Hypersomatotropism and Hypercortisolism Caused by a Plurihormonal Pituitary Adenoma in a Dog
Funding: The authors received no specific funding for this work.
ABSTRACT
A 12-year-old, male Labrador Retriever was presented because of polyuria, polydipsia, polyphagia, joint pain, and physical features consistent with acromegaly. Circulating insulin-like growth factor-1 (IGF-1) concentration was increased (> 1000 ng/mL; reference interval [RI], 42–449), suggestive of hypersomatotropism. An abnormal low-dose dexamethasone suppression test and increased circulating adrenocorticotropic (ACTH) concentration indicated pituitary-dependent hypercortisolism. Computed tomography identified an enlarged pituitary gland. Treatment with cabergoline initially decreased circulating IGF-1 and ACTH concentrations and urinary cortisol-to-creatinine ratio (UCCR), with a notable reduction in acromegalic physical features. However, 7 months after the start of cabergoline treatment, IGF-1, ACTH, and UCCR had increased again, although pituitary gland size remained stable. Because of worsening joint pain, euthanasia was performed. On necropsy, double immunohistochemistry identified pituitary tumor cells with cytoplasmic co-expression of both growth hormone (GH) and ACTH, consistent with a monomorphic plurihormonal macroadenoma. This case shows that concurrent hypersomatotropism and hypercortisolism can occur in dogs caused by a plurihormonal pituitary adenoma.
Abbreviations
-
- ACTH
-
- adrenocorticotropic hormone
-
- CLIA
-
- chemiluminescent immunometric assay
-
- CT
-
- computed tomography
-
- D2
-
- dopamine receptor subtype 2
-
- DM
-
- diabetes mellitus
-
- GH
-
- growth hormone
-
- IGF-1
-
- insulin-like growth factor-1
-
- P/B
-
- pituitary height-to-brain area ratio
-
- SSTR
-
- somatostatin receptor
-
- SSTR2
-
- somatostatin receptor subtype 2
-
- SSTR5
-
- somatostatin receptor subtype 5
-
- TSH
-
- thyroid-stimulating hormone
-
- TT4
-
- Total thyroxine
-
- UCCR
-
- urinary cortisol-to-creatinine ratio
1 Introduction
Hypersomatotropism (excess growth hormone [GH] and subsequent increased insulin-like growth factor-1 [IGF-1]) may lead to physical changes known as acromegaly, as well as insulin resistance and diabetes mellitus (DM) [1]. Hypersomatotropism in dogs usually is caused by either the influence of progesterone produced during the luteal phase of the estrous cycle or administration of progestagens [1]. Progesterone and progestagens result in GH production in the mammary gland, and this mammary GH may enter the systemic circulation in dogs [2]. Also, hypothyroidism may result in GH excess in dogs [3]. Excessive GH secretion from a pituitary tumor (somatotropinoma), the main cause of hypersomatotropism in humans and cats, is very rare in dogs, and only 5 cases have been documented to date [4-9]. Except for the first reported case [5], all dogs with hypersomatotropism exhibited features of acromegaly, including soft tissue overgrowth (e.g., excessive skin folds, particularly on the neck and head, and macroglossia) and skeletal overgrowth (e.g., widened interdental spaces). The majority also developed DM requiring high insulin doses, except one case [6], which had insulin resistance without DM. Treatment in these cases consisted of radiotherapy [7], the somatostatin analogue pasireotide [8], and hypophysectomy [9], along with insulin treatment. Although all cases improved clinically, only the dog treated with pasireotide achieved DM remission.
Cabergoline, a dopaminergic agonist, effectively decreases circulating IGF-1 concentration in approximately 26% of cats, improves glycemic control, and may induce DM remission in cats with hypersomatotropism [10]. Cabergoline also has been shown to decrease circulating GH and IGF-1 concentrations in some human patients with acromegaly [11]. Cabergoline has been used to treat pituitary-dependent hypercortisolism in dogs and one cat, showing an efficacy of approximately 30% in dogs, and achieving remission of DM in the affected cat [12, 13]. However, its use in dogs with hypersomatotropism has not yet been reported.
We describe the diagnosis and follow-up after treatment with cabergoline in a dog with both hypersomatotropism, resulting in acromegalic features, and hypercortisolism, caused by a plurihormonal pituitary adenoma expressing both GH and ACTH.
2 Case Description
A 12-year-old male Labrador Retriever (body weight, 40 kg) was presented because of polyuria, polydipsia, polyphagia, and joint pain. Additionally, the owner described progressive physical changes over the past 4 years, including head and neck enlargement and macroglossia (Figure 1A–D). Physical examination confirmed these findings, identifying prominent skin folds on the head and neck, along with widening of the extremities (Figure 2A) and interdental spaces (Figure 2B). Laboratory findings disclosed clinically relevant abnormalities, including increased alkaline phosphatase activity (1800 U/L; reference interval [RI], < 250 U/L), alanine aminotransferase activity (173 U/L; RI, < 50 U/L), total cholesterol concentration after 10 h of fasting (390 mg/dL; RI, < 220 mg/dL), and fasting blood glucose concentration (255 mg/dL; RR, 70–125 mg/dL). Diabetes mellitus was ruled out based on a 24-h blood glucose curve (< 120 mg/dL). However, hyperinsulinemia (114 μU/L; RI, < 20 μU/L) with fasting normoglycemia (104 mg/dL) suggested insulin resistance.


Based on clinical signs and biochemical changes, the dog was suspected to have hypercortisolism. Subsequently, hypercortisolism was diagnosed based on a low-dose dexamethasone suppression test (basal cortisol concentration: 10 μg/dL; 4 h: 2.1 μg/dL; 8 h: 2.3 μg/dL; RI for 4 and 8 h, < 1.4 μg/dL; chemiluminescent immunometric assay [CLIA], Immulite 1000) and an increased urinary cortisol-to-creatinine ratio (UCCR, 71 × 10−6; RI, < 30 × 10−6). Abdominal ultrasonography disclosed bilaterally symmetrical adrenomegaly, with caudal pole thicknesses of 8.2 mm (left adrenal) and 8.4 mm (right adrenal). The circulating ACTH concentration (55.5 pg/mL; RI, < 10–38 pg/mL; CLIA, Immulite 1000) was increased. Together, these findings pointed to pituitary-dependent hypercortisolism.
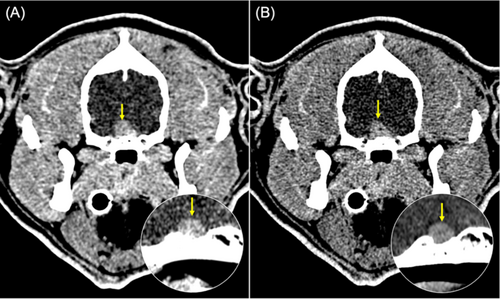
Because clinical signs suggested acromegaly, circulating IGF-1 concentration, measured in two samples collected on separate days, was used to confirm hypersomatotropism, with concentrations of 993 ng/mL and > 1000 ng/mL (RI, 42–449 ng/mL; CLIA, Immulite 2000) [6, 14]. Taking into account the reasons for hypersomatotropism in dogs, hypothyroidism was ruled out (thyroid-stimulating hormone [TSH] concentration, 0.12 ng/mL; RI, < 0.6 ng/mL and total thyroxine [TT4] concentration, 1.2 μg/dL; RI, 1–3 μg/dL). In addition, there was no history of use of progestagens. Endogenous progesterone was not assessed because of the low likelihood of excess in a male dog. Computed tomography (CT) identified an enlarged pituitary gland with a pituitary height-to-brain area ratio (P/B) of 0.6 (RI, < 0.31; Figure 3A).
Because hypophysectomy, somatostatin analogues, and radiotherapy were unavailable to us, we considered using cabergoline, considering its beneficial role in humans, dogs, and a cat with hypercortisolism, as well as in humans and cats with hypersomatotropism [11-13]. Two weeks after initiating cabergoline (5 μg/kg q72h, Dostinex, Pfizer), the dog developed DM, evidenced by severe hyperglycemia, glucosuria, and worsening of polyuria and polydipsia, with mild body weight loss (< 1 kg). Insulin treatment with levemir (20 IU, SC q24h, Detemir, Novo Nordisk) and a commercial diet for joint disease, administered q12h, was added. Meanwhile, the cabergoline dose was adjusted to 6.5 μg/kg q24h, as the proposed regimen of 10 μg/kg every 48 h caused diarrhea and loss of appetite.
Three months after the start of cabergoline treatment, a decrease in the circulating concentrations of IGF-1 (890 ng/mL; previously > 1000) and ACTH (24.8 pg/mL; previously 55.5 pg/mL) were found. However, given the coefficients of variation (CV) of the immunoassay used (approximately 4.7% for IGF-1 and up to 10.7% for ACTH at these concentrations) as well as possible biological variation, these changes were interpreted with caution. The UCCR had decreased to 53.9 × 10−6 (previously 71 × 10−6), along with resolution of polyuria and polydipsia. On an average insulin dose of 20 IU q12h, the home glucometer concentrations were < 200 mg/dL. Interestingly, a notable decrease in soft tissue overgrowth and body weight (29 kg) were observed with stable DM (Figure 4).

Four months later, the circulating concentrations of IGF-1 (> 1000 ng/mL) and ACTH (57.8 pg/mL) and the UCCR (71.3 × 10−6) had increased to results similar to before the start of cabergoline treatment, whereas pituitary size remained stable on CT scan (Figure 3B). The dog required an average insulin dose of 27 IU q12h to maintain good glycemic control (home glucose concentrations consistently < 250 mg/dL). A decrease in alkaline phosphatase activity was noted (1231 U/L compared to 1800 U/L), but no change was observed in alanine aminotransferase activity (303 U/L) or total cholesterol concentration (471 mg/dL; Supporting Information). Based on increased UCCR and ACTH, combined treatment with trilostane and cabergoline was proposed. However, because of worsening of the joint pain as well as the possibility of further growth of the pituitary gland, the owner declined the change in treatment.
One month after last follow-up, the owner elected euthanasia because of severe mobility limitations caused by advanced joint disease. Necropsy identified a 1.1 cm pituitary tumor with well-defined borders and a yellowish-brown coloration (Figure 5A). Histopathology confirmed a pituitary acidophil adenoma (Figure 5B). Immunohistochemistry identified GH immunopositivity (Figure 5C,D) in the majority of the neoplasm, along with ACTH immunopositivity in a specific region within the neoplasm (Figure 5E,F). Double immunohistochemistry for GH and ACTH confirmed cytoplasmic co-expression of both GH and ACTH in the cells initially identified as ACTH immunopositive (Figure 5G,H). The proliferation marker Ki-67 was low (< 1%). The final histological diagnosis was a plurihormonal pituitary adenoma. Immunostaining for prolactin, thyroid stimulating hormone (TSH), follicle-stimulating hormone, and luteinizing hormone could not be performed.
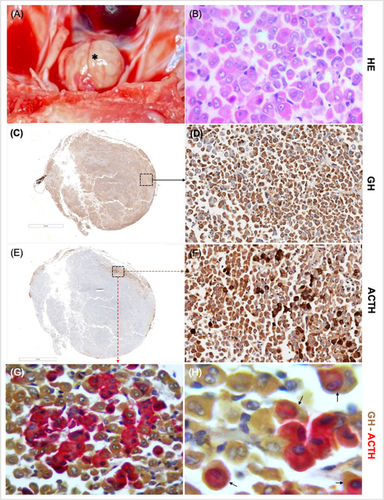
Histological evaluation of the thyroid gland, parathyroid glands, pancreas, and adrenal glands showed no clinically relevant changes, except for marked bilateral hyperplasia of the adrenal cortex, specifically in the fascicular zone.
3 Discussion
We report a rare case of a plurihormonal pituitary adenoma expressing both GH and ACTH, leading to hypersomatotropism and hypercortisolism in a dog that was subsequently treated with cabergoline. To our knowledge, it is the first reported case of a functional plurihormonal pituitary adenoma in a dog and of cabergoline use in a dog with hypersomatotropism.
The biochemical diagnosis of hypersomatotropism was based on increased circulating IGF-1 concentrations, after recognition of acromegalic changes [6-9]. The pituitary origin was confirmed by positive GH immunoreactivity [6-9]. Dogs with hypothyroidism generally have lower circulating IGF-1 concentrations [3] than the few reported cases of dogs with acromegaly caused by a functional somatotrope adenoma [6-9]. Although total thyroxine concentration (TT4) was at the lower reference limit, TSH was in the normal range and thyroid gland appearance and histology on necropsy were normal. Progestagens were never administered, and endogenous progesterone excess is rare in male dogs, previously reported only in a progesterone-secreting adrenocortical tumor, which was ruled out in our case [15].
Hypercortisolism was diagnosed based on biochemical abnormalities, positive LDDST, and an increased UCCR [16]. A circulating ACTH concentration above the RI, ACTH immunoexpression within the somatotropinoma, and bilateral adrenal fascicular zone hyperplasia confirmed pituitary-dependent hypercortisolism [16]. Clinically, hypercortisolism overlaps with hypersomatotropism, manifesting as polyuria, polydipsia, and polyphagia, although acromegalic features predominate.
Human patients with untreated Cushing's disease may have a mildly increased IGF-1 concentration, which decreases after remission [17]. In addition, long-term prednisone treatment is associated with suppressed GH concentrations and with a mild increase in IGF-1 concentrations [18]. Consequently, diagnosing acromegaly in a dog with concurrent hypercortisolism requires more evidence than only an increased IGF concentration.
Among functional pituitary tumors in dogs, corticotropinomas are most common, followed by somatotropinomas and prolactinomas [19]. The coexistence of hypersomatotropism and hypercortisolism was documented in an 8-year-old diabetic cat with a double pituitary adenoma, consisting of a somatotropinoma and a plurihormonal adenoma expressing ACTH, alpha-melanocyte-stimulating hormone, and follicle-stimulating hormone [20]. To date, simultaneous GH and ACTH excess has not been described in dogs, although by using immunohistochemistry a plurihormonal adenoma expressing both GH and ACTH was reported in an 11-year-old Labrador retriever, but there was no evidence of excessive hormone secretion [21]. In four of the five previously reported cases of acromegaly in dogs caused by somatotropinoma, testing for hypercortisolism was negative [6-9]. In none of these cases was the circulating ACTH concentration evaluated, and the adrenal glands appeared normal in size and shape based on necropsy [5], ultrasonography [6, 7], or CT [9]. Additionally, histopathological evaluation of three pituitary tumors showed no ACTH immunoreactivity [5, 6, 9]. Therefore, the findings in our case indicate a unique presentation.
In humans, plurihormonal pituitary adenomas produce two or more hormones, with acromegaly being the most common clinical presentation [22]. These tumors are often larger, more aggressive, and highly recurrent [22]. They are classified as monomorphic (single cell type producing multiple hormones) or pleomorphic (different cell types producing distinct hormones) [22]. In our case, expression of GH predominated over that of ACTH in the cells that constituted the pituitary adenoma, which is consistent with the predominant physical features of acromegaly. Double immunohistochemistry allowed for the identification of a group of cells with cytoplasmic co-expression of both GH and ACTH. Therefore, these findings point to a monomorphic plurihormonal macroadenoma that was non-aggressive based on the absence of local invasion and low Ki-67 index.
Although hormonal tests, increased circulating ACTH, and ACTH immunoexpression in the pituitary tumor were consistent with ACTH-dependent hypercortisolism, the predominant signs were those associated with GH excess, as also reported in humans with plurihormonal pituitary adenomas co-secreting ACTH and GH [22]. The increase in serum alkaline phosphatase activity (which was normal in three of the cases in dogs reported with acromegaly caused by a somatotropinoma) and bilateral adrenal enlargement on ultrasonography (absent in the four dogs reported with acromegaly caused by a somatotropinoma) were key findings, because without them, hypercortisolism might have gone undetected [6-9].
Various treatment options exist for the management of somatotropinomas and corticotropinomas in humans, dogs, and cats, including transsphenoidal surgery, radiotherapy, somatostatin receptor ligands, dopaminergic agonists, and GH receptor antagonists such as pegvisomant [7-13, 22-25]. In our case, only medical treatment with cabergoline was available. Cabergoline is hypothesized to decrease GH/IGF-1 secretion (as reported in humans and cats with hypersomatotropism) and ACTH secretion (as observed in humans and dogs with hypercortisolism) by activating dopamine receptor subtype 2 (D2) [12]. However, both D2 receptor expression and drug efficacy may be limited in pituitary macroadenomas [26, 27].
Cabergoline dosage and frequency of administration in dogs with hypersomatotropism have not been previously established, nor has D2 receptor expression been characterized in somatotropinomas in dogs. In our case, a dosage of 10 μg/kg was attempted, similar to that used in cats with hypersomatotropism, but it was not tolerated because of adverse gastrointestinal effects [10]. Additional studies are needed to evaluate the role of D2 receptor expression in somatotropinomas and the effective dosage of cabergoline in affected dogs.
Despite these uncertainties, a decrease in circulating IGF-1 concentration, accompanied by a decrease in soft tissue overgrowth, was observed after 3 months, along with a decrease in circulating ACTH concentration. In addition to these initial hormonal changes, the owner reported improvement in the dog's quality of life, despite the fact that the dog developed DM that required treatment with insulin. However, at the four-month follow-up, the insulin dose had to be increased to control DM, coinciding with increases in both circulating IGF-1 and ACTH concentrations and UCCR. This deterioration may be attributed to a potential loss of cabergoline efficacy because of drug resistance, as previously described in humans with acromegaly [11]. The pituitary tumor did not decrease in height on CT during cabergoline treatment, which confirms that there was no relevant tumor shrinkage induced by D2 receptor agonism in the present case.
Use of the multi-receptor somatostatin analogue pasireotide previously was reported in a dog with a somatotropinoma, in combination with radiotherapy, leading to resolution of signs of acromegaly and DM remission [8]. This finding suggests that, as in cats with somatotropinomas, the predominant receptor population to target in dogs with a somatotropinoma may be somatostatin receptors (SSTRs), such as somatostatin receptor 2 (SSTR2) and subtype 5 (SSTR5), rather than dopamine D2.
In conclusion, our case shows that concurrent hypersomatotropism and hypercortisolism can occur in dogs with plurihormonal pituitary adenomas. In our case, hypersomatotropism predominated clinically over hypercortisolism, suggesting that it might be useful to assess other pituitary axes in dogs with hypersomatotropism. Finally, cabergoline seemed to have a transient beneficial effect, leading to decreases in IGF-1, ACTH, and UCCR, although biochemical remission was not achieved. However, these changes should be interpreted with caution given potential assay and biological variability. Additional long-term studies are needed to determine the role of cabergoline in hormonal control and tumor progression in dogs with hypersomatotropism caused by somatotropinoma.
Acknowledgments
The authors thank the Albeitar Laboratory, Intensivet Veterinary Hospital, and Dr. Santiago Teyssandier for the oral presentation of the partial results of this case at the Summer School of Endocrinology in Bologna, Italy (2024).
Disclosure
Authors declare no off-label use of antimicrobials.
Ethics Statement
Authors declare no institutional animal care and use committee or other approval was needed. Authors declare human ethics approval was not needed.
Conflicts of Interest
The authors declare no conflicts of interest.




