Long-Term Outcomes of Mitral Valve Repair With Artificial Chordae and Annuloplasty for Myxomatous Mitral Valve Disease in Dogs
Funding: The authors received no specific funding for this work.
ABSTRACT
Background
Myxomatous mitral valve disease (MMVD) in dogs commonly progresses to congestive heart failure, which carries a poor prognosis. Mitral valve repair (MVR) is a recognized treatment for advanced-stage MMVD.
Hypothesis/Objectives
Identify the risk factors and prognosis in dogs undergoing MVR.
Animals
We enrolled 1019 dogs with MMVD (ACVIM stages B2, C, and D) that underwent MVR between January 2017 and December 2020.
Methods
Medical records from a single institution were retrospectively reviewed. Early and late postoperative periods were defined as < 100 days and 100–1400 days after surgery, respectively. The outcome was time to a composite of all-cause mortality, onset of postoperative congestive heart failure, or undergoing a second MVR (surgical revision).
Results
In the early postoperative period, 61 dogs experienced the composite outcome; in the late period, 211 dogs did. No dogs underwent a second MVR. The incidence rate was 19.6 and 7.1 events per 1000 dog-months in the early and late periods, respectively. Compared with stage B2 dogs, stage D dogs had 2.2 times the daily hazard of experiencing an early postoperative event. In the late period, increasing age (per year; hazard ratio [HR], 1.3), higher body weight (per kilogram; HR, 1.1), Cavalier King Charles Spaniels compared with Chihuahuas (HR, 2.2), and preoperative tricuspid valve regurgitant velocity > 3.7 m/s (HR, 2.5) were associated with the events.
Conclusions and Clinical Importance
A higher incidence of the event was observed in the early postoperative period rather than later, with the outcome varying according to MMVD condition and patient-specific factors.
Abbreviations
-
- ACVIM
-
- American College of Veterinary Internal Medicine
-
- CI
-
- confidence interval
-
- E
-
- peak velocity of early diastolic transmitral flow
-
- HR
-
- hazard ratio
-
- LA:Ao
-
- left atrial to the aortic ratio
-
- LVIDDN
-
- left ventricular end-diastolic internal diameter normalized to body weight
-
- LVIDSN
-
- left ventricular systolic internal diameter normalized to body weight
-
- MMVD
-
- myxomatous mitral valve disease
-
- MVR
-
- mitral valve repair
-
- TRV
-
- tricuspid regurgitant velocity
-
- VHS
-
- vertebral heart score
1 Introduction
Myxomatous mitral valve disease (MMVD) is the most common heart disease in dogs, primarily affecting small breeds [1]. The prognosis of MMVD dogs with a history of congestive heart failure is poor, with a median survival time of less than 1 year with medical management alone [2]. Mitral valve repair (MVR) recently has emerged as a potentially curative treatment for MMVD, representing an alternative to medical management [3, 4]. The American College of Veterinary Internal Medicine (ACVIM) consensus guidelines (2019) recommend considering MVR in dogs with MMVD at advanced stage B2 and above, highlighting its potential to improve outcomes in dogs with severe disease [1].
Several studies have demonstrated the efficacy of MVR in dogs, primarily focusing on surgical techniques and early postoperative hemodynamics. For example, a report described the surgical procedure and early postoperative results in 48 small-breed dogs and reported significant improvements in heart size after surgery [3]. Some studies described institution-specific chordae tendineae reconstruction techniques applied across different clinical stages, including the loop-in-loop and four-point surgical techniques [5, 6]. Additionally, the quality of life of dogs and owners after surgical interventions was reported [7, 8]. Recently, a report described the geometric and hemodynamic changes after MVR in dogs up to 3 months after the procedure [9]. Although these studies emphasize the potential benefits of MVR, they are limited in scope, focused primarily on short-term results, and lacked comprehensive long-term data.
Some case reports indicated that MVR can remain durable for more than 5 years after surgery in individual cases [10, 11]. These observations, however, were based on small sample sizes and lacked statistical power to detect treatment effects. The long-term survival and risk factors associated with MVR have not yet been thoroughly investigated in a larger cohort of dogs with MMVD.
We aimed to identify possible risk factors for both early postoperative mortality and long-term survival in dogs that underwent MVR for the treatment of MMVD. By analyzing data from a larger cohort of dogs, our study provides valuable information for future veterinary practice. Overall, our findings have the potential to enhance our understanding of the outcomes of MVR, guide clinical decision-making, and support the development of evidence-based guidelines for the treatment of MMVD in dogs.
2 Materials and Methods
2.1 Animals
The target population for our study comprised all client-owned dogs with MMVD that can access the JASMINE Veterinary Cardiovascular Medical Center, Kanagawa Prefecture, Japan. The source population comprised members of the target population with MMVD diagnosed as ACVIM stages B2, C, or D (confirmed by echocardiographic measurements) and, for stages C and D, those that had a documented history of congestive heart failure [1], with owners who had elected to pursue MVR. Dogs eligible for the study were members of the source population that received MVR at the institution between January 2017 and December 2020 (inclusive). From this inclusive cohort, dogs were excluded if their survival status after discharge was unknown or if they had already undergone MVR before inclusion. Statistical analyses were conducted using the final dataset. Postoperative clinical courses were obtained from medical records and by telephone interviews with owners or referring veterinarians. Follow-up data were updated in April 2025. Data were collected only after obtaining consent from owners. The study protocol was approved by the Institutional Ethics Committee of the JASMINE Veterinary Cardiovascular Medical Center (approval number: 230315–02).
2.2 Surgical Technique
All surgical procedures were performed by a single team, consisting of a primary surgeon (MU) and an assistant surgeon (MM). The primary surgeon was responsible for all surgeries conducted during the study period. The surgery involved cardiopulmonary bypass (CPB) with hypothermic management [12]. Before CPB cannulation, heparin sodium (200 U/kg) was administered IV, and the activated clotting time was confirmed to exceed 300 s using an analyzer (Sonoclot; Sienco Inc. Colorado, USA) with a cuvette (gbACT+ Kit; Sienco Inc. Colorado, USA). An arterial CPB cannula was subsequently inserted into the left carotid artery, and a venous CPB cannula was inserted into the right atrium via the left jugular vein [3].
The cardioplegia used in the study consisted of a St. Thomas II cardioplegic solution (Miotecter, Mochida Pharmaceutical Co. Ltd. Tokyo, Japan) containing 50% autologous blood and augmented with potassium until a concentration of 40–50 mEq/L was achieved. This solution was administered as an equal-part mixture of 5 mL/kg St. Thomas II solution and 5 mL/kg blood withdrawn from the CPB circuit. A total of 10 mL/kg was administered as the initial dose. During the cross-clamp phase, cardioplegic administration was repeated every 5–10 min at a half of the initial dosage [13]. The cardioplegia was delivered in an antegrade manner into the coronary artery via an aortic root cannula [3, 14], while maintaining an intra-circuit pressure of 150 mmHg at a temperature ranging from 10°C to room temperature. To ensure the effectiveness of cardioplegia, the surgeon and anesthesiologist continuously monitored cardiac activity using an ECG and directly observed the heart for signs of contractile activity. Additional doses of cardioplegia were administered if any resumption of cardiac activity was observed. After atriotomy, artificial chordae were placed, and annuloplasty was performed in accordance with previously described methods [15, 16]. The target minimum core body temperature was set at 27°C–30°C during the cross-clamp phase. Left atrial appendage occlusion was not performed in any of the dogs during the study period.
Dogs were closely monitored during the postoperative period using continuous ECG and pulse oximetry. Monitoring was performed while the dogs were in the intensive care unit with an oxygen supply set to maintain the fraction of inspired oxygen at 30%–40% for 48 h after surgery. We performed routine echocardiograms 12 h postoperatively and again before discharge (typically 5 days after surgery). The use of pimobendan and loop diuretics was determined based on these examinations.
Postoperative inpatient antithrombotic treatment was initiated with dalteparin sodium (Sawai Pharmaceutical Co. Ltd. Osaka, Japan) at a dose of 25 IU/kg at 6 h and 50 IU/kg at 12 h postoperatively. Starting the day after surgery, treatment included clopidogrel (Plavix, Sanofi-Aventis, Paris, France) at 3.0 mg/kg PO q24h, along with either dalteparin sodium at 100 IU/kg SC q8h or rivaroxaban (Xarelto, Bayer, Leverkusen, Germany) at 0.5–1.0 mg/kg PO q24h during hospitalization. Dogs also received IV or PO antibiotics (cefazolin [Cefazolin Sodium Injection, Nichi-Iko Pharmaceutical Co. Ltd. Toyama, Japan] 20 mg/kg IV q8h; cephalexin [Cefaclear, Kyoritsu Seiyaku, Tokyo, Japan] 20 mg/kg PO q12h) during surgery and until 5 days after the surgery. After discharge from the hospital, clopidogrel was continued at 3.0 mg/kg PO q24h for 3 months.
2.3 Measurements
Thoracic radiographic and echocardiographic measurements were obtained at four time points: preoperatively, and at three, six, and twelve months postoperatively. Vertebral heart score (VHS) was determined using thoracic radiographic images taken in right lateral recumbency [17]. Echocardiography was performed by several veterinary cardiologists at the institution using a Vivid E95 machine (GE HealthCare Japan, Tokyo, Japan) with a 1.0–5.0 MHz sector probe (6S-D, GE HealthCare Japan, Tokyo, Japan). The ratio of the left atrial dimension to the aortic annulus dimension (LA:Ao) was determined using a right parasternal short-axis view at the level of the heart base, as proposed previously [18]. Left ventricular internal dimensions at end-diastole and systole, normalized to body weight (LVIDDN, LVIDSN) were obtained using M-mode from a right parasternal short-axis view at a level of the chordae tendineae. Each investigator used an allometric approach as proposed previously [19], applying a scaling exponent of 0.294 for LVIDDN and 0.315 for LVIDSN. Using pulsed-wave Doppler echocardiography, peak velocity of the early diastolic transmitral flow (E) wave and the tricuspid regurgitant velocity (TRV) were measured based on a left apical four-chamber view. The TRV values were evaluated to determine whether they exceeded 3.7 m/s, which may indicate pulmonary hypertension that is less responsive to treatment [20].
2.4 Outcome
The study outcome was time to a composite of all-cause mortality, the onset of postoperative congestive heart failure, or undergoing a second MVR (surgical revision). Congestive heart failure included left-sided congestive heart failure (cardiogenic pulmonary edema) and right-sided congestive heart failure secondary to pulmonary hypertension (pleural effusion or ascites). Cardiac-related deaths were documented as a subgroup of mortality and were defined as euthanasia due to a cardiac cause or other cardiac-related events, including sudden death from ventricular arrhythmia and low-output syndrome secondary to myocardial dysfunction, which may be related to interventricular septal hematoma or myocardial damage after surgery [21, 22]. Suspected causes of noncardiac death also were recorded. A second MVR may be considered in cases of artificial chordae rupture, sudden worsening of regurgitant fraction, or progressive mitral annular dilatation during postoperative follow-up.
2.5 Statistical Analysis
All statistical analyses were performed using the R software (version 4.2.2, Foundation for Statistical Computing, Vienna, Austria). The Shapiro–Wilk test was used to assess the distribution of each continuous variable for normality. Normally distributed continuous variables are expressed as the mean ± SD, non-normally distributed continuous variables are expressed as the median (interquartile range [IQR]), and categorical variables as counts. The Tukey test was used to compare continuous values among groups for normally distributed data, whereas the Steel-Dwass test was used for non-normally distributed data. In addition, Fisher's exact test was used for categorical variables, with the Holm-Bonferroni correction applied to account for multiple testing.
The study outcome was measured as the number of days from MVR to the occurrence of the event. Dogs that were alive 1400 days after surgery or lost to follow-up were treated as censored observations. The Kaplan–Meier method and landmark analysis were utilized [23], dividing the analysis into two time points based on a cutoff of 100 days post-surgery: the early postoperative period (< 100 days) and the late postoperative period (100–1400 days). This cutoff was chosen to include the period during which the postoperative clinical course was empirically considered stable, marking the transition out of the perioperative phase. Based on prior research, we anticipated that more postoperative events would occur during the early postoperative period than during the later phase [5, 13]. Given the likely differences in basic characteristics associated with events across these intervals, incidence rate calculations and a piecewise Cox regression model were applied to each time frame.
The incidence rates during the early and late postoperative periods were calculated as the number of new events (incident cases) divided by the total dog-months at risk (i.e., the sum of observation time across all population members during the specified time period). These rates represent the probability of experiencing an event during each specified interval and are expressed as the number of events per 1000 dogs-month. Ninety-five percent confidence intervals (CI) for the incidence rates were estimated using a Poisson approximation [24].
For the piecewise Cox regression model, the proportional hazards assumption was first assessed separately for each time-defined model using the Grambsch–Therneau method with Schoenfeld residuals [25]. After confirming that the assumption was not violated in each segment, the overall hazard ratios (HR) and 95% CI were estimated [26]. The Wald test was used to evaluate the significance of the variables of interest. The variables included in the Cox proportional hazards regression models comprised baseline characteristics on the day of surgery, including ACVIM stage, age, body weight, heart rate, breed, surgical year, and preoperative echocardiographic measurements (LVIDDN, LA:Ao, fractional shortening [FS], E wave, and TRV). These factors were selected based on their clinical relevance. The generalized variance inflation factor was used to assess the degree of collinearity, with a threshold of 10 indicating the presence of multicollinearity [27]. Missing data was addressed using the multiple imputation by chained equations method in the ‘mice’ package in R, which employed predictive mean matching to generate five imputed datasets. This approach was applied only to the Cox proportional hazards regression models, whereas other analyses used data before imputation. The threshold for statistical significance was set at p < 0.05 for all comparative analyses, including continuous variable comparisons, categorical variable analyses, and outcomes.
3 Results
A total of 1019 of 1033 potentially eligible cases were included, after excluding 14 cases owing to the absence of contact information after discharge in 13 cases (overseas cases) and one dog that had already undergone MVR before inclusion. In the early postoperative period, 61 dogs experienced the composite outcome (57 dogs of all-cause mortality, 4 dogs of postoperative congestive heart failure, and no dogs of second MVR), whereas in the late postoperative period, 211 dogs experienced the outcome (167 dogs of all-cause mortality, 44 dogs of postoperative congestive heart failure, and no dogs of second MVR). The breeds included were Chihuahua (n = 473), mixed breed (n = 113), Toy/Miniature Poodle (n = 91), Cavalier King Charles Spaniel (n = 66), Maltese (n = 62), Pomeranian (n = 47), Shih Tzu (n = 35), Norfolk Terrier (n = 24), Miniature Schnauzer (n = 20), Miniature Dachshund (n = 16), Yorkshire Terrier (n = 15), Papillon (n = 13), Boston Terrier (n = 8), Beagle (n = 4), Jack Russell Terrier (n = 4), Miniature Pinscher (n = 4), Shetland Sheepdog (n = 4), American Cocker Spaniel (n = 2), Bichon Frise (n = 2), Japanese Chin (n = 2), Japanese Spitz (n = 2), Havanese (n = 2), and one each of American Eskimo, Bolognese, Border Collie, Chinese Crested, Italian Greyhound, Lhasa Apso, Pekingese, Shiba, Toy Manchester Terrier, and Whippet.
The baseline characteristics of the study population were as follows: median age, 10.5 years (IQR, 9.2–11.8); median body weight, 3.7 kg (IQR, 2.8–5.2); and sex distribution, 547 males and 472 females. Atrial fibrillation was observed in 14 dogs; the prevalence of atrial fibrillation was higher in stage D. The median age of dogs in stages C and D was higher than that of those in stage B2. Regarding body condition score, the underweight condition was more prevalent in stage D. Heart rate and heart murmur intensity increased progressively from stage B2 to stage D, whereas systolic, diastolic, and mean blood pressures were lower in stages C and D than in stage B2. In addition, laboratory variables indicated an increase in blood urea nitrogen and creatinine concentrations and a decrease in sodium, potassium, and chloride concentrations, particularly in stages C and D, compared with stage B2. Medication usage also varied across stages, with loop diuretics, spironolactone, and vasodilators being used more frequently in advanced stages; pimobendan was used almost universally across all ACVIM stages (Table 1). The surgical survival prevalence at each ACVIM stage was 318/320 dogs for stage B2, 560/562 for stage C, and 136/137 for stage D (p = 0.78). Furthermore, the prevalence of discharge at each ACVIM stage was 305/320 dogs for stage B2, 538/562 for stage C, and 125/137 for stage D (p = 0.09). The year-to-year basic characteristics and intraoperative findings are shown in Table S1.
| Variables | ACVIM stage | ||
|---|---|---|---|
| B2 | C | D | |
| Number | 320 | 562 | 137 |
| Age (years) | 10.2 [8.8–11.8] | 10.6 [9.3–11.8]a | 11.0 [9.8–12.1]a |
| Sex (female/male) (n) | 135/185 | 276/286 | 61/76 |
| Body weight (kg) | 3.7 [2.9–5.1] | 3.6 [2.8–5.1] | 3.9 [2.9–5.5] |
| Body condition score (underweight [1–3]/normal [4, 5]/overweight [6–9]) (n) | 9/249/29 | 35/429/47 | 17/88/11 |
| Heart rate (beats/min) | 144 [120–160] | 146 [120–165] | 154 [132–179]a, b |
| Heart murmur intensity (mild [grade 1, 2]/moderate [grade 3, 4]/severe [grade 5, 6]) (n) | 5/120/177 | 2/203/332 | 1/32/92 |
| Blood pressure (mmHg) | |||
| Systolic | 132 [121–141] | 127 [117–138]a | 119 [109–132]a |
| Diastolic | 76 [66–85] | 72 [63–81]a | 69 [60–80]a |
| Mean | 99 [91–106] | 95 [86–105]a | 90 [82–100]a, b |
| Atrial fibrillation (n) | 0 | 1 | 13a, b |
| Laboratory variables | |||
| Hematocrit (%) | 47.2 [43.6–51.0] | 47.1 [43.7–50.7] | 47.4 [44.1–51.7] |
| Total protein (g/dL) | 6.5 [6.1–6.9] | 6.5 [6.1–6.8] | 6.3 [6.0–6.8] |
| Blood urea nitrogen (mg/dL) | 21.6 [17.3–28.0] | 27.4 [20.9–37.5]a | 41.4 [28.9–64.1]a, b |
| Creatinine (mg/dL) | 0.70 [0.60–0.90] | 0.80 [0.67–1.04]a | 1.02 [0.80–1.49]a, b |
| Sodium (mEq/L) | 149 [147–150] | 148 [145–149]a | 147 [144–149]a |
| Potassium (mEq/L) | 4.1 [3.8–4.3] | 3.7 [3.4–4.0]a | 3.5 [3.2–3.8]a, b |
| Chloride (mEq/L) | 111 [108–114] | 108 [104–111]a | 106 [100–110]a, b |
| Medications | |||
| Pimobendan (No/Yes) (n) | 2/308 | 0/554 | 0/136 |
| Loop diuretics (No/Yes) (n) | 162/148 | 40/514a | 0/136a, b |
| Spironolactone (No/Yes) (n) | 268/42 | 410/144a | 88/48a, b |
| RAAS inhibitors (No/Yes) (n) | 102/208 | 117/437a | 37/99 |
| Vasodilators (No/Yes) (n) | 221/89 | 380/174 | 75/61a, b |
- Note: Continuous variables are presented as medians [interquartile range], and categorical variables as counts. Loop diuretics include furosemide and torsemide. RAAS (renin-angiotensin-aldosterone system) inhibitors include angiotensin-converting-enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and angiotensin receptor/neprilysin inhibitors (ARNIs). Vasodilators comprise amlodipine, hydralazine, and isosorbide dinitrate.
- a p < 0.05 compared with stage B2.
- b p < 0.05 compared with stage C.
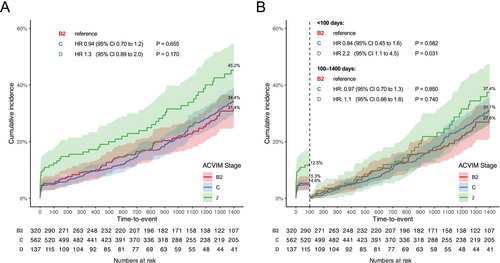
The incidence rate during the early postoperative period among all dogs at risk was 19.6 events per 1000 dogs-month and was particularly high in stage D dogs (43.0 events per 1000 dogs-month). In contrast, the incidence rate during the late postoperative period among all dogs at risk was 7.1 events per 1000 dogs-month, with 8.7 events per 1000 dogs-month in stage D dogs (Table 2). The suspected causes of death within the early postoperative period were primarily cardiac-related, respiratory disorders, bleeding, and neurologic disease. Conversely, in the late postoperative period, the most common suspected causes of death included kidney disease, neoplasia, and neurologic disease (Table S2).
| Group | Dogs at risk (n) | Events (n) | Dog-months | Incidence ratea (per 1000 dogs-month) (95% CI) |
|---|---|---|---|---|
| Early postoperative period | ||||
| All dogs | 1019 | 61 | 3114 | 19.6 (15.0–25.2) |
| ACVIM stage | ||||
| B2 | 320 | 17 | 979 | 17.4 (10.1–27.8) |
| C | 562 | 27 | 1740 | 15.5 (10.2–22.6) |
| D | 137 | 17 | 395 | 43.0 (25.1–68.9) |
| Late postoperative period | ||||
| All dogs | 923 | 211 | 29 925 | 7.1 (6.1–8.1) |
| ACVIM stage | ||||
| B2 | 289 | 61 | 9494 | 6.4 (4.9–8.3) |
| C | 519 | 119 | 16 888 | 7.0 (5.8–8.4) |
| D | 115 | 31 | 3543 | 8.7 (5.9–12.4) |
- a Incidence rate refers to the incidence density of the postoperative events (all-cause mortality and onset of congestive heart failure; no dogs underwent a second mitral valve repair) occurring during the specified time interval and is expressed as the number of events per 1000 dogs-month.
The proportional hazards assumption was visually confirmed to hold using Schoenfeld residuals for each multivariable Cox regression model (Figure S1). Postoperative events within the early postoperative period were more common in stage D, but no differences were observed among the ACVIM stages in the late postoperative period (Figure 1). In the early postoperative period, the Cox proportional hazards regression model indicated that stage D dogs had 2.2 (95% CI, 1.1–4.5) times the daily hazard of early postoperative events when compared with stage B2 dogs, and surgeries performed in the most recent year (2020) had 0.29 (95% CI, 0.12–0.70) times the daily hazard compared to surgeries conducted in 2017. In the late postoperative period, increasing age (per year: HR, 1.3; 95% CI, 1.2–1.4), higher body weight (per kilogram: HR, 1.1; 95% CI, 1.0–1.2), Cavalier King Charles Spaniel compared to Chihuahua (HR, 2.2; 95% CI, 1.2–4.1), and TRV > 3.7 m/s (HR, 2.5; 95% CI, 1.2–5.0) were associated with late postoperative events (HRs are summarized in Table 3; full statistical details are available in Table S3).
| Variables | Early postoperative period (events/dogs = 61/1019) | Late postoperative period (events/dogs = 211/923) | ||||
|---|---|---|---|---|---|---|
| HRa | 95% CI | p | HRa | 95% CI | p | |
| ACVIM stage | ||||||
| C vs. B2 | 0.84 | 0.45−1.6 | 0.58 | 0.97 | 0.70–1.3 | 0.85 |
| D vs. B2 | 2.2 | 1.1−4.5 | 0.03 | 1.1 | 0.66–1.8 | 0.74 |
| Age (years) | 1.1 | 0.98−1.3 | 0.09 | 1.3 | 1.2–1.4 | < 0.01 |
| Body weight (kg) | 1.1 | 0.95−1.2 | 0.35 | 1.1 | 1.0 to 1.2 | 0.02 |
| Heart rate (per 10 beats/min) | 1.1 | 0.95−1.2 | 0.26 | 1.0 | 0.97 to 1.1 | 0.47 |
| Breed | ||||||
| CKCS vs. Chihuahuas | 0.58 | 0.18−1.8 | 0.35 | 2.2 | 1.2 to 4.1 | 0.01 |
| Others vs. Chihuahuas | 0.57 | 0.31−1.1 | 0.08 | 1.3 | 0.93–1.8 | 0.12 |
| Years of surgery | ||||||
| 2018 vs. 2017 | 0.89 | 0.44−1.8 | 0.75 | 0.82 | 0.52–1.3 | 0.37 |
| 2019 vs. 2017 | 0.59 | 0.28−1.2 | 0.16 | 0.74 | 0.48–1.2 | 0.18 |
| 2020 vs. 2017 | 0.29 | 0.12−0.70 | 0.01 | 0.93 | 0.61–1.4 | 0.75 |
| Preoperative echocardiographic measurements | ||||||
| LVIDDN (per 0.1 unit) | — | — | — | 0.95 | 0.89 to 1.0 | 0.09 |
| LA:Ao (per 0.1 unit) | — | — | — | 1.0 | 0.96 to 1.0 | 0.91 |
| FS (%) | — | — | — | 1.0 | 0.99–1.0 | 0.29 |
| E wave (per 0.1 m/s) | — | — | — | 1.0 | 0.97–1.1 | 0.35 |
| TRV > 3.7 m/s | — | — | — | 2.5 | 1.2–5.0 | 0.01 |
- Note: Full statistical details are available in Table S3.
- Abbreviations: ACVIM, the American College of Veterinary Internal Medicine; CI, confidence interval; CKCS, Cavalier King Charles Spaniel; E, peak velocity of early diastolic transmitral flow; FS, fractional shortening; LA:Ao, left atrial to aortic ratio; LVIDDN, left ventricular end-diastolic internal diameter normalized to body weight; TRV, tricuspid regurgitant velocity.
- a Hazard ratios (HR) represent the relative daily hazard of postoperative events (all-cause mortality and onset of congestive heart failure; no dogs underwent a second mitral valve repair) compared to the reference category, adjusted for baseline variables. For example, within the first 100 days after surgery, dogs classified as ACVIM stage D had a 2.2-fold higher daily hazard compared to those in stage B2.
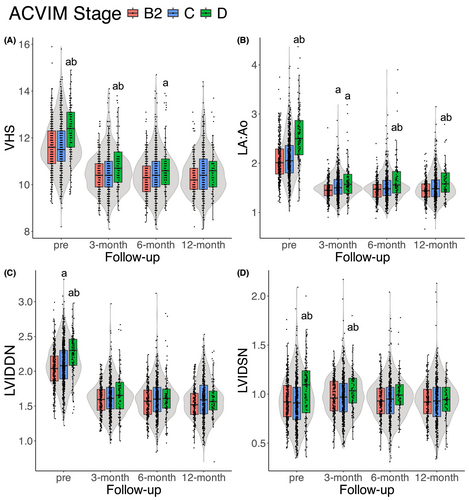
Regarding the preoperative and postoperative radiographic and echocardiographic measurements, dogs in stage D exhibited higher values for each preoperative variable than those in other ACVIM stages. Postoperatively, VHS, LA:Ao ratio, and LVIDSN were higher in stage D than in the other ACVIM stages (Figure 2). In addition, stage D dogs were more likely to receive postoperative medications (Table S4).
4 Discussion
Previous reports on the medical treatment of MMVD with congestive heart failure have reported a median time-to-event of less than 1 year [2, 28, 29]. In comparison, our outcomes after MVR suggest a substantial effect on this disease, particularly in stages C and D. Overall, our results reinforce the validity of MVR as a treatment option for MMVD. However, it remains unclear whether the perioperative events associated with surgical intervention outweigh the clinical outcome of medical treatment in stage B2. The EPIC study previously reported that the median time to event for dogs with MMVD and without congestive heart failure treated with pimobendan was 1228 days [30]. Consequently, future studies are required to compare the efficacy of medical treatment with that of surgical intervention in stage B2 and should involve more extended survival tracking.
In addition, the definition of advanced stage B2, which is identified as a potential candidate for surgery according to the consensus guidelines [1], remains ambiguous. At our institution, we consider stage B2 dogs eligible for surgery based on gradually progressive cardiac enlargement, persistently high E wave, presence of atrial fibrillation, or atrial rupture, but we have no specific cut-off values of echocardiographic variables to define advanced stage B2. Many dogs in our study were prescribed preoperative diuretics despite being classified as stage B2, suggesting that their disease was considerably advanced. Diuretics were given to stage B2 dogs in our study as a short-term management approach to prevent pulmonary edema until the surgical date. Notably, the administration of diuretics is currently not a standard treatment protocol, and the characteristics of the stage B2 dogs in our study may not exactly represent a typical group of dogs receiving medical treatment. To determine the optimal timing for surgery more precisely, specific criteria are needed to distinguish high-risk cases among stage B2 dogs.
Regarding the baseline characteristics in our study, disease progression was found to be associated with a decrease in body condition scores. This phenomenon may be a result of cachexia, which is linked to a variety of metabolic alterations including inflammation, oxidative stress, and energy metabolism [31-33]. Similar to the findings of a previous study [34], we observed a decrease in systolic, diastolic, and mean blood pressures in progressive cases. These findings may be secondary to disease-related left ventricular function impairment or the use of vasodilators, which are both likely to decrease cardiac afterload [1, 35]. Laboratory variables showed significant increases in blood urea nitrogen and creatinine concentrations along with decreases in sodium, potassium, and chloride concentrations in stages C and D. These changes are related to the use of diuretics and decreased renal perfusion owing to decreased cardiac output [36, 37], which can cause electrolyte imbalances in dogs with advanced MMVD [38].
A Cox proportional hazards regression model further determined that older age was associated with mortality during the late postoperative period. The major causes of death in our study were kidney disease, neoplasia, and neurologic disease, which have been commonly linked to aging [39, 40]. In contrast, respiratory disorders and neurologic conditions were the major causes of death in the early postoperative period. Neurologic problems are most likely caused by intraoperative cerebral hypoperfusion or postoperative cerebral thrombosis [5, 12, 41, 42]. Respiratory disorders may be associated with diaphragmatic paralysis and CPB-related lung injury [43, 44]. In addition, preoperative pulmonary conditions may be related to postoperative respiratory distress. In human medicine, preoperative heart conditions and arterial oxygenation have been identified as risk factors for pulmonary complications after cardiac surgery [45]. Additionally, our study showed that blood urea nitrogen and creatinine concentrations tended to increase as the disease progressed, and a previous report suggested that azotemia may cause pulmonary abnormalities in dogs [46]. In view of these findings, the progressive exacerbation of pulmonary conditions with MMVD may lead to respiratory distress in the postoperative period.
In our study, dogs classified as stage D tended to have larger heart sizes both before and after surgery. Clinical indices such as left ventricular diameter and ejection fraction may reflect histological changes associated with MMVD, including fatty replacement and myocardial vacuolization [47]. Moreover, myocardial fibrosis may be linked to cardiac remodeling [48]. Such irreversible structural and functional changes in the ventricle may have affected postoperative cardiac reverse remodeling and residual mitral regurgitant volume [35]. This hypothesis may help explain why heart sizes remained larger in stage D dogs compared with other stages, even after surgery. Furthermore, one report on dogs undergoing MVR noted a larger decrease in the regurgitant jet area-to-left atrial area ratio at 1 month postoperatively in stage C than in stage D [6], highlighting the potential difficulty of achieving effective repair in more severe cases. To explore this issue further, a controlled, prospective study focusing on detailed echocardiographic assessments is warranted.
In our study, Cavalier King Charles Spaniels showed a higher HR in the late postoperative period than did Chihuahuas. Interestingly, previous research has indicated that the morphology of the mitral valve annulus in Cavalier King Charles Spaniels can differ from that of other breeds [49, 50], with this breed tending to have shorter and thicker mitral valve leaflets [51]. These differences may affect the surgical outcomes of regurgitant reduction. In general, the life expectancies of Chihuahuas and Cavalier King Charles Spaniels in Japan are 11.8 and 13.1 years, respectively [52]. However, consistent with our findings that higher body weight is associated with late-term events, previous reports have stated that larger dog breeds tend to have shorter lifespans [53, 54]. Multifaceted investigations considering genetic, pathological, and pathophysiological perspectives are required to clarify the differences in surgical outcomes among breeds.
Pulmonary hypertension commonly occurs in severe cases of MMVD and is associated with increased mortality during medical management [20]. Our study showed that a TRV > 3.7 m/s was associated with an increased incidence of postoperative events, whereas a previous report suggested that pulmonary hypertension caused by MMVD can be improved by MVR [55]. Another report on MVR in dogs with pulmonary hypertension suggested that some dogs experienced residual or late recurrence of pulmonary hypertension after MVR [56]. This phenomenon may be attributed to pulmonary arterial and venous remodeling, which can lead to irreversible pulmonary hypertension [57, 58]. This condition also may have been exacerbated by cardiovascular surgery with CPB, which involve pulmonary vasomotor dysfunction, cyclooxygenase, the thromboxane A2 and prostacyclin pathway, the nitric oxide pathway, inflammation, and oxidative stress [59, 60]. In future studies, strict preoperative diagnosis of pulmonary hypertension in accordance with ACVIM consensus guidelines [61], along with comprehensive evaluation of perioperative factors (including intraoperative and postoperative contributors) is warranted to better understand their impact on surgical prognosis. Furthermore, right heart catheterization and pulmonary histopathological assessment would be ideal for elucidating the relationship between pulmonary hypertension and surgical outcomes.
The proficiency of the surgical team also appears to be important, because the HRs of early postoperative events decreased annually from 2017 to 2020. We believe that the accumulated experience of the surgeons, surgical assistants, perfusionists, anesthesiologists, and intensive care staff contributed to the outcomes. In our study, a single primary surgeon was responsible for all procedures, thereby ensuring consistency in surgical technique. By contrast, in studies involving multiple independent surgeons, the identity of the operating surgeon should be considered a potential confounding factor, as noted in MVR in humans [62, 63]. Additionally, given that surgical outcomes are likely influenced by the overall expertise of the surgical team rather than the individual surgeon alone [64], future multicenter studies also should account for variation in surgical case volume and institutional protocols.
During the study period, cardiac medications (e.g., pimobendan, loop diuretics, renin-angiotensin-aldosterone system [RAAS] inhibitors, vasodilators) were prescribed at the discretion of each attending clinician, and their potential effects on long-term outcomes remain unknown. At our institution, pimobendan was commonly used as a first-line agent for postoperative systolic dysfunction and often was continued as long-term supportive treatment. Loop diuretics generally were avoided because of their potential long-term adverse effects on the RAAS system [65], but are reserved for dogs with severely enlarged cardiac chambers, residual severe mitral regurgitation, secondary mitral stenosis, or postoperative congestive heart failure. Indeed, dogs classified as stage D in our study were more likely to receive these medications postoperatively. Other medications typically were prescribed in more stable or milder cases. Treatment decisions were based on a combination of factors, including preoperative ACVIM stage, postoperative blood chemistry results, blood pressure, clinical signs, echocardiographic findings, and their changes over time. To better understand the impact of postoperative medical treatment on time-to-event outcomes, future prospective studies that incorporate detailed echocardiographic variables and medical treatments (including their potential interactions) as time-dependent covariates are warranted.
Our study had some limitations. Firstly, owing to the retrospective study design, attending clinicians were not controlled for, and the focus of treatment was primarily on general variables. Although no predefined contraindications for surgery were established, dogs with severe pulmonary hypertension with or without right-sided congestive heart failure were carefully considered as surgical candidates. Additionally, dogs with serious systemic diseases (e.g., severe gastrointestinal signs, pancreatitis, neoplasia, acute kidney injury) were less likely to be deemed eligible for surgery or faced delays in undergoing the procedure. These scenarios may influence interpretation of the results. The suspected causes of death were estimated from medical records and telephone interviews and were not confirmed by necropsy, which may have led to misclassification. Approximately half of the dogs in our study were Chihuahuas, which differs from a previous report from England [8], and thus the results should be interpreted with caution. The differences in VHS and LVIDSN among stages tended to decrease and were not significant at six and twelve months after surgery, possibly because of survival bias from early mortality in stage D dogs or decreased statistical power because of the smaller sample size. Finally, stage B2 dogs in our study were diagnosed with advanced stage by attending clinicians to incorporate MVR and were prescribed diuretics before surgery, meaning these dogs may not exactly represent the characteristics of a typical stage B2 group with respect to medical treatment.
5 Conclusions
In conclusion, MVR appears to offer a favorable prognosis in dogs with advanced-stage MMVD. The incidence rate of early postoperative mortality was higher than that in the later period. Although dogs in stage D exhibited a relatively higher incidence of early postoperative events, no significant difference was found between stages B2 and C. In the late postoperative period, the HRs did not differ among clinical stages but were associated with age, body weight, breed, and TRV.
Acknowledgments
We thank Editage (www.editage.com) for English language editing. We further extend our profound gratitude to all the medical staff members at the JASMINE Veterinary Cardiovascular Medical Center for their invaluable contributions and unwavering support. This study did not receive any specific grants from public, commercial, or non-profit funding agencies. This subject was presented as an abstract at the 32nd European College of Veterinary Internal Medicine—Companion Animals (ECVIM-CA) Congress, September 2022.
Disclosure
Authors declare no off-label use of antimicrobials.
Ethics Statement
Approved by the Institutional Ethics Committee of JASMINE Veterinary Cardiovascular Medical Center (approval number: 230315–02). Authors declare human ethics approval was not needed.
Conflicts of Interest
The authors declare no conflicts of interest.




