 1.34 | 1.57 | 1.31 | 1.14 | 1.12 | 0.83 | 0.15 | 0.64 | 0.95 |
| (0.96–1.73) | (0.96–1.72) | (0.95–1.67) | (0.86–1.42) | (0.75–1.48) | ||||||
| CBD | 1.40 | 1.58 | 1.36 | 1.26 | 1.47 | |||||
| (1.00–1.81) | (0.99–2.17) | (0.98–1.74) | (0.96–1.55) | (1.09–1.86) | ||||||
| Monocyte (×103 uL−1) | Placebo | 0.48 | 0.47 | 0.51 | 0.32 | 0.31 | 0.28 | 0.02 | 0.99 | 0.93 |
| (0.25–0.70) | (0.24–0.69) | (0.25–0.77) | (0.17–0.47) | (0.15–0.47) | ||||||
| CBD | 0.67 | 0.54 | 0.62 | 0.30 | 0.47 | |||||
| (0.43–0.91) | (0.31–0.78) | (0.35–0.90) | (0.14–0.45) | (0.30–0.64) | ||||||
| Eosinophil (×103 uL−1) | Placebo | 0.15 | 0.48 | 0.31 | 0.49 | 0.37 | 0.5 | 0.07 | 0.56 | 0.26 |
| (0–0.31) | (0–1.03) | (0.11–0.50) | (0.15–0.83) | (0.18–0.57) | ||||||
| CBD | 0.22 | 0.37 | 0.25 | 0.54 | 0.26 | |||||
| (0.05–0.38) | (0–0.96) | (0.04–0.45) | (0.18–0.90) | (0.05–0.46) | ||||||
| Platelet (×103 uL−1) | Placebo | 302 | 287 | 354 | 318 | 296 | 0.15 | 0.06 | 0.06 | 0.65 |
| (210–396) | (167–406) | (258–451) | (237–400) | (227–365) | ||||||
| CBD | 225 | 351 | 302 | 275 | 317 | |||||
| (126–322) | (225–477) | (201–404) | (189–361) | (244–390) | ||||||
| Mean platelet volume (fL) | Placebo | 12.4 | 13.2 | 13.4 | 13.2 | 14.2 | 0.4 | 0.17 | 0.85 | 0.58 |
| (10.6–14.2) | (10.7–15.7) | (11.3–15.6) | (11.0–15.5) | (12.4–16.1) | ||||||
| CBD | 13.0 | 13.3 | 12.9 | 12.5 | 13.2 | |||||
| (11.0–14.9) | (10.7–16.0) | (10.6–15.1) | (10.2–15.5) | (11.2–15.2) | ||||||
- Note: Values are presented as least square means (LSM) and 95% confidence intervals. The total white blood cell count, neutrophils, monocytes, eosinophils and platelets were log transformed variables. All 19 dogs had a complete blood count completed at each timepoint.
Possible attributable hematological AEs were graded and summarized in Table 4. No grade 4 or 5 adverse events were observed. There were no significant differences between groups for any of the toxicoses recorded across low HCT (p = 0.25), neutropenia (p = 0.20), or thrombocytopenia (p = 0.27). Gastrointestinal AEs were reported for each visit (Table 4). There were no significant differences between the two groups for vomiting (p = 0.18) and diarrhea (p = 0.52). However, there was a significant increase in grade 1 inappetence in the intervention group (p = 0.02).
| Adverse event | Treatment group | Grade 0 | Grade 1 | Grade 2 | Grade 3 | p |
|---|---|---|---|---|---|---|
| Diarrhea | Placebo | 40 | 2 | 8 | 0 | 0.52 |
| CBD | 36 | 4 | 5 | 0 | ||
| Vomiting | Placebo | 44 | 3 | 3 | 0 | 0.18 |
| CBD | 40 | 5 | 0 | 0 | ||
| Inappetence | Placebo | 40 | 0 | 0 | 0 | 0.02 |
| CBD | 50 | 5 | 0 | 0 | ||
| Low HCT | Placebo | 39 | 11 | 0 | 0 | 0.25 |
| CBD | 39 | 5 | 1 | 0 | ||
| Neutropenia | Placebo | 29 | 18 | 3 | 0 | 0.2 |
| CBD | 32 | 13 | 0 | 0 | ||
| Thrombocytopenia | Placebo | 45 | 3 | 1 | 1 | 0.27 |
| CBD | 41 | 1 | 3 | 0 |
3.4 Serum Biochemistry
Table 5 summarizes the serum chemistry results. A mild increase across both groups was observed in creatinine over time, with significantly higher serum concentrations within the normal range at the end of the protocol than at baseline (p = 0.02). Albumin was significantly higher at baseline than study week 3 and study week 5 (P = < 0.001), while potassium showed a time effect that was no longer significant with post hoc testing between groups (p = 0.02). Similarly, cholesterol showed a significant treatment × time effect (p = 0.02), which was lost during post hoc data assessment. A significant age effect was observed for potassium and globulin, with increases and a decrease with age for cholesterol (p = 0.02; p = 0.02; p = 0.002, respectively).
| Metabolites | Treatment group | Study week 0 (n = 19) | Study week 3 (n = 15) | Study week 5 (n = 18) | p-value | |||
|---|---|---|---|---|---|---|---|---|
| Tx | Time | Tx × Time | Age | |||||
| Glucose (mg dL−1) | Placebo | 113 (105–121) | 106 (99–114) | 110 (103–118) | 0.18 | 0.21 | 0.37 | 0.85 |
| CBD | 102 (93–111) | 102 (94–111) | 106 (97–114) | |||||
| Creatinine (mg dL−1) | Placebo | 0.84 (0.68–1.00) | 1.14 (0.95–1.35) | 1.07 (0.94–1.20) | 0.16 | 0.03 | 0.17 | 0.1 |
| CBD | 1.00 (0.82–1.19) | 0.94 (0.76–1.11) | 1.10 (0.96–1.25) | |||||
| Serum urea nitrogen (mg dL−1) | Placebo | 19 (14–23) | 16 (10–22) | 16 (11–20) | 0.71 | 0.15 | 0.24 | 0.32 |
| CBD | 18 (12–24) | 16 (10–23) | 18 (13–23) | |||||
| Phosphorus (mg dL−1) | Placebo | 4.3 (3.7–4.9) | 4.0 (3.5–4.4) | 3.9 (3.4–4.4) | 0.77 | 0.19 | 0.63 | 0.45 |
| CBD | 4.4 (3.7–5.1) | 3.9 (3.4–4.4) | 4.1 (3.6–4.8) | |||||
| Calcium (mg dL−1) | Placebo | 10.0 (9.6–10.3) | 9.9 (9.6–10.2) | 10.0 (9.6–10.3) | 0.87 | 0.43 | 0.63 | 0.08 |
| CBD | 10.0 (9.6–10.4) | 9.9 (9.5–10.2) | 9.9 (9.4–10.3) | |||||
| Total protein (g dL−1) | Placebo | 6.3 (6.0–6.6) | 6.1 (5.6–6.6) | 6.4 (6.0–6.8) | 0.77 | 0.33 | 0.53 | 0.21 |
| CBD | 6.3 (6.0–6.7) | 6.1 (5.7–6.5) | 6.2 (5.8–6.6) | |||||
| Albumin (g dL−1) | Placebo F | 3.3 (3.1–3.5) | 3.0 (2.8–3.3) | 3.1 (2.9–3.2) | 0.73 | < 0.001 | 0.79 | 0.59 |
| CBD | 3.3 (3.1–3.6) | 3.1 (2.8–3.2) | 3.2 (3.0–3.3) | |||||
| Globulin (g dL−1) | Placebo | 3.0 (2.7–3.2) | 3.1 (2.9–3.3) | 3.4 (3.1–3.7) | 0.5 | 0.11 | 0.31 | 0.02 |
| CBD | 3.0 (2.7–3.3) | 3.1 (2.8–3.4) | 3.1 (2.8–3.4) | |||||
| Alanine aminotransferase (ALT, U L−1) | Placebo | 77 (29–83) | 41 (26–56) | 56 (29–83) | 0.79 | 0.12 | 0.87 | 0.06 |
| CBD | 71 (27–115) | 46 (29–64) | 40 (9–70) | |||||
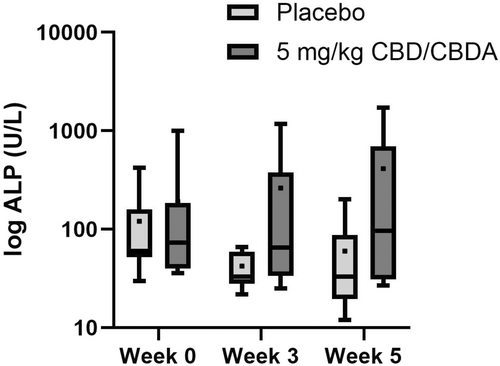
| Alkaline phosphatase (ALP, U L−1) | Placebo | 108 (0–236) | 93 (0–250) | 79 (0–325) | 0.19 | 0.2 | 0.22 | 0.1 |
| CBD | 205 (57–353) | 234 (52–417) | 417 (142–694) | |||||
| GGT (U L−1) | Placebo | 3 (2–5) | 2 (0–3) | 1 (0–2) | 0.54 | 0.1 | 0.39 | 0.14 |
| CBD | 2 (1–4) | 1 (0–3) | 2 (0–3) | |||||
| Total bilirubin (mg dL) | Placebo | 0.2 (0.1–0.3) | 0.2 (0.1–0.3) | 0.2 (0.1–0.3) | 0.69 | 0.83 | 0.75 | 0.45 |
| CBD | 0.2 (0.1–0.3) | 0.2 (0.1–0.3) | 0.2 (0.2–0.3) | |||||
| Cholesterol (mg dL) | Placebo | 242 (196–288) | 245 (190–331) | 235 (173–297) | 0.98 | 0.3 | 0.02 | 0.03 |
| CBD | 218 (166–271) | 231 (167–288) | 261 (190–332) | |||||
| Sodium (mmoL) | Placebo | 148.5 (146.0–151.1) | 148.1 (145.3–151.0) | 148.5 (146.3–150.8) | 0.35 | 0.67 | 0.39 | 0.68 |
| CBD | 149.5 (147.5–151.2) | 150.0 (147.7–154.3) | 148.6 (146.0–151.1) | |||||
| Potassium (mmoL) | Placebo | 4.3 (4.1–4.4) | 4.6 (4.3–4.9) | 4.6 (4.4–4.8) | 0.66 | 0.02 | 0.59 | 0.002 |
| CBD | 4.3 (4.1–4.5) | 4.6 (4.2–4.9) | 4.4 (4.2–4.6) | |||||
| Chloride (mmoL) | Placebo | 111.4 (109.5–113.3) | 112.5 (110.5–114.4) | 112.7 (110.7–114.6) | 0.64 | 0.18 | 0.64 | 0.91 |
| CBD | 112.1 (110.0–114.4) | 113.6 (111.3–115.8) | 112.5 (110.3–114.7) | |||||
- Note: Values are presented as least square means (LSM) and 95% confidence intervals. All 19 dogs had a chemistry panel completed at week 0, 15 dogs at week 3 (n = 8 placebo and n = 7 CBD), and 18 dogs at week 5 (n = 9 placebo and n = 9 CBD).
Laboratory AEs were graded according to VCOG-CTCAE v2 guidelines. Pre-existing abnormal values were assessed for worsening over time and, if present, were classified as possible AEs. No grade 4 or 5 AEs were observed. One dog developed a grade 2 elevation of BUN that improved over time to a grade 1. One dog developed a grade 2 elevation in ALT at the end of the protocol. Two dogs developed a grade 2 ALP elevation, whereas one developed an asymptomatic grade 3 ALP elevation. Four dogs developed a progressive mild elevation in cholesterol. Due to the low prevalence, statistics were not performed. Figure 1 shows ALP across time and treatment.
3.5 Quality of Life Survey
Ninety-one percent of surveys were completed (Table 6). Questions 3,5,6,8,9, and 15 were primarily 0 across nearly all respondents. There were no differences across the sum of questions between treatment group or regarding treatment × time effect. We observed significant differences in only three QoL measurements. Notably, the intervention group exhibited higher levels of “my dog enjoyed being touched/pet” (p = 0.04), and in measurements concerning mobility, such as “my dog had trouble getting up or down” (p = 0.05) with the upper limit of the range being 4 days per week, and “my dog had troubles going for a walk” (p = 0.05) with the upper limit of the range being 5 days per week.
| Question | Tx | Mean | Median | Range | p-value Tx |
|---|---|---|---|---|---|
| 1. My dog had a lack of energy. | CBD | 1.97 | 1.0 | (0, 7) | 0.22 |
| Placebo | 1.02 | 0 | (0, 7) | ||
| 2. My dog's appetite was decreased. | CBD | 1.07 | 0 | (0, 7) | 0.16 |
| Placebo | 0.34 | 0 | (0, 7) | ||
| 3. My dog was reluctant to get up. | CBD | 0.62 | 0 | (0, 6) | 0.36 |
| Placebo | 0.45 | 0 | (0, 7) | ||
| 4. My dog had pain or discomfort. | CBD | 0.52 | 0 | (0, 5) | 0.1 |
| Placebo | 0.54 | 0 | (0, 4) | ||
| 5. My dog's treatment interfered with his/her enjoyment of life. | CBD | 0.85 | 0 | (0, 6) | 0.27 |
| Placebo | 0.65 | 0 | (0, 5) | ||
| 6. My dog enjoyed being near me (less). | CBD | 0.02 | 0 | (0, 1) | 0.92 |
| Placebo | 0.02 | 0 | (0, 1) | ||
| 7. My dog was less playful. | CBD | 1.3 | 1.0 | (0, 6) | 0.49 |
| Placebo | 1.1 | 1.0 | (0, 4) | ||
| 8. My dog showed a less normal amount of affection. | CBD | 0.37 | 0 | (0, 3) | 0.22 |
| Placebo | 0.13 | 0 | (0, 2) | ||
| 9. My dog enjoyed being pet or touched less. | CBD | 0.17 | 0 | (0, 2) | 0.04 |
| Placebo | 0.04 | 0 | (0, 2) | ||
| 10. My dog did his/her favorite activities less. | CBD | 0.82 | 0 | (0, 4) | 0.22 |
| Placebo | 1.1 | 0 | (0, 7) | ||
| 11. My dog slept less well at night. | CBD | 0.55 | 0 | (0, 4) | 0.68 |
| Placebo | 0.45 | 0 | (0, 3) | ||
| 12. My dog acted less like his/her normal self. | CBD | 0.82 | 0 | (0, 4) | 0.44 |
| Placebo | 0.7 | 0 | (0, 5) | ||
| 13. My dog had trouble getting up or lying down | CBD | 0.40 | 0 | (0, 3) | 0.05 |
| Placebo | 0.04 | 0 | (0, 1) | ||
| 14. My dog had trouble going for a walk. | CBD | 0.55 | 0 | (0, 5) | 0.04 |
| Placebo | 0.21 | 0 | (0, 2) | ||
| 15. My dog fell or lost balance. | CBD | 0.35 | 0 | (0, 4) | 0.06 |
| Placebo | 0.0 | 0 | 0 | ||
| 16. My dog did not eat his/her normal food. | CBD | 0.87 | 0 | (0, 7) | 0.38 |
| Placebo | 0.17 | 0 | (0, 2) | ||
| 17. My dog had trouble getting comfortable. | CBD | 0.52 | 0.0 | (0, 5) | 0.73 |
| Placebo | 0.47 | 0 | (0, 4) | ||
|
Mean sum of weekly score (maximum 119) |
Placebo | 7.56 | 4.0 | (0, 35) | 0.35 |
| CBD | 10.75 | 5.5 | (0, 56) |
- Note: Owners were instructed to list the number of days per week each statement applied to their dog's behavior. Zero days indicates the dog never exhibits stated behavior, and 7 indicates the dog exhibits stated behavior at least daily and is shown as the mean, median, and range. The overall response rate throughout the study was 91%.
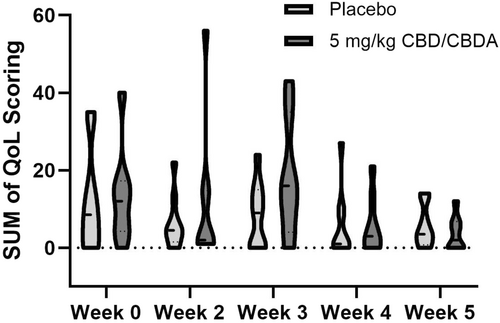
Figure 2 depicts the sum of all variables measured over time in each group, showing the relative mean and range over time between groups, which was not significant for time (p = 0.06), treatment (p = 0.39), or treatment × time (p = 0.45).
3.6 Pharmacokinetic Studies
3.6.1 Doxorubicin PK
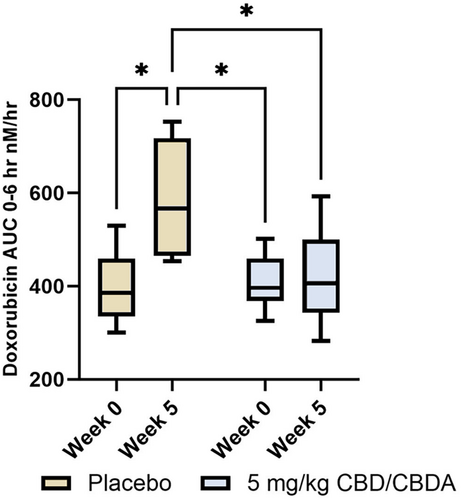
Seven dogs in the intervention group and 6 in the placebo group had doxorubicin PK performed at week 0 and week 5. The mean AUC was significantly higher at study week 5 (572.6 nM/h) (range 448.3–731.2 nM/h) compared with study week 0 (390.8 nM/h (range 318.6–479.4 nM/h)) in the placebo group (p = 0.015); however, there was no difference between week 0 (403.4 nM/h (range 351.9–462.5 nM/h)) and 5 (406.8 nM/h (range 3.23.2–551.8 nM/h)) in the intervention group (p = 0.99). The doxorubicin AUC was not different between groups when comparing study week 0 (p = 1.0), yet was significantly different between the two groups at week 5 (p = 0.026). Placebo group week 5 had a higher doxorubicin AUC compared with the CBD/CBDA- rich hemp treatment group at week 0, (p = 0.017). Figure 3 summarizes the data.
3.6.2 Serum Cannabinoids
Eight dogs had CBD serum concentrations checked at week 0, and nine dogs had serum CBD steady-state concentrations checked at week 5 of the study. Cannabinoids were not detectable in any dog before CBD therapy at week 0. Serum CBD, CBDA, THC, and THCA were the only cannabinoids shown to be consistently quantifiable, while CBC and CBGA concentrations were only detectable in 2 of the 9 dogs at the end of the protocol, with no other cannabinoids being detectable at the lower limit of quantitation. CBD and CBDA serum concentrations were most abundant, with smaller amounts of THC and THCA observed in the serum. Table 7 summarizes the data.
| Cannabinoid | Median serum concentration ng/mL median (range) | |
|---|---|---|
| Week 0 | Week 5 | |
| Cannabidiol (CBD) | Below quantification level | 335 (15–1693) |
| Cannabidiolic acid (CBDA) | Below quantification level | 15 (0–1347) |
| Δ9-tetrahydrocannabinol (THC) | Below quantification level | 12 (0–47) |
| Tetrahydrocannabinolic acid (THCA) | Below quantification level | 5 (0–47) |
4 Discussion
Administration of CBD/CBDA-rich hemp oil concurrently with one cycle of CHOP chemotherapy did not lead to significantly increased hematological or biochemical AEs between intervention and control groups. CBD isolates and CBD-rich hemp extracts have minimal adverse effects that can include a transient elevation in alkaline phosphatase (ALP), gastrointestinal upset, and hypersalivation [16, 19-25]. The only statistically significant changes found in select blood count or serum biochemistry variables were mild and time-related. Hematocrit and red blood cell count were lower in study week 2 than in study week 5 in both groups. In both groups, total white blood cell and neutrophil counts were increased at study week 2 compared with week 5. We suspect these mild changes are secondary to chemotherapy rather than the CBD/CBDA-rich hemp treatment. A difference in inappetence was observed between the groups. However, this was only seen for low grade 1 events, which are usually mild and do not typically necessitate medical intervention. Similarly, most AEs observed on biochemistry panels were mild and low in number. Only two grade 2 and one grade 3 elevations in ALP were seen in dogs in the intervention group and remained asymptomatic. Interestingly, no statistically significant rise in ALP value was noted, but trends suggest there was a rise in ALP in the intervention group, while the placebo group trended toward static or even decreasing ALP. In addition, ALT concentrations trended toward a decrease over time in both groups. While this data suggests CBD use might be safe during CHOP chemotherapy, we only assessed the effects of supplementation over 5 weeks, and a nadir CBC post doxorubicin was not performed at week 6, a week after the second doxorubicin treatment. Therefore, the study could not capture potential long-term effects or changes over time. Longitudinal studies with extended follow-up would provide a more comprehensive understanding of the safety of CBD in conjunction with chemotherapy.
Some dogs received supportive medications, including anti-nausea and anti-diarrheal agents, which could interact with CBD. Determining whether any observed adverse events resulted from these interactions remains challenging.
No published data assesses the safety and tolerability of CBD-rich hemp oil use during chemotherapy for lymphoma and its effect on QoL. Some evidence suggests that CBD interacts with various receptors involved in regulating fear- and anxiety-related behaviors, specifically antagonism to CB1 receptor; and agonists to the serotonin 5-HT1A receptor, and TRPV1 receptor [26, 27]. These effects could improve the QoL for pets with terminal illnesses. Owners of such pets often prioritize QoL over longevity and are willing to trade survival time to enhance their pets' well-being [28, 29].
When scoring in the QoL surveys was visually assessed, the intervention group had higher scores than placebo at 2 and 3 weeks, which appeared due to questions related to lethargy and ability to rise (Q13) dog and dog going for a walk (Q14), which might have been different for treatment if a larger cohort were assessed. These differences might be related to the sedation effects observed with cannabinoid use in other studies [6, 18]. This finding did not lead to alteration of the chemotherapy protocol, was mild, and appeared to dissipate by weeks 4 and 5. Of the QoL measurements around lethargy, activity, and enjoyment of life, few changes were observed other than the dog wanting to be touched (Q9). Statistically, the mild primary effects related to QoL differences were likely chemotherapy related, and the AEs of chemotherapy appeared to be minimal. Therefore, a larger group with more AEs due to chemotherapy is needed to determine the positive or negative effects of CBD-rich hemp utilization for QoL.
In companion animals, CBD appears to have adequate bioavailability depending on the medium used to deliver the oral treatment, leading to decreased pain and increased activity [19, 22, 23, 30]. Though the serum cannabinoids measured were only a “snapshot” in time with no assessment regarding the timing of the last dose or whether dogs missed their morning dose due to food being withheld, serum CBD and CBDA concentrations were highly variable. Serum concentrations of CBD measured at the end of our study were typically less than 0.3 ug/mL and less than 1 ug/mL for all cannabinoids, making anti-neoplastic activity unlikely. However, some dogs had serum concentrations in the 2 ug/mL range similar to concentrations observed to have anti-neoplastic effects in canine lymphoma cells [7]. These data suggest that there might be differences in metabolism and retention across dogs and that there is potential for effective anti-neoplastic dosing, which requires further study, however, the dosing would need to be relatively high [7, 9, 21].
It is possible that some of this variability could have been due to owner compliance and CBD/CBDA dose administration, although skipped doses were rare. Serum concentrations of the primary psychotropic cannabinoid, THC, exhibited significant variability, ranging up to 47 ng/mL. AEs associated with THC intoxication might be correlated to the decreased ability to rise in some but not all dogs. This was relatively mild and dissipated completely by weeks 4 and 5 of treatment. Similarly, no acute physiological indicators of toxicosis were observed when utilizing a comparable whole hemp product at a dose of 5 mg kg−1 [31]. Consequently, administering up to 5 mg kg−1 every 12 h of a full-spectrum hemp product appears to be well tolerated without manifesting the adverse effects attributed to THC.
Doxorubicin PK analysis showed no significant variation in AUC in the intervention group over time, suggesting that using CBD concurrently with doxorubicin does not lead to significant variation in this chemotherapy drug disposition in this small cohort. As our study looked at only one cycle of CHOP, additional long-term data is needed to evaluate for any change with a more chronic CBD administration.
Doxorubicin AUC was significantly higher in the placebo group at study completion compared with week 0 and compared with the intervention group at week 5. Doxorubicin is mainly metabolized by liver cells, kidney cells, and red blood cells, and the renal clearance of doxorubicin metabolites is low. None of the dogs showed signs of impaired liver function (based on pseudo-function biochemical values), nor any significant change in body surface area.
Ideally, pharmacokinetic analyses should have been conducted for all chemotherapy agents utilized in this study to provide a comprehensive understanding of the effect of CBD/CBDA on drug distribution, metabolism, and excretion, which could inform dose optimization and adverse events management. However, due to budgetary constraints, we were limited to analyzing only doxorubicin. This agent was selected because, at the time, it was deemed to have the highest potential for adverse events (AEs) based on its known toxicity profile, including gastrointestinal upset and myelosuppression, which could decrease the dog's quality of life during chemotherapy.
5 Conclusions
In summary, administration of CBD/CBDA-rich hemp oil alongside one cycle of CHOP chemotherapy in dogs with lymphoma showed no significant increase in hematological or biochemical AEs compared with control groups. Nor were there any persistent positive or negative QoL measurements that could be attributed to the treatment. Dosing of 5 mg Kg−1 PO q12 h led to variable serum concentrations of CBD and CBDA in our study cohort.
Disclosure
Authors declare no off-label use of antimicrobials.
Ethics Statement
Approved by the University of Florida Institutional Animal Care and Use Committee (protocol #202110448). Authors declare human ethics approval was not needed.
Conflicts of Interest
Joseph J. Wakshlag is a paid consultant of ElleVet Sciences. To reduce bias, the dog enrollment and randomization were blinded to him. Data analysis (stats) were led and performed by Francisco A. Leal Yepes. The other authors declare no conflicts of interest.




