Exploring the Importance of Repeated Health Screening in Healthy Older Dogs
Funding: The authors received no specific funding for this work.
ABSTRACT
Background
The clinical relevance of repeated health screenings in aging dogs remains unclear. Moreover, which physical or laboratory variables are associated with survival have not been thoroughly studied.
Objectives
Evaluate the health status of apparently healthy older dogs and determine which diseases are manifested in confirmed healthy dogs within 2 years, based on extensive health screening. Assess the predictive value for mortality of various clinicopathological variables at baseline in confirmed healthy older dogs.
Animals
A total of 122 apparently healthy senior and geriatric dogs.
Methods
Prospective, longitudinal study. History, physical examination, blood testing, and urinalysis were performed at baseline and every 12 months for 2 years.
Results
At baseline, 20% of apparently healthy older dogs were diagnosed with ≥ 1 disease, resulting in 98 confirmed healthy dogs included in the follow-up study. The most common emerging disorders within 2 years were neoplasia (cumulative incidence, 12%), azotemic chronic kidney disease (CKD, 8%), neurologic disease (11%), or orthopedic disorders (5%). Malignant neoplasia could be detected in 47% (8/17) of dogs on physical examination, including rectal, skin, and mammary gland palpation. Only age group was associated with survival in confirmed healthy dogs at baseline, with geriatric dogs being more likely to die compared with senior dogs.
Conclusions and Clinical Importance
Routine health screening helps to detect unrecognized diseases in older dogs. Repeated screening for CKD, rectal palpation, and assessment of neurologic and orthopedic health seem most relevant.
Abbreviations
-
- ALKP
-
- alkaline phosphatase
-
- ALT
-
- alanine transaminase
-
- BCS
-
- WSAVA body condition score
-
- CKD
-
- chronic kidney disease
-
- KCS
-
- keratoconjunctivitis sicca
-
- MCS
-
- WSAVA muscle condition score
-
- QOL
-
- quality of life
-
- RI
-
- reference interval
-
- SBP
-
- systolic blood pressure
-
- SD
-
- standard deviation
-
- SDMA
-
- symmetric dimethyl arginine
-
- STT
-
- Shimer tear testing
-
- T
-
- time point
-
- UPC
-
- urinary protein: creatinine ratio
-
- USG
-
- urine specific gravity
-
- WSAVA
-
- World Small Animal Veterinary Association
1 Introduction
Routine healthcare visits are considered vital for the well-being of aging dogs, with annual or biannual health screening advised [1, 2]. Senior animals have specific needs and are more likely to develop chronic illness [3]. Initial clinical signs of chronic conditions can be vague and difficult to recognize for the owner or disregarded as “normal for the animal's age” [3-5]. Healthy aging is defined as a low probability of disease or disease-related disability, both in humans and companion dogs [1, 6-8]. Although results of health screening studies in dogs are available in the literature [5, 9-12], to date no studies have been published about the incidence of abnormalities in older dogs when followed longitudinally. Such data could clarify the value of repeated health screening [13, 14]. Serial data also could help in distinguishing age-related, clinically unimportant laboratory changes from clinically relevant alterations [15-18]. More scientific evidence could guide clinical decision-making to allow timely intervention when indicated but also to avoid unnecessary procedures [18]. For example, hematocrit, albumin concentration and alanine transaminase (ALT) activity are significantly lower in older people [15, 16, 19]. Decreases are associated with increased mortality and linked with frailty, and therefore considered not physiological aging effects [15, 19]. A decreasing hematocrit and serum albumin concentration also have been described in aging clinically healthy dogs, but a potential link with survival has not yet been studied [5, 20]. In contrast to people, significantly higher activities of liver enzymes, including ALT, are present in aged compared to adult dogs [20]. Periodontal disease is associated with multiple systemic conditions in dogs, such as cardiac, renal and hepatic disease, but effect on longevity in older dogs has not been studied [21, 22].
Our first objective was to describe the prevalence of conditions detected during extensive health screening in a group of apparently healthy, older dogs. A second aim was to assess which diseases older dogs, confirmed healthy at baseline, develop within 2 years by performing repeated health screening. Thirdly, we evaluated the predictive value on mortality for baseline values of age group, body weight, body and muscle condition score (MCS), dental score, hematocrit, albumin concentration, and ALT and alkaline phosphatase (ALKP) activities in healthy confirmed older dogs.
2 Material and Methods
2.1 Study Cohort
The study was completed between July 2019 and June 2023. All dogs were privately owned. Owners signed an informed consent form, and the study was approved by the local ethical committee (EC2019/39).
Apparently healthy senior or geriatric dogs, as defined based on a previously published human/pet analogy chart [3] were prospectively recruited as previously described [23]. Weight categories were adapted slightly to avoid exclusion of certain weights (≤ 9.1 kg: ≥ 8 years; > 9.1–≤ 22.7 kg: ≥ 7 years; > 22.7–≤ 54.5 kg: ≥ 6 years; > 54.5 kg: ≥ 4 years). Confirmed healthy dogs were included in the follow-up study if baseline examinations did not indicate metabolic or systemic disease (e.g., malignant neoplasia, azotemic chronic kidney disease [CKD], diabetes mellitus, urolithiasis or clinically relevant cardiovascular disease [cardiac disease ≥ stage B2 or hemodynamically relevant arrhythmia]). Chronic kidney disease was diagnosed if serum creatinine concentration was ≥ 1.8 mg/dL (159 μmol/L) or symmetric dimethyl arginine (SDMA) was > 18 μg/dL in combination with urine specific gravity (USG) ≤ 1.030. International Interest Renal Society (IRIS) CKD stage 1 was diagnosed if persistent urinary protein: creatinine ratio [UPC] ≥ 0.5 or persistent USG ≤ 1.020 in the absence of azotemia was present. All dogs were fasted overnight and underwent complete health screening at 12-month intervals [5]. During the 2-year follow-up, medical or surgical treatment was allowed.
2.2 Health Screening Protocol
The study protocol was based on a previously published health study [5]. In brief, at each visit a history questionnaire [5] was completed and a standard physical examination was performed including assessment of body condition score (BCS) and MCS according to World Small Animal Veterinary Association (WSAVA) guidelines [24, 25] and rectal examination [5]. For dental disease, a categorization system previously described in cats was applied to assign each dog a calculus score of 0–3 and a gingivitis score of 0–3. The combination of both scores resulted in a dental disease score of 0–6, implying no (score 0), mild (score 1–2), moderate (score 3–4) or severe (score 5–6) dental disease [26]. Standard orthopedic, neurologic, and brief ophthalmic examinations were performed [5]. Systolic blood pressure (SBP) measurement (Doppler ultrasonography according to the American College of Veterinary Internal Medicine [ACVIM] consensus statement) [27] and direct fundoscopy were performed, and results are described in detail elsewhere [23, 28]. Fine needle aspiration and cytology of cutaneous or SC masses were performed. At each time point, blood was collected from the jugular vein (21 G needle) and urine was taken by ultrasonographic-guided cystocentesis (22 G needle) or voiding. Complete blood count and serum biochemistry profile, including SDMA and electrolytes (Procyte One Hematology, IDEXX SDMA ELISA and Catalyst Dx Chemistry Analyzer, IDEXX laboratories Inc., United States) were performed on each blood sample. Urinalysis consisted of measurement of USG using a manual refractometer (master refractometer, ATAGO CO. LTD), UPC (protein: colorimetric reaction with pyrogallol red molybdate, Beckman Coulter; creatinine: modified Jaffe assay, Beckman Coulter), urinary dipstick (iChem velocity stick, Beckman Coulter) and sediment analysis. Urinary sediment, prepared by centrifuging 5–10 mL of urine at 447 g for 3 min at 2°C (Jouan B4i, Thermo Scientific) was considered active in the presence of bacteriuria or > 3 casts or > 5 red blood cells, white blood cells, or epithelial cells per high power field (40× objective), respectively [29]. Aerobic bacterial culture was performed at baseline only.
Diagnosed diseases were grouped into specific categories (e.g., malignant neoplasia, neurologic disorder, gastrointestinal disease, orthopedic disorder, dermatologic disorder, urogenital disorder, CKD, cardiac disorder, hematologic disorder). Neurologic disorder was defined as neurologic abnormalities beyond hearing or vision deficiency or both, decreased or absent patellar reflexes, or previously known abnormalities (e.g., facial nerve paresis or paralysis). Dogs with unexplained increased liver enzyme activities (ALKP > 600 U/L or ALT > 300 U/L) or confirmed pancreatitis or cholecystitis, or cholelithiasis also were grouped under gastrointestinal disease. Renal disease was defined as the presence of azotemic CKD, persistent renal proteinuria (UPC ≥ 0.5) or USG ≤ 1.020 based on ≥ 2 urine samples. Dogs with orthopedic problems necessitating surgery or medical treatment beyond nutraceuticals were grouped under orthopedic disorder. Time and reason of death during or after the study period were noted. All owners of dogs alive at the end of the study period were contacted in March 2024 for follow-up.
2.3 Statistical Analysis
Statistical analysis was performed using the statistical software package R (R module of SAS version 9.3, SAS Institute, NC, United States). Baseline comparison between the senior and geriatric age groups was performed, based on the Welch two-sample t-test, for body weight, dental score, presence of systolic murmur, BCS, MCS, SBP, Schirmer tear testing results, USG, UPC, CBC variables, biochemistry variables, SDMA, and electrolytes. The predictive value of the following variables at baseline to onset of death was assessed for the confirmed healthy older dogs at baseline, using the Cox proportional hazards model to manage right censoring: age group, body weight, body and MCS, dental score, hematocrit, albumin, ALT, and ALKP. The level of significance was set at 5%.
3 Results
3.1 Study Cohort
One-hundred twenty-two apparently healthy older dogs were included at baseline, with 67 senior and 55 geriatric dogs and 41, 33, 46, and 2 dogs in each weight category [3]. Fifty-one (42%) dogs were male (30 neutered) and 71 (58%) were female (43 spayed). The study cohort consisted of 14 mixed breed dogs, 10 Border Collies, 9 Belgian Shepherds, 8 Golden Retrievers, 6 Chihuahuas, 6 Labrador Retrievers, 6 Dachshunds, 5 Shetland Sheepdogs, 5 Jack Russell Terriers, 4 Cavalier King Charles Spaniels, 4 Shih Tzus, 4 English Cocker Spaniels, 4 Rottweilers, and ≤ 3 dogs of 28 other breeds. Follow-up data were available for 88 (time point T12) and 77 (T24) of the 98 dogs confirmed healthy at baseline (Table 1).
| RI | Baseline (T0) (n = 122) | Confirmed healthy T0 (n = 98) | T12 (n = 88) | T24 (n = 77) | |
|---|---|---|---|---|---|
| Age (years) | / |
9.7 (±2.2) 9.5 (5.1–15.5) |
9.7 (±2.1) 9.4 (6.1–15.4) |
10.7 (±2.1) 10.4 (7.1–16.6) |
11.3 (±2.0) 11.2 (7.7–16.9) |
| BW (kg) | / |
18.5 (±12.9) 16.5 (1.2–73.8) |
17.4 (±11.9) 16.3 (1.2–59) |
16.9 (±11.2) 14.9 (1.9–46.8) |
16.5 (±10.9) 14.0 (2.0–42.4) |
| BCS [25] | / | ||||
| Optimal (4–6/9) | 107/122 (87%) | 89/98 (91%) | 78/87 (88%) | 69/77 (90%) | |
| Below optimal (1–3/9) | 2/122 (2%) | 2/98 (2%) | 2/87 (2%) | 2/77 (2%) | |
| Above optimal (7–9/9) | 13/122 (11%) | 7/98 (7%) | 7/87 (8%) | 6/77 (8%) | |
| MCS [24] | / | ||||
| Normal (1/4) | 116/122 (10%) | 93/98 (95%) | 76/87 (87%) | 59/76 (77%) | |
| Decreased (2–4/4) | 6/122 (5%) | 5/98 (5%) | 11/87 (13%) | 17/76 (22%) | |
| Dental disease score [26] | / | ||||
| Absent (0/6) | 33/122 (27%) | 29/98 (29%) | 26/86 (30%) | 17/73 (23%) | |
| Mild (1–2/6) | 47/122 (39%) | 36/98 (37%) | 33/86 (38%) | 30/73 (41%) | |
| Moderate (3–4/6) | 28/122 (23%) | 22/98 (22%) | 23/86 (27%) | 20/73 (27%) | |
| Severe (5–6/6) | 14/122 (12%) | 11/98 (11%) | 4/86 (5%) | 6/73 (8%) | |
| Lymphadenopathy | / | ||||
| (n) 1 region | 17/122 (14%) | 15/98 (15%) | 19/88 (2%) | 24/76 (32%) | |
| (n) Multiple regions | 3/122 (2%) | 2/98 (2%) | 4/88 (5%) | 3/76 (4%) | |
| HR (bpm) [30] |
60–120 |
91.5 (±22.6) 81.0 (60.0–180.0) |
90.6 (±20.9) 80.0 (60.0–180.0) |
91.4 (±19) 84.0 (52.0–140.0) |
99.8 (±18.8) 100.0 (60.0–180.0) |
| (n) New systolic murmur present | 9/122 (7%) | 7/98 (7%) | 5/88 (6%) | 3/76 (4%) | |
| RR (/min) [30] | 10–30 |
31.2 (±11.1) 24.0 (16.0–60.0) |
30.2 (±10.3) 24.0 (20.0–60.0) |
32.5 (±13.6) 24.0 (20.0–60.0) |
31.6 (±12.6) 24.0 (24.0–60.0) |
| T (°C) [30] | 38–39.2 |
38.5 (±0.5) 38.6 (37.5–39.9) |
38.5 (±0.4) 38.6 (37.6–39.5) |
38.6 (±0.5) 38.6 (37.7–40.2) |
38.9 (±0.4) 38.8 (37.9–40.2) |
- Abbreviations: BCS, WSAVA body condition score (/9); BW, body weight; HR, heart rate; MCS, WSAVA muscle condition score (/4); n, number; RI, reference interval; RR, respiratory rate; T, temperature.
3.2 Health Screening: Findings in History and Physical Examination
Results of the completed questionnaires, at baseline and during follow-up, can be found in Table S1. The majority of owners reported good to excellent quality of life (QOL) for their dogs at baseline. However, > 25% of owners of confirmed healthy dogs reported decreased QOL by the end of the study, most commonly related to orthopedic (13/77, 17%) or neurologic (10/77, 13%) issues. In 10 confirmed healthy dogs, owners reported weight loss at baseline, but the majority (8/10) of these had an ideal BCS (4–5/9). Weight gain also was reported at baseline in 10 confirmed healthy dogs, of which 6 were overweight (BCS 6–7/9). The majority (80%, 85/107) of owners went to the veterinarian with their dog annually, with 10% (11/107) reporting a biannual visit. Vaccination in accordance with recent guidelines [31] and quarterly deworming was not up to date in 11% (12/110) and 24% (26/107) of dogs at baseline, respectively.
Findings on physical examination, at baseline and during follow-up, are presented in Tables 1 and 2. At baseline, only body weight was significantly (p < 0.05) higher in the geriatric compared to the senior age group. The majority of confirmed healthy dogs (90%) had an optimal BCS throughout the study, whereas a worse MCS was more commonly seen at the end (22%) of the study compared to T0 (5%). Overall, BCS and MCS were similar between apparently healthy and confirmed healthy dogs at baseline. None of the 13 of 122 (11%) dogs with obesity (BCS ≥ 7/9) at baseline showed signs of muscle wasting. Eight confirmed healthy dogs developed a new systolic murmur within 2 years of follow-up (grade 1–2, n = 6; grade 3–4, n = 2), and in all cases the murmur was asymptomatic. Echocardiography was performed in 2/8 dogs and identified myxomatous mitral valve disease ACVIM stage B1. None of the confirmed healthy dogs had an irregular heart rhythm at any time point, other than respiratory sinus arrhythmia. Throughout the study period, rectal examination identified 5 palpable anal sac masses (right-sided, n = 3; left-sided, n = 2). The majority (4/5) were surgically removed; 1 dog was euthanized because of concurrent hypercalcemia and renal azotemia. In 3/4 surgical cases, histology of the mass confirmed an anal sac adenocarcinoma; in the remaining dog, no histopathology was performed. Six female dogs had undergone partial or complete unilateral mastectomy before inclusion. In three intact female dogs where a mammary gland nodule was observed at baseline, histopathology identified a benign process. These dogs therefore still met the criteria for “confirmed healthy” and were included for follow-up. One of these dogs developed a new mammary gland nodule at a different location by T12. Subcutaneous masses were commonly observed at baseline and during follow-up. Of nodules on which cytology was performed, the majority (> 90%) were classified as lipomas or sebaceous gland cysts.
| RI | Baseline (T0) (n = 122) | Confirmed healthy T0 (n = 98) | T12 (n = 88) | T24 (n = 76) | |
|---|---|---|---|---|---|
| SBP (mmHg) [27] | < 160 | 148.9 (±22.9) | 148.6 (±23.2) | 153.9 (±29.4) | 145.5 (±31.2) |
| 148.0 (105.0–239.0) | 148.6 (105.0–239.0) | 153.4 (108.4–250.0) | 155.0 (104.0–214.0) | ||
| (n) ≥ 160 mmHg | 32/118 (27%) | 24/97 (25%) | 28/79 (35%) | 27/68 (40%) | |
| Orthopedic exam | |||||
| (n) normal | 78/122 (64%) | 58/98 (59%) | 62/88 (70%) | 40/76 (53%) | |
| (n) 1 abnormal joint | 32/122 (26%) | 30/98 (31%) | 18/88 (20%) | 25/76 (33%) | |
| (n) multiple abnormal joints | 12/122 (10%) | 10/98 (10%) | 8/88 (9%) | 11/76 (14%) | |
| Neurologic exam | |||||
| (n) normal | 84/122 (69%) | 71/98 (72%) | 61/88 (7%) | 47/76 (70%) | |
| (n) decreased response to auditory stimuli | 16/122 (13%) | 9/98 (9%) | 14/88 (16%) | 10/76 (13%) | |
| (n) decreased vision status | 16/122 (13%) | 8/98 (8%) | 12/88 (14%) | 13/76 (17%) | |
| (n) discomfort neck/back | 14/122 (12%) | 5/98 (5%) | 10/88 (11%) | 16/76 (21%) | |
| (n) decreased patellar reflexes | 13/122 (11%) | 11/98 (11%) | 6/88 (7%) | 5/76 (7%) | |
| Tense abdominal palpation | / | 25/122 (21%) | 18/98 (18%) | 21/88 (24%) | 28/76 (33%) |
| Palpable (sub)cutaneous masses | |||||
| 0 | 77/122 (63%) | 64/98 (65%) | 54/88 (61%) | 42/76 (55%) | |
| 1–2 | 29/122 (24%) | 23/98 (23%) | 24/88 (27%) | 22/76 (29%) | |
| 3–4 | 12/122 (10%) | 8/98 (8%) | 8/88 (9%) | 8/76 (11%) | |
| ≥ 5 | 4/122 (3%) | 3/98 (3%) | 2/88 (2%) | 4/76 (5%) | |
| Inspection of external genital tract | |||||
| Balanoposthitis | 19/51 (37%) | 14/41 (34%) | 6/35 (17%) | 4/32 (13%) | |
| New mammary gland nodule(s) | 4/122 (3%) | 3/98 (3%) | 3/88 (3%) | 1/76 (1%) |
| Rectal palpation | |||||
| Prostatomegaly | 8/51 (16%) | 5/41 (12%) | 2/35 (6%) | 2/32 (6%) | |
| New anal sac gland nodule | 3/109 (3%) | 0/89 (0%) | 0/72 (0%) | 2/66 (3%) | |
| Ophtalmological exam | |||||
| Lens opacification | |||||
| Mild | 43/122 (35%) | 33/89 (37%) | 34/88 (39%) | 42/76 (55%) | |
| Moderate | 4/122 (3%) | 4/89 (4%) | 3/88 (3%) | 2/76 (3%) | |
| Severe | 1/122 (1%) | 0/89 (0%) | 0/88 (0%) | 0/76 (0%) | |
| STT left eye [32] | ≥ 15.0 mm/min | 18.2 (±3.8) (n = 60) | 18.3 (±4.0) (n = 51) | 16.3 (±3.9) (n = 15) | / |
| 20.0 (9.0–32.0) | 20.0 (9.0–32.0) | 16.0 (10.0–24.0) | |||
| STT right eye [32] | ≥ 15.0 mm/min | 18.7 (±3.3) (n = 60) | 18.7 (±3.4) (n = 51) | 15.3 (±3.2) (n = 15) | / |
| 20.0 (12.0–27.0) | 20.0 (12.0–27.0) | 15.0 (11.0–20.0) | |||
| Ocular discharge | 13/122 (11%) | 10/97 (10%) | 11/89 (12%) | 12/76 (16%) | |
| Scleral abnormalities | 13/118 (11%) | 9/97 (9%) | 8/85 (9%) | 5/76 (7%) | |
| Conjunctival abnormalities | 6/122 (5%) | 4/97 (4%) | 3/89 (3%) | 8/75 (11%) |
- Abbreviations: n, number; RI, reference interval; SBP, systolic blood pressure; STT, Schirmer tear test.
Schirmer tear testing (STT) was performed in 50% of dogs at baseline. In 50 of 63 (79%) apparently healthy older dogs, STT was bilaterally normal, in 11 of 63 dogs (17%) unilaterally decreased (< 15 mm/min) and in 2 dogs bilaterally decreased, of which one was previously diagnosed with keratoconjunctivitis sicca (KCS). In three confirmed healthy dogs with normal STT at baseline, STT decreased to < 15 mm/min by T12. Lens opacification was the most commonly observed abnormality on ophthalmic examination, with an increasing frequency by T24. Of 52 confirmed healthy dogs without lens opacification at baseline, 24 (46%) showed mild opacification by T24.
3.3 Health Screening: Laboratory Findings
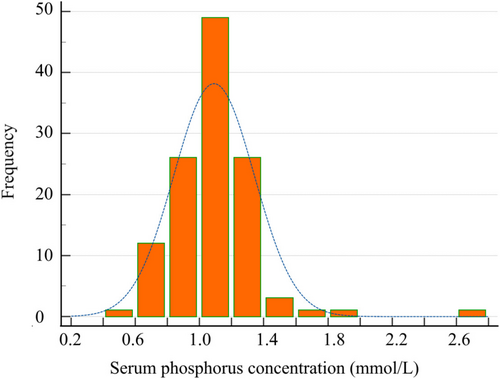
Results of routine blood tests and urinalysis at baseline and during follow-up are presented in Tables 3 and 4. The most common deviations on CBC in dogs at baseline were mild anemia (12%; of which 17% [2/12] showed signs of regeneration [reticulocyte count > 60 K/μL]), mild lymphocytosis (18%), mild leukopenia (8%) and mild neutropenia (6%); these frequencies remained stable over time (Table 3). Increased ALKP or ALT activity was observed in > 10% of dogs at baseline and during follow-up. In ≥ 60% of cases, these increases were mild (≤ 2 times the upper limit of the reference interval [RI]). Of the six dogs with moderate to severely (> 2 times the upper limit of the RI) increased liver enzyme activities, four underwent further evaluation; in two dogs, vacuolar hepatopathy was found, and in two dogs, abdominal ultrasonography did not identify an obvious cause. Deviations in electrolytes were rare and usually minor, with the exception of hypophosphatemia. At baseline, 11% of dogs had a decreased serum phosphorus concentration, with a lowest concentration of 0.42 mmol/L. Because of the high number of apparently healthy dogs with hypophosphatemia, an age-specific RI (0.72 [0.42–0.75]–1.74 [1.38–2.6] mmol/L) was calculated using a non-parametric percentile method according to the American Society of Veterinary Clinical Pathology (ASVCP) guidelines [33, 34]. A histogram is provided in Figure 1 to illustrate the distribution of serum phosphorus concentration in our study cohort.
| Variable | RI | Baseline (T0) (n = 122) | Confirmed healthy T0 (n = 98) | T12 (n = 88) | T24 (n = 76) |
|---|---|---|---|---|---|
| Leukocytes (×109/L) | 5.05–16.76 |
7.90 (±2.56) 7.40 (2.30–15.4) |
7.61 (±2.41) 7.18 (2.3–15.4) |
7.16 (±1.17) 7.30 (4.30–8.50) |
7.47 (±2.64) 6.80 (3.30–17.50) |
| (n) > RI | 0/121 (0%) | 0/97 (0%) | 2/87 (2%) | 1/76 (1%) | |
| (n) < RI | 10/121 (8%) | 8/97 (8%) | 8/87 (9%) | 5/76 (7%) | |
| Neutrophils (×109/L) | 2.95–11.64 |
5.20 (±1.93) 4.90 (1.30–13.10) |
5.03 (±1.87) 4.64 (1.34–13.10) |
5.14 (±0.77) 5.20 (4.00–6.30) |
4.89 (±2.16) 4.20 (2.10–13.10) |
| (n) > RI | 2/121 (2%) | 1/97 (1%) | 0/87 (0%) | 1/76 (1%) | |
| (n) < RI | 7/121 (6%) | 6/97 (6%) | 6/87 (7%) | 5/76 (7%) | |
| Monocytes (×109/L) | 0.16–1.12 |
0.50 (±0.23) 0.50 (0.20–1.40) |
0.51 (±0.21) 0.46 (0.16–1.36) |
0.77 (±0.38) 0.60 (0.40–1.50) |
0.42 (±0.21) 0.40 (0.20–1.10) |
| (n) > RI | 3/121 (2%) | 1/97 (1%) | 1/87 (1%) | 0/76 (0%) | |
| (n) < RI | 0/121 (0%) | 0/97 (0%) | 0/87 (0%) | 0/76 (0%) | |
| Lymphocytes (×109/L) | 1.05–5.10 |
1.60 (±0.58) 1.50 (0.30–3.90) |
1.62 (±0.59) 1.52 (0.53–3.86) |
1.49 (±0.58) 1.60 (0.50–2.20) |
1.69 (±0.66) 1.40 (0.90–3.40) |
| (n) > RI | 0/121 (0%) | 0/121 (0%) | 0/87 (0%) | 0/76 (0%) | |
| (n) < RI | 21/121 (17%) | 18/97 (19%) | 10/87 (11%) | 12/76 (16%) | |
| Eosinophils (×109/L) | 0.06–1.23 |
0.5 (±0.68) 0.40 (0.00–4.70) |
0.45 (±0.53) 0.35 (0.04–4.66) |
0.31 (±0.22) 0.30 (0.10–0.70) |
0.30 (±0.19) 0.30 (0.10–0.70) |
| (n) > RI | 6/121 | 3/97 (3%) | 2/87 (2%) | 2/76 (3%) | |
| (n) < RI | 5/121 | 3/97 (3%) | 1/87 (1%) | 3/76 (4%) | |
| Basophils (×109/L) | 0.00–0.10 |
0.0 (±0.02) 0.00 (0.00–0.20) |
0.02 (±0.02) 0.02 (0.00–0.16) |
0.03 (±0.02) 0.00 (0.00–0.10) |
0.03 (±0.03) 0.00 (0.00–0.10) |
| (n) > RI | 3/121 (2%) | 2/97 (2%) | 1/87 (1%) | 1/76 (1%) | |
| Thrombocytes (K/μL) | 148–484 |
271 (±130) 268 (11–813) |
267 (±129) 266 (11–813) |
305 (±98) 265 (201–459) |
286 (±149) 284 (8–664) |
| (n) > RI | 7/121 (6%) | 4/97 (4%) | 6/87 (7%) | 5/76 (6%) | |
| (n) <RIa | 16/121 (13%) | 13/97 (13%) | 3/87 (3%) | 4/76 (5%) | |
| Hematocrit (%) | 37.3–61.7 |
45.4 (±6.7) 45.5 (29.3–60.1) |
44.9 (±6.9) 44.7 (29.3–60.1) |
44.5 (±2.5) 45.2 (40.0–48.2) |
44.6 (±8.5) 45.6 (23.5–63.5) |
| (n) > RI | 0/121 (0%) | 0/97 (0%) | 0/87 (0%) | 0/76 (0%) | |
| (n) < RI | 15/121 (12%) | 12/97 (12%) | 14/87 (16%) | 8/76 (11%) | |
| Mean corpuscular volume (fL) | 61.6–73.5 |
64.8 (±3.1) 64.9 (55.1–71.4) |
64.9 (±0.5) 65.0 (55.1–71.4) |
64.0 (±2.2) 64.6 (60.3–66.2) |
62.7 (±3.5) 62.8 (51.8–68.8) |
| (n) > RI | 0/121 (0%) | 0/97 (0%) | 0/87 (0%) | 0/76 (0%) | |
| (n) < RI | 17/121 (14%) | 13/97 (13%) | 13/87 (15%) | 10/76 (13%) | |
| Mean corpuscular hemoglobin concentration (g/dL) | 32.0–37.9 |
35.7 (±0.9) 35.7 (32.8–38.3) |
35.8 (±0.9) 35.8 (33.2–37.9) |
35.1 (±1.1) 23.0 (20.2–23.2) |
35.7 (±0.9) 35.8 (34.0–37.0) |
| (n) > RI | 1/121 (1%) | 0/97 (0%) | 1/87 (1%) | 0/76 (0%) | |
| (n) < RI | 0/121 (0%) | 0/97 (0%) | 0/87 (0%) | 0/76 (0%) | |
| Total protein (g/L) | 52–82 |
63 (±6) 63 (49–82) |
63 (±6) 63 (49–82) |
69 (±5) 63 (60–73) |
65 (±5) 63 (56–75) |
| (n) > RI | 0/122 (0%) | 0/98 (0%) | 0/88 (0%) | 1/97 (1%) | |
| (n) < RI | 3/122 (2%) | 2/98 (2%) | 1/88 (1%) | 0/97 (0%) | |
| Albumin (g/L) | 22–39 |
33 (±4) 32 (23–48) |
33 (±4) 32 (25–48) |
36 (±4) 36 (29–43) |
32 (±6) 32 (13–42) |
| (n) > RI | 5/122 (4%) | 3/98 (3%) | 2/88 (2%) | 2/76 (3%) | |
| (n) < RI | 0/122 (0%) | 0/98 (0%) | 0/88 (0%) | 1/76 (1%) | |
| Globulin (g/L) | 25–45 |
30 (±5) 30 (17–51) |
30 (±5) 29.5 (17.0–51.0) |
31 (±5) 29.5 (25.0–33.0) |
32 (±4) 31 (25–42) |
| (n) > RI | 2/122 (2%) | 1/98 (1%) | 0/88 (0%) | 0/76 (0%) | |
| (n) < RI | 12/122 (10%) | 9/98 (9%) | 5/88 (6%) | 3/76 (4%) | |
| Alanine aminotransferase (U/L) | 10–125 |
73 (±106) 48 (10–1000) |
72 (±105) 50.5 (12–1000) |
79 (±52) 62 (24–215) |
84 (±60) 63 (22–297) |
| (n) > RI | 13/122 (11%) | 10/98 (10%) | 14/88 (16%) | 12/76 (16%) | |
| (n) < RI | 0/122 (0%) | 0/98 (0%) | 0/88 (0%) | 0/76 (0%) | |
| Alkaline phosphatase (U/L) | 23–212 |
115 (±207) 58.5 (12–1898) |
100 (±121) 60 (12–740) |
123 (±160) 49 (30–557) |
125 (±104) 108 (28–432) |
| (n) > RI | 12/122 (10%) | 8/98 (8%) | 10/88 (11%) | 10/76 (13%) | |
| (n) < RI | 17/122 (14%) | 13/98 (13%) | 0/88 (0%) | 5/76 (7%) | |
| Serum creatinine (mg/dL) | 0.50–1.80 |
1.02 (±0.26) 1.00 (0.46–1.93) |
1.04 (±0.25) 1.01 (0.46–1.76) |
0.87 (±0.19) 0.96 (0.53–1.10) |
0.93 (±0.38) 0.86 (0.51–2.4) |
| (n) > RI | 2/122 (2%) | 0/98 (0%) | 3/88 (3%) | 4/76 (5%) | |
| (n) < RI | 2/122 (2%) | 1/98 (1%) | 0/88 (0%) | 1/76 (1%) | |
| SDMA (μg/dL) | 0–14 |
11.30 (±2.94) 11 (5–22) |
11.31 (±2.84) 11 (5–19) |
12.19 (±3.07) 12 (7–24) |
13.28 (±6.20) 13 (8–59) |
| (n) > RI | 16/121 (13%) | 16/97 (16%) | 5/88 (6%) | 20/74 (27%) | |
| Blood urea nitrogen (mmol/L) | 2.5–9.6 |
5.4 (±2.3) 4.9 (1.5–17.7) |
15.2 (±6.9) 5.0 (1.5–12.7) |
6.0 (±2.5) 5.6 (3.2–10.7) |
8.0 (±7) 6.6 (2.5–34.0) |
| (n) > RI | 6/122 (5%) | 3/98 (3%) | 1/88 (1%) | 6/76 (8%) | |
| (n) < RI | 7/122 (6%) | 5/98 (5%) | 0/88 (0%) | 2/76 (3%) | |
| Glucose (mmol/L) | 3.89–7.95 |
5.20 (±0.64) 5.20 (2.50–7.10) |
5.20 (±0.68) 5.15 (2.46–7.11) |
5.24 (±0.69) 5.5 (4.2–6.0) |
5.48 (±0.51) 5.5 (4.5–6.5) |
| (n) > RI | 0/122 (0%) | 0/98 (0%) | 0/88 (0%) | 0/76 (0%) | |
| (n) < RI | 2/122 (2%) | 1/98 (1%) | 5/88 (6%) | 0/76 (0%) | |
| Sodium (mmol/L) | 144–160 |
155 (±3) 155 (149–167) |
155.17 (±2.93) 155 (149–167) |
154.3 (±2.41) 154.5 (150.0–158.0) |
151.63 (±2.66) 152 (145–157) |
| (n) > RI | 6/122 (5%) | 5/97 (5%) | 3/88 (3%) | 0/76 (0%) | |
| (n) < RI | 0/122 (0%) | 0/97 (0%) | 0/88 (0%) | 0/76 (0%) | |
| Potassium (mmol/L) | 3.5–5.8 |
4.7 (±0.5) 4.7 (3.8–6.5) |
4.8 (±0.5) 4.7 (3.8–6.5) |
4.8 (±0.5) 4.6 (4.3–5.5) |
4.6 (±0.7) 4.6 (3.4–7.3) |
| (n) > RI | 5/122 (4%) | 4/97 (4%) | 2/88 (2%) | 2/75 (3%) | |
| (n) < RI | 0/122 (0%) | 0/97 (0%) | 0/88 (0%) | 1/75 (1%) | |
| Chloride (mmol/L) | 109–122 |
117 (±3) 117 (108–126) |
117 (±3) 117 (108–126) |
115 (±3) 114.5 (110.0–119.0) |
113 (±2) 112 (107–118) |
| (n) > RI | 11/122 (9%) | 10/97 (10%) | 0/88 (0%) | 0/76 (0%) | |
| (n) < RI | 2/122 (2%) | 1/97 (1%) | 2/88 (2%) | 3/76 (3%) | |
| Calcium (mmol/L) | 1.98–3.00 |
2.40 (±0.14) 2.40 (2.20–2.90) |
2.40 (±0.13) 2.39 (2.16–2.85) |
2.40 (±0.15) 2.4 (2.1–2.8) |
2.37 (±0.13) 2.4 (2.2–2.7) |
| (n) > RI | 0/122 (0%) | 0/97 (0%) | 1/88 (1%) | 0/73 (0%) | |
| (n) < RI | 0/122 (0%) | 0/97 (0%) | 1/88 (1%) | 0/73 (0%) | |
| Phosphorus (mmol/L) | 0.81–2.20 |
1.10 (±0.21) 1.10 (0.42–1.83) |
1.08 (±0.22) 1.07 (0.42–1.83) |
1.09 (±0.22) 1.1 (0.70–1.50) |
1.11 (±0.24) 1.10 (0.60–2.10) |
| (n) > RI | 0/122 (0%) | 0/97 (0%) | 1/88 (1%) | 0/73 (0%) | |
| (n) < RI | 14/122 (11%) | 11/97 (11%) | 9/88 (10%) | 3/73 (4%) |
- Abbreviations: n, number; RI, reference interval; T, time point (months).
- a Presence of pseudothrombocytopenia was confirmed by blood smear in all dogs with decreased thrombocyte count.
| Variable | Baseline (T0) (n = 122) | Confirmed healthy T0 (n = 98) | T12 (n = 88) | T24 (n = 76) |
|---|---|---|---|---|
| Urine specific gravity | 1.033 (±0.011) | 1.035 (±0.011) | 1.034 (±0.010) | 1.036 (±0.011) |
| 1.035 (1.005–1.055) | 1.036 (1.005–1.055) | 1.034 (1.009–1.058) | 1.036 (1.011–1.060) | |
| Crystalluria | ||||
| Amorphous | 31/118 (26%) | 26/92 (28%) | 13/79 (16%) | 10/72 (14%) |
| Struvite | 24/118 (20%) | 18/92 (20%) | 9/79 (11%) | 8/72 (11%) |
| Calcium oxalate | 5/118 (4%) | 5/92 (5%) | 3/79 (4%) | 2/72 (3%) |
| Red blood cells | ||||
| Absent | 50/118 (42%) | 36/92 (39%) | 53/86 (62%) | 60/72 (83%) |
| ≤ 5 | 57/118 (48%) | 45/92 (48%) | 26/86 (30%) | 10/72 (14%) |
| > 5 | 15/118 (13%) | 11/92 (12%) | 7/86 (8%) | 2/72 (3%) |
| White blood cells | ||||
| Absent | 97/118 (82%) | 77/92 (84%) | 78/87 (90%) | 68/72 (94%) |
| ≤ 5 | 21/97 (22%) | 15/92 (16%) | 8/87 (9%) | 4/72 (5%) |
| > 5 | 0/97 (0%) | 0/92 (0%) | 1/87 (1%) | 0/72 (0%) |
| Bacteriuria | 18/118 (15%) | 10/92 (11%) | 12/87 (14%) | 7/72 (10%) |
| pH | 7.0 (±1.1) | 6.9 (±1.2) | 7.2 (±1.2) | 6.3 (±1.4) |
| 7.0 (5.0–9.0) | 7.0 (5.0—9.0) | 7.0 (5.0–9.0) | 6.5 (5.0–9.0) | |
| Glucosuria | 2/118 (2%) | 1/98 (1%) | 1/88 (1%) | 1/74 (1%) |
| Ketonuria | 8/118 (7%) | 6/98 (6%) | 5/88 (6%) | 8/74 (11%) |
| Bilirubinuria | 26/118 (22%) | 22/98 (22%) | 7/88 (8%) | 10/74 (14%) |
| UPC | 0.3 (±0.5) | 0.14 (±0.09) | 0.29 (±0.86) | 0.23 (±0.28) |
| 0.1 (0.1–2.8) | 0.1 (0.1–0.7) | 0.1 (0.1–7.5) | 0.1 (0.1–1.7) | |
| Positive urine culture | 9/121 (7%) | 7/97 (7%) | NP | NP |
- Note: White and red blood cell were counted per high power field (40× objective).
- Abbreviations: n, number; NP, not performed; T, time point (months).
At baseline, only USG was significantly (p < 0.05) lower in the geriatric compared with the senior age group. Bacteriuria was observed at baseline in 11% of confirmed healthy dogs; in 7% it was confirmed by positive urine culture (three voided and four cystocentesis samples). These dogs did not show lower urinary tract signs, and this subclinical bacteriuria was not treated, in accordance with recent guidelines [35]. The incidence of subclinical microscopically detected bacteriuria remained similar during follow-up (10%–14%; Table 4).
3.4 New or Unknown Diseases Detected at Baseline
Based on the complete health evaluation at baseline, 24/122 (20%) apparently healthy older dogs were diagnosed with ≥ 1 disease, excluding them from further inclusion in the longitudinal health screening study (Table 5). In total, five dogs were diagnosed with a malignant neoplasia at baseline (n = 3, anal sac tumors; n = 1, mammary gland tumor and pulmonary mass; n = 1, cutaneous mastocytoma). Azotemic CKD [31] was not diagnosed at baseline, but 16% (20/122) showed CKD IRIS stage 1 [31] (n = 15, persistent renal proteinuria; n = 5, persistent USG ≤ 1.020) and one other dog was diagnosed with non-obstructive sterile struvite urolithiasis and excluded from follow-up.
| All dogs (n = 122) | Senior dogs (n = 67) | Geriatric dogs (n = 55) | |
|---|---|---|---|
| Health screening conclusion | |||
| Healthy | 98 (80%) | 54 (81%) | 44 (80%) |
| Excluded from follow-up study | 24 (20%) | 13 (19%) | 11 (20%) |
| Disorders diagnosed at baseline | |||
| Malignant neoplasia | 5 (4%) | 3 (4%) | 2 (4%) |
| CKD IRIS stage 1 | 20 (16%) | 10 (15%) | 10 (18%) |
| Urolithiasis | 1 (< 1%) | 1 (1%) | 0 (0%) |
| Orthopedic disorder | 2 (2%) | 0 (0%) | 2 (4%) |
| Neurologic disorder | 2 (2%) | 1 (1%) | 1 (2%) |
| Dermatological disorder | 1 (< 1%) | 0 (0%) | 1 (2%) |
| Gastrointestinal disorder | 4 (3%) | 2 (3%) | 2 (4%) |
- Note: Diagnosis of malignant neoplasia, CKD IRIS stage 1, and urolithiasis led to exclusion for follow-up analysis. Two dogs were diagnosed with ≥ 1 disease.
- Abbreviation: n, number.
3.5 New or Unknown Diseases Detected During Follow-Up
During the first year of follow-up, 1 dog dropped out for owner-related reasons and 7 dogs were euthanized. During the second year of follow-up, 15 dogs died (euthanasia, n = 14; hit-by-car, n = 1). In 43% (9/21) euthanasia was elected because of malignant neoplasia (lymphoma, n = 2; hemangiosarcoma, n = 2; mammary and pulmonary mass, n = 1; intramedullary sarcoma, n = 1; pancreatic adenocarcinoma, n = 1; gastric adenocarcinoma, n = 1; heart base tumor, n = 1). Remaining dogs were mainly euthanized because of a (progressive) neurologic disorder (n = 9, 41%) including cognitive dysfunction, and less frequently because of progressive CKD (n = 1), hematologic disorder (n = 1) or gastrointestinal disorder (n = 1). In 4 confirmed healthy dogs, health screening was repeated at T24 but not at T12.
Diseases that confirmed healthy dogs developed within 2 years are listed in Table 6. In 3/12 dogs where malignant neoplasia developed, it was detected on health screening (n = 2, anal sac tumor; n = 1, indolent splenic lymphoma). In the nine other dogs, a diagnosis was made independent of health screening time points. Including the 5 dogs diagnosed with neoplasia at baseline, these 8/17 (47%) malignant neoplasms were detected during health screening.
| Confirmed healthy (n = 98) | Senior dogs (n = 54) | Geriatric dogs (n = 44) | |
|---|---|---|---|
| Available follow-up data | |||
| Completed the study period | 77/98 (79%) | 49/54 (91%) | 26/44 (59%) |
| Remained “healthy” during 2 years follow-up | 58/98 (59%) | 40/54 (74%) | 18/44 (41%) |
| Died within 2 years | 22/98 (22%) | 5/54 (9%) | 17/44 (34%) |
| Lost to follow-up | 1/98 (1%) | 0/54 (0%) | 1/44 (2%) |
| Newly diagnosed diseases within 2 years | |||
| Malignant neoplasia | 12/98 (12%) | 3/54 (6%) |
9/44 (20%) |
| Chronic kidney disease | 12/98 (12%) | 5/54 (9%) | 7/44 (16%) |
| IRIS stage 1 | 4/98 (4%) | 2/54 (4%) | 2/44 (5%) |
| ≥ IRIS stage 2 | 8/98 (8%) | 3/54 (6%) | 5/44 (11%) |
| Neurologic disorder | 11/98 (11%) | 3/54 (6%) | 8/44 (18%) |
| Orthopedic disorder | 5/98 (5%) | 0/54 (0%) | 5/44 (11%) |
| Dermatological disorder | 4/98 (4%) | 2/54 (4%) | 2/44 (5%) |
| Respiratory disorder | 2/98 (2%) | 0/54 (0%) | 2/44 (5%) |
| Gastrointestinal disease | 2/98 (2%) | 1/54 (2%) | 1/44 (2%) |
| Uro-genital disorder | 3/98 (3%) | 2/54 (4%) | 1/44 (2%) |
| Cardiac disorder ≥ ACVIM stage B2 | 1/98 (1%) | 1/54 (2%) | 0/44 (0%) |
| Hematological disorder | 1/98 (1%) | 1/54 (2%) | 0/44 (0%) |
- Note: Six dogs were diagnosed with > 1 disease during the study period and are therefore represented more than once. “Healthy” during 2 years follow-up was defined as absence of metabolic/systemic disease (e.g., malignant neoplasia, azotemic chronic kidney disease [CKD]; serum creatinine ≥ 1.8 mg/dL (159 μmol/L) or SDMA > 18 μg/dL in combination with urine specific gravity (USG) ≤ 1.030), CKD IRIS stage 1 [persistent urinary protein: Creatinine ratio (UPC) ≥ 0.5 or persistent USG ≤ 1.020 in the absence of azotemia], diabetes mellitus, urolithiasis), or clinically relevant cardiovascular disease (e.g., third-degree atrioventricular block, cardiomyopathy ACVIM stage ≥ B2).
- Abbreviation: n, number.
3.6 Prediction of Mortality
The normal distribution assumption for the biomarkers was not rejected based on the Shapiro-Wilks test. Age group had a significant predictive value for onset of death (p < 0.0001) and was implemented as a stratification factor for the remaining analysis. None of the remaining assessed variables showed a predictive value for onset of death in our cohort of 98 confirmed healthy older dogs at baseline (Table S2).
4 Discussion
Our prospective and longitudinal study on health screening shows that: (1) in 20% of apparently healthy older dogs, an unrecognized systemic disease can be detected at the first health screening; (2) subjects of interest for repeated annual health screening in confirmed healthy dogs are assessment of neurologic and orthopedic health and screening for CKD; (3) malignant neoplasia could be suspected in 47% on physical health screening, indicating the benefit of semi-annual evaluation of skin and mammary glands and rectal palpation; and (4) only age group was associated with survival in confirmed healthy dogs at baseline.
Health screening has added value in older apparently healthy dogs, because 1/5 had a sub-clinical or unnoticed systemic disease. In contrast to recent findings in cats, geriatric dogs were not diagnosed more frequently with a condition than senior dogs [36]. The prevalence of disease in our study (20%) is higher compared with the 5% found in a previous study in a similar cohort of dogs [5]. The difference is most likely related to the inclusion of dogs with IRIS stage 1 CKD within the CKD group in our study. Doing so was only possible because of repeated urinalysis in dogs with proteinuria or USG ≤ 1.020. The significantly lower USG in geriatric dogs compared with senior dogs at baseline is suggestive of an age-related decline in renal function, but was previously not reported in a similar cohort of dogs using the same methodology [5]. A detailed description of the renal health in this cohort of dogs can be found elsewhere [23]. A previous study [5] found significant differences between both age groups for platelet count, hematocrit, and serum albumin concentration, which was supported by another study [20], but similar differences were not confirmed in our cohort. The contrasting results indicate the need to confirm certain research findings in various, similar study populations. A clear difference was identified between senior and geriatric dogs in terms of the number that remained healthy. Therefore, performance of repeated health screening should be emphasized in geriatric dogs. That said, a quarter of senior dogs also developed disease within 2 years.
Physical examination did identify several abnormalities requiring further diagnostic or therapeutic intervention, or at least further monitoring. These included the presence of moderate to severe dental disease (34%), the presence of cutaneous or SC masses (37%) or abnormalities on orthopedic (36%) or neurologic (31%) examination. Despite the link between periodontal disease and cardiac, hepatic, or renal conditions, no effect on longevity was identified in our study cohort of confirmed healthy older dogs at baseline [21, 22]. Obesity was observed in 11% of apparently healthy dogs. When including dogs with BCS > 5/9, 26% (32/122) were overweight, which is lower compared with a previous study in a similar cohort of dogs (39%) [5]. This finding may indicate more awareness of the negative implications of obesity in dogs among owners compared with a decade ago. Of note, BCS was not associated with survival in our cohort of confirmed healthy dogs at baseline, probably reflecting the rather low percentage of obesity. Muscle wasting (MCS, 2–4/4) was more commonly seen at T24 (22%) compared with baseline (5%). In a recently published study, the presence of frailty, linked with decreased muscle strength, was observed in up to 42% of healthy-appearing older medium-sized (> 20 kg) dogs [37]. These findings are important because sarcopenia (i.e., loss of skeletal muscle and poor muscle quality leading to decreased muscle strength) is associated with decreased lifespan in people, dogs, and cats, and could become a therapeutic target in older dogs [38]. Neoplasia, congestive heart failure, CKD, and aging are the most common causes of muscle and fat loss in dogs [38]. No predictive value of MCS for survival could be found in dogs confirmed healthy at baseline in our cohort.
Blood examination identified mostly mild alterations. Except for measuring hematocrit, repeated performance of CBC appears to be of limited value in older dogs that remain clinically healthy according to their owners. Similar to a previous study [5], mild to moderate increases in ALKP and ALT activity were quite common, but are considered clinically unimportant and were not associated with survival. Repeated assessment of ALKP and ALT might be recommended in breeds predisposed to chronic liver diseases, but it can be debated whether annual assessment in all healthy-appearing older dogs provides added value. A similar conclusion can be drawn for electrolyte measurements. The need for an age-specific RI for serum phosphorus concentration was confirmed and was calculated based on our data [5]. The most common diseases that developed within 2 years in confirmed healthy older dogs were malignant neoplasia, CKD or neurologic disorder, followed by orthopedic disorder. Focusing on these disorders therefore seems appropriate during repeated annual health screening in older dogs by performing urinalysis or completing questionnaires (e.g., Liverpool OsteoArthritis in Dogs [LOAD]) [39], rather than repeating extensive blood testing, in particular in senior dogs. Our data support the inclusion of urinalysis in health screening protocols for aging dogs, which often is neglected in practice [14, 40]. Besides CKD, urinalysis also can provide relevant information when screening for other diseases such as diabetes mellitus, urolithiasis or hyperadrenocorticism (e.g., UPC, urinary cortisol: creatinine ratio). Subclinical or occult bacteriuria is reported in 2%–12% of healthy dogs [35, 41, 42]. Although the prevalence of bacteriuria can be high in elderly people [43], in our cohort of confirmed healthy older dogs its frequency (7%) was similar to that of the more general dog population [35, 42]. We emphasize that urine culture should not be part of routine health screening protocols, but can be considered in cases where it is deemed clinically relevant.
None of the assessed laboratory variables were associated with survival in our study cohort of aging confirmed healthy dogs at baseline, which contrasts with reports in humans [15, 16, 19]. Despite previous suggestive findings in dogs, neither serum albumin concentration nor hematocrit was associated with increased risk of mortality in our cohort, but additional larger-scale studies are indicated to investigate this possibility further [5, 20].
Early detection and diagnosis of cancer in older dogs is a crucial aspect in providing optimal care. Physical examination is considered an important first step in the diagnosis, which is confirmed by our study data, because almost half of all malignant neoplastic disorders were detected during health screening [44]. A recent large-scale study in middle-aged to older dogs of breeds at risk for cancer detected 83% of all confirmed cancers on physical examination [45]. Anal gland tumors were detected in our study in 4% of dogs. This finding was unexpected, because such tumors are relatively uncommon (2% of all skin or SC tumors), although most frequently reported in older dogs [46]. As such, this example indicates why health screening adds value, given that the prognosis of anal gland adenocarcinoma is strongly dependent on the size of the tumor as well as the stage of the disease. Therefore, including an annual or semiannual rectal palpation in the health screening protocol of older dogs seems advisable [45]. It must be acknowledged, however, that the remaining half of the neoplastic disorders could not be detected based on health screening. Rapidly advancing diseases, such as lymphoma, typically will cause clinical disease before screening tests can detect it [47].
Our study had some limitations. First we defined “confirmed healthy” as the absence of certain systemic diseases, which means that dogs with mild orthopedic or neurologic alterations, as well overweight dogs, were included for follow-up. This design feature could have contributed to the relative high cumulative incidence of these disorders within 2 years. However, we aimed to obtain a cohort representative of the average older dog population. One dog previously diagnosed with KCS was included in the confirmed healthy group. In the majority of cases, KCS in dogs is caused by a local immune-mediated disease, making this decision for inclusion debatable. Because no clear data suggest that KCS in dogs is associated with onset of systemic neoplasia or chronic illness, this dog still was considered systemically healthy. Secondly, because 22% of confirmed healthy dogs died within 2 years, this factor influenced the cumulative incidences. Thirdly, our study provides no evidence that screening improves overall outcome, a conclusion that would require comparison of outcomes in dogs that are screened with those that were not screened.
In conclusion, our study shows the merit of routine health screening because in 20% of apparently healthy older dogs an unrecognized systemic disease was detected. Next to repeated screening for CKD, assessment of neurologic and orthopedic health seems most relevant. Furthermore, malignant neoplasia could be detected in 47% of the dogs on physical health screening, indicating the benefit of annual or semiannual examination of the skin and mammary glands and in particular performing rectal palpation. Beyond age group, no clinicopathological variables were associated with survival in our study cohort.
Disclosure
Authors declare no off-label use of antimicrobials.
Ethics Statement
Approved by the ethics committee of Ghent University, 2019-39 (Lab LA 1400075). Authors declare human ethics approval was not needed.
Conflicts of Interest
The authors declare no conflicts of interest.




