Twenty-Four-Hour Electrocardiographic Monitoring for Assessment of Cardiac Arrhythmias in Healthy and Hospitalized Goats
Funding: The authors received no specific funding for this work.
ABSTRACT
Background
Continuous 24-h electrocardiographic (ECG) recording is a well-established method for the detection of intermittent arrhythmias in veterinary medicine. The clinical utility of 24-h ECG for the detection of intermittent arrhythmias in hospitalized goats has not been reported.
Hypothesis/Objectives
(1) Determine the clinical feasibility of continuous 24-h ECG monitoring in goats; (2) Report the frequency of ventricular arrhythmias in healthy and hospitalized medically ill goats.
Animals
Eleven healthy goats, 20 hospitalized medically ill goats.
Methods
Prospective clinical study. Continuous 24-h ECG recordings were performed. Electrocardiograms were analyzed for rhythm diagnosis. The number of ventricular premature depolarizations (VPD) was compared between groups using the Wilcoxon rank sum test.
Results
The ECG monitors were well-tolerated in 30/31 goats, with no adverse effects. Twenty-eight recordings were of sufficient quality for analysis with a median readable time of 23 h (range, 15–24 h). Eleven goats had ventricular arrhythmias (4 healthy, 7 medically ill), consisting of single VPDs only in 7 goats (3 healthy, 4 medically ill), VPDs and ventricular couplets in 4 goats (1 healthy, 3 medically ill), and ventricular rhythm consistent with accelerated idioventricular rhythm (AIVR) or ventricular tachycardia in 2 goats (2 medically ill). A significant difference in the number of VPDs in healthy goats (median, 0; range, 0–9) and medically ill goats was not identified (median, 0; range, 0–201; p = 0.66), but a larger sample size is required.
Conclusion and Clinical Importance
Most goats tolerated 24-h ECG monitoring well, although a few recordings were of poor quality. Ventricular arrhythmias were seen in healthy and medically ill goats.
Abbreviations
-
- AIVR
-
- accelerated idioventricular rhythm
-
- cTnI
-
- cardiac troponin I
-
- ECG
-
- electrocardiographic
-
- VPD
-
- ventricular premature depolarization
1 Introduction
Continuous 24-h electrocardiographic (ECG) recording is a well-established method for detection of intermittent arrhythmias in veterinary medicine in many species, including companion animals, horses, and cattle [1-4]. However, the feasibility and clinical utility of continuous 24-h ECGs have not previously been reported in goats. Goats often are admitted to veterinary hospitals in a critically ill state, with common conditions including urolithiasis, endoparasitism, and pregnancy toxemia. Although the cardiac effects of a variety of diseases have been described in goats [5-11], information regarding cardiology in goats is scarce, with only a few studies focusing on echocardiography and single timepoint electrocardiograms (ECGs) [8, 12-19]. In addition to the applications to clinical veterinary medicine, the use of ECG monitoring in goats has applications to research in human medicine. Goats are a useful model for cardiology research because they have a body and heart size similar to humans and are easier to handle than many other commonly used research species [14, 20-22].
No information is currently available regarding the incidence of arrhythmias in ill goats, despite the fact that many disorders in goats could cause myocardial injury through perfusion deficits and impacts on myocardial function, or might result in serum electrolyte concentration abnormalities that can adversely affect cardiac function [10, 23]. Further characterizing the frequency of cardiac arrhythmias in medically ill goats is an important step in advancing knowledge of cardiac conditions in goats and improving their medical care. Ventricular arrhythmias in particular can have life-threatening physiologic consequences, and thus the ability to accurately detect them is crucial. Sudden death caused by ventricular arrhythmias has been described in several species including humans, dogs, cats, and horses [24-27].
Our primary objective was to determine the clinical feasibility of continuous 24-h ECG monitoring in goats. A secondary objective was to report the frequency of ventricular arrhythmias in healthy goats and in hospitalized medically ill goats. We hypothesized that ventricular arrhythmias would be more frequent in hospitalized medically ill goats than in healthy goats.
2 Materials and Methods
2.1 Animals
The study was approved by the University of Florida Institutional Animal Care and Use Committee (IACUC 202011285). Ours was a prospective study enrolling 11 healthy adult goats and 20 hospitalized client-owned medically ill adult goats. Goats were all ≥ 1 year of age. The 11 healthy goats consisted of 7 healthy goats from the University of Florida College of Veterinary Medicine teaching and research herd (IACUC 201808131) and 4 privately owned goats. Goats were deemed healthy based on history and normal physical examination findings before study enrollment. Healthy goats had no known history of systemic or cardiovascular illness and had normal cardiac auscultation findings, heart rate (beats per minute, bpm), respiratory rate, and temperature on physical examination. The hospitalized medically ill group (n = 20) consisted of goats presented to the University of Florida Large Animal Hospital and hospitalized for treatment as recommended by the supervising veterinarian. Inclusion criteria for the hospitalized, medically ill group were adult (≥ 1 year) goats admitted and hospitalized for at least 24 h. Information recorded for each patient included: signalment, body weight (kilograms), admission date, presenting complaint, clinicopathologic findings, final diagnosis, survival to discharge, and necropsy results (where applicable). Goats were housed in box stalls, bedded on shavings, and access to feed and water was determined by the supervising veterinarian.
2.2 Electrocardiographic Monitoring
All goats were fitted with a continuously recording 3-channel 5-lead electrocardiographic monitor (Trillium 7000, Forest Medical, Syracuse, NY), with data recorded onto a compact flash memory card. Elastic adhesive tape (Sher-light tape, Covidien, Mansfield, Massachusetts) was used to secure electrodes, followed by veterinary elastic wrap (Vetrap, 3 M, St. Paul, Minnesota) placed over the ECG leads and equipment to provide security and minimize goat interaction with the equipment. Preliminary assessment of the best lead placement in 3 goats before the study determined that a modified base apex placement as described below produced an adequate signal for analysis. The left arm lead was placed on the left pectoral muscle; the right arm lead was placed on the right pectoral muscle; the left leg lead was placed 2 cm caudal and 2 cm ventral to the left side of the withers; the right leg lead was placed 2 cm caudal and 2 cm ventral to the right side of the withers; the ground lead was placed in the right axillary region (Figure 1). The ECG monitors were placed within 24 h of admission and remained in place for 24 h.
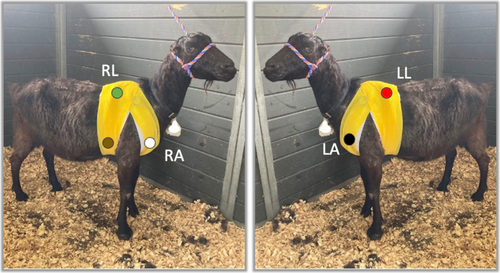
Electrocardiographic recordings were analyzed for the presence of arrhythmias using 24-h Holter software (Trillium Platinum Vet Software, Forest Medical, Syracuse, NY). Each Holter ECG was manually reviewed in a full disclosure review and corrected if necessary for rhythm accuracy (AS, DL). Sinus rhythm was diagnosed based on a regular rhythm with a narrow QRS complex with associated P waves. If P waves were not discernible but the QRS morphology matched definitive sinus beats and the rhythm was regular at an unchanged rate, the rhythm was presumed sinus. Ventricular ectopy was diagnosed if QRS complexes were approximately < 90% like the sinus beats (wide and bizarre) without an associated P wave on subjective assessment. Supraventricular ectopy was diagnosed if QRS complexes were approximately > 90% like the sinus beats with or without a premature P wave on subjective assessment. Twenty-four hour Holter ECG variables that were used for statistical analysis were number of heartbeats, maximum heart rate, minimum heart rate, and number of ventricular premature depolarizations (VPDs), ventricular couplets, and ventricular rhythms with ≥ 3 consecutive ventricular beats. The instantaneous rate of consecutive ventricular beats was recorded.
2.3 Cardiac Troponin I Concentrations
After acclimation to the stall and at the time of placement of the ECG monitors, 3 mL of whole blood were collected from each goat by jugular venipuncture into lithium heparin-containing blood tubes. Plasma cardiac troponin I (cTnI) concentrations were measured within 30 min of sample collection using a point-of-care analyzer that has been validated for use in goats (Vetscan i-STAT 1, Abaxis, Union City, CA) [10], according to the manufacturer's instructions for the first 12 goats (6 healthy, 6 ill). Because of the lack of availability of the cartridges for the aforementioned analyzer during a portion of the study period, for the remaining 19 goats (5 healthy, 14 ill), samples were immediately centrifuged and 1.5 mL of plasma was frozen at −80 C for later analysis. These samples were analyzed using a commercially available two-site chemiluminescent immunoassay (University of Pennsylvania School of Veterinary Medicine New Bolton Center Cardiology Laboratory, Siemens Stratus CS, Dade Behring Inc., Deerfield, IL, USA).
2.4 Echocardiography
To assess for underlying structural cardiac disease that might predispose the goats to arrhythmias, echocardiography was performed. Subjective assessment of echocardiographic images was performed for the purpose of the study. Echocardiographic evaluation was performed at the end of the 24-h period of Holter recording by individuals trained in echocardiography in goats (AS, DL), with images reviewed by a board-certified large animal internist (DL). Six standard 2-dimensional right parasternal views were obtained using a 5 MHz phased-array transducer (Philips CX50, Koninklijke Philips Electronics N.V., The Netherlands), as previously described in goats [12, 19]. These views included: right parasternal cranial long axis view of the right ventricular outflow tract, right parasternal long axis view of the left ventricular outflow tract, right parasternal long axis view of the left atrium and ventricle, right parasternal short axis view of the left ventricle (at the chordal level), right parasternal short axis view of the mitral valve, and right parasternal short axis view of the aortic valve (also allowing visualization of the left atrium and left atrial appendage). Additionally, M mode images were obtained of the left ventricle (at the chordal level) and mitral valve from the right parasternal short axis view, and a 2-dimensional left parasternal long axis view of the left atrium and left ventricle was obtained. All images were stored using software of the ultrasound machine (Philips CX50, Koninklijke Philips Electronics N.V., The Netherlands).
2.5 Statistical Analysis
A statistical a priori power analysis (Type II error = 0.2; Type I error = 0.05, www.openepi.com and G*Power software) showed that 7 healthy animals and 21 ill animals, for a total sample size of 28 animals, were necessary to detect a significant (40%) difference in heart rate parameters between groups based on results of preliminary findings in 2 healthy goats and previously performed studies in dogs. Initial study plans were to enroll 10 healthy and 30 ill goats to account for possible incomplete data or poor quality recordings, but because of difficulties in enrollment of client-owned animals as well as other constraints, the study was ended once 11 healthy and 20 ill goats were enrolled.
Statistical analysis was performed using standard statistical software (Stata 16.1IC, StataCorp, State College, TX). All analyses were conducted using two-sided tests of hypotheses, and a p-value of < 0.05 was set as the criterion for statistical significance. Continuous data was assessed for normality using the Shapiro–Wilk test. For non-normally distributed data, results are reported as median and range. For normally distributed data, results are reported as mean and SD. Continuous variables (age, weight, ECG measurements, cardiac troponin concentration) were compared between groups (healthy vs. medically ill; goats with and without VPDs) using a Student's t test if normally distributed or a Wilcoxon rank sum test if non-normally distributed. The proportion of animals with arrhythmias was compared between groups using a χ2 test.
3 Results
3.1 Patient Signalment
Thirty-one goats were enrolled in the study, consisting of 11 healthy goats and 20 hospitalized medically ill goats. Table 1 reports the signalment of the healthy and medically ill groups. No significant differences in age, weight, or sex were found between healthy and medically ill goats. A significant breed difference was noted between groups, with LaMancha being more common in the healthy group and Nigerian Dwarf only seen in the medically ill group (p = 0.01).
| All animals (n = 30) | Healthy (n = 11) | Medically ill (n = 20) | p | |
|---|---|---|---|---|
| Age (years) | 5 (1–14) | 7 (1–14) | 3.5 (1–12) | 0.06 |
| Breed | 0.01 | |||
| LaMancha | 11 | 9 | 2 | |
| Nigerian Dwarf | 8 | 0 | 8 | |
| Oberhasli | 3 | 0 | 3 | |
| Nubian | 2 | 0 | 2 | |
| Toggenburg | 2 | 1 | 1 | |
| Alpine | 2 | 1 | 1 | |
| Pygmy | 1 | 0 | 1 | |
| Boer | 1 | 0 | 1 | |
| Tennessee fainting | 1 | 0 | 1 | |
| Sex | 0.07 | |||
| Female | 22 | 10 | 12 | |
| Castrated male | 9 | 1 | 8 | |
| Body weight (kg) | 54 ± 23 | 67 ± 10 | 50 ± 24 | 0.11 |
| Cardiac troponin concentration (ng/mL) | 0.02 (0.00–0.91) | 0.00 (0.00–0.02) | 0.08 (0.00–0.91) | < 0.01 |
- Note: Values are reported as median (range) or mean ± SD. P value reported for comparison between healthy and ill goats. One goat excluded from further analysis due to no ECG recording available.
3.2 Clinical Findings
The most common presenting complaints for the medically ill goats included inappetence (5), straining to urinate (4), fever (2), gastrointestinal signs (2), mastitis (2) and dystocia (2). Final diagnoses included reproductive conditions (7), urolithiasis (4), neoplasia (2), gastrointestinal disease (2), endoparasitism (1), liver disease (1), pneumonia (1), neurologic disease (1), and severe musculoskeletal deformity (1).
Seventeen of the 20 medically ill goats (85%) survived to hospital discharge. Of the 3 goats that were euthanized because of the severity of clinical disease, necropsy results yielded final diagnoses of mediastinal thymoma, Mycoplasma infection (with secondary otitis externa and media, osteomyelitis and neuritis), and severe hepatic lipidosis.
3.3 Cardiac Troponin I
Median cardiac troponin I (cTnI) for all goats was 0.02 ng/mL (range, 0.00–0.91 ng/mL). Cardiac troponin I was lower in healthy goats (median, 0.00 ng/mL; range, 0.00–0.02 ng/mL) than in medically ill goats (median, 0.08 ng/mL; range, 0.00–0.91 ng/mL; p < 0.01).
The final diagnoses in the goats with the two highest cTnI concentrations were obstructive urolithiasis (0.74 ng/mL) and severe cachexia caused by severe endoparasitism (0.91 ng/mL).
3.4 Echocardiography
Echocardiography was performed in all goats. No clinically relevant valvular regurgitation or structural cardiac abnormalities were noted. Further comparisons between groups in echocardiographic measurements were not pursued because of the wide variability in goat breed and size. Goats were determined to have no structural abnormalities based on standard echocardiographic views and color Doppler to assess valvular function [12].
3.5 Electrocardiographic Monitoring
Continuous ECG monitors were well-tolerated in 30/31 goats. One healthy goat was excluded from further analysis because of destruction of the Holter monitor. No adverse effects associated with the ECG monitors occurred in any goats. Sufficient quality ECGs for rhythm analysis were obtained in 28/31 goats. Median readable time for the 28 sufficient quality ECGs was 23 h (range, 15–24 h).
Table 2 reports heart rate variables for all goats. Average heart rate over the 24-h recording was higher in medically ill goats than in healthy goats (p < 0.01). Minimum heart rate over the 24-h recording was higher in medically ill goats than in healthy goats (p < 0.01). Maximum heart rate was not significantly different between groups (p = 0.67).
| Healthy (n = 10) | Medically ill (n = 20) | p | |
|---|---|---|---|
| Average heart rate (bpm) | 82 ± 13 | 103 ± 14 | < 0.01 |
| Minimum heart rate (bpm) | 59 ± 12 | 75 ± 14 | < 0.01 |
| Maximum heart rate (bpm) | 159 ± 41 | 164 ± 22 | 0.67 |
| Body weight (kg) | 67 ± 10 | 50 ± 24 | 0.11 |
- Note: P value reported for comparison between healthy and ill goats.
The predominant rhythm was regular and of supraventricular morphology. Discernible P waves were not present in many areas of the Holter recordings, but the rhythm even in these areas was consistent with sinus rhythm based on regularity, lack of prematurity, and similar QRS morphology compared to areas where P waves were discernible. No clear atrial ectopy was present during full manual review.
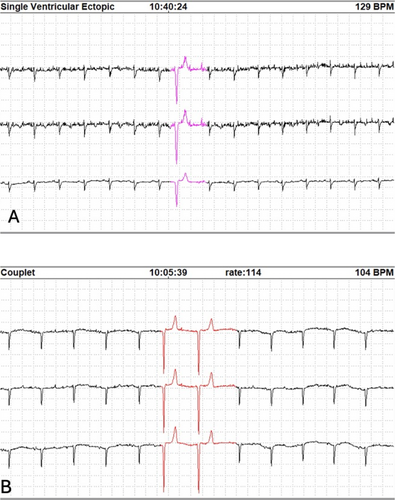
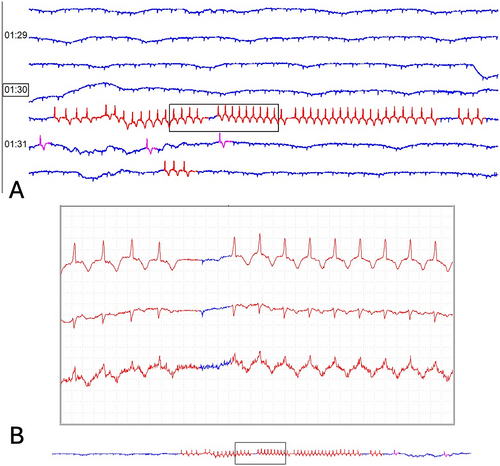
Eleven goats had ventricular arrhythmias detected on 24-h ECG recordings (4 healthy, 7 medically ill). Single ventricular premature depolarizations (VPDs) were seen in 7 goats (3 healthy, 4 medically ill; Figure 2a) and ventricular couplets were seen in 4 goats (1 healthy, 3 medically ill; Figure 2b). The median number of VPDs in healthy goats was 0 (range, 0–9). The median number of VPDs in medically ill goats was 0 (range, 0–201). A significant difference in the number of VPDs between groups was not identified, but findings were limited by small sample size (p = 0.66). Similarly, no significant difference was identified in the number of goats with VPDs between groups (χ2 = 0.003, p = 0.95). Non-sustained, self-limiting ventricular rhythms (consistent with accelerated idioventricular rhythm [AIVR] or ventricular tachycardia [VT]; Figure 3) were identified in 2 medically ill goats that also displayed ventricular couplets. One of these 2 goats had 11 instances of a ventricular rhythm with a mean instantaneous heart rate of 88 bpm (range, 76–121 bpm) and the other goat had two instances of a ventricular rhythm with a mean instantaneous heart rate of 143 bpm (132 and 155 bpm). These two goats had a final diagnosis of obstructive urolithiasis and enteritis and had cTnI concentrations of 0.06 ng/mL and 0.31 ng/mL, respectively.
The median cTnI in goats without VPDs (median [range]: 0.02 [0–0.51] ng/mL) was not significantly different from goats with VPDs (median [range]: 0.06 [0–0.74] ng/mL; p = 0.19).
4 Discussion
We demonstrated the clinical feasibility of continuous 24-h ECG monitoring in goats. The majority of goats tolerated 24-h ECG monitoring well, but a few recordings were of poor quality. Ventricular arrhythmias were seen in both healthy and medically ill goats, but non-sustained ventricular rhythms consistent with either AIVR or VT were only seen in medically ill goats.
The prevalence of cardiac arrhythmias in sick goats has not been reported previously. One study described cardiac arrhythmias in clinically healthy goats in Iran [28]. In that study, only 0.5% of healthy goats had an arrhythmia considered to be abnormal (atrial [supraventricular] premature complexes). No other studies have described the frequency of arrhythmias in healthy or medically ill goats, and the clinical relevance of many arrhythmias in goats is not well understood [28]. In our study, approximately one-third of all goats had ventricular arrhythmias noted, including healthy goats. Ventricular ectopic activity has been identified in healthy humans and animals [2, 29, 30]. Although no data exist in goats, one ventricular ectopic beat per hour can be seen in normal horses, and this number is considered clinically unimportant if the abnormal beats disappear with exercise [26]. The number of VPDs noted in the healthy goats and the majority of the medically ill goats would be considered within the range of normal for other species, with only 2 medically ill goats having frequent VPDs considered outside the range of normal for other species (up to 200 in a 24-h period). Ventricular arrhythmias that are complex (couplets, triplets, bigeminy or trigeminy patterns, R-on-T, multiform ventricular tachycardia) are cause for greater clinical concern; 4 goats in our study were noted to have ventricular couplets, 2 of which also were found to have a ventricular rhythm consistent with AIVR or VT [26]. Additionally, although underlying structural heart disease worsens prognosis in horses, the exact number of VPDs in horses above which the risk of sudden death increases remains unknown [26, 31]. Given the fact that a single VPD with early coupling could lead to R-on-T, a safe number of VPDs is difficult to predict, and rather a consideration of the acceptable risk for a species (including goats) should be considered.
Ventricular rhythms consistent with AIVR or VT were noted in 2 medically ill goats in our study, but no studies have defined the rate distinction between AIVR and VT in goats. This type of arrhythmia can occur due to primary or secondary heart disease, but it also can occur in the absence of cardiac disease and has been identified in normal humans and animals [2, 30, 32]. A clear definition of AIVR in large animals has not been established, but it is often described as a rhythm of ventricular origin with a rate faster than sinus rhythm but slower than the criteria for ventricular tachycardia [33]. In other species, heart rate cut-offs are utilized to distinguish between AIVR and ventricular tachycardia. No clear definition or heart rate cut-offs for AIVR and VT exist in goats.
Goats are becoming increasingly popular as companion animals, and as such the demand for specialized medical care is increasing. Currently, limited published information is available regarding cardiology in goats in comparison to the volume of literature available for other companion animals. Cardiac arrhythmias can be caused by sympathetic and parasympathetic imbalance or could be a result of cardiomyocyte injury caused by inadequate perfusion, as well as electrolyte or acid–base abnormalities associated with the primary disease process [34]. Of note, the average heart rate was higher in the medically ill goats than in the healthy goats, which could be attributed to stress, pain, shock, hypovolemia, hypotension, anemia, endotoxemia, or sepsis, systemic inflammatory response syndrome, or myocardial disease, among others. Many causes of tachycardia also can predispose to cardiac arrhythmias, which warrants further investigation in ill goats. Identification and management of arrhythmias are essential, because the arrhythmias can negatively impact patient outcome, with the potential to result in poor perfusion or unexpected death. The ability to accurately detect arrhythmias is the first step in improving the ability to identify and treat arrhythmias, thereby providing more specialized critical care medicine for goats.
Cardiac troponin I (cTnI) is a biomarker of myocardial injury that has been utilized in a variety of species, including goats. It allows for non-invasive detection of cardiomyocyte injury from a variety of causes, and correlates with arrhythmias in many studies [10, 11, 35-38]. Cardiac troponin I was measured in our study to assess for underlying myocardial injury that could predispose to the development of arrhythmias. The medically ill goats had higher cTnI concentrations than healthy goats, but no difference was detected between goats with and without arrhythmias. Although there is an association between increased cTnI and ventricular arrhythmias in other species [26], it is possible that the arrhythmias in our goats were not all related to cardiomyocyte injury or the low numbers of arrhythmias and detectable cTnI prevented detection of a relationship.
One limitation of our study was that we presumed sinus rhythm in many areas of the Holter despite not being able to visualize P waves. Therefore, although we did not clearly identify atrial ectopy on these Holter recordings, it is possible that we classified some areas of rhythm incorrectly by presuming sinus rhythm where P waves were not discernible. However, no other indicators of supraventricular or atrial ectopy such as prematurity or paroxysmal tachycardia were present, and the Holter recordings were manually reviewed, and thus the likelihood of misclassification is low. The inability to consistently detect P waves could have been related to lead placement and baseline artifact. A limitation in our group classified as healthy goats was the lack of prior cardiac evaluations, although these animals were part of a teaching and research herd and therefore had many years without a history of known cardiac disease. Additionally, heart rate variability calculations could be useful to assess the risk of arrhythmic events and cardiovascular-related deaths [39, 40]. Heart rate variability has been utilized to predict outcome in a variety of critically ill veterinary patients [41, 42]. The large disparity in goat size did not allow for direct comparisons to be made regarding echocardiographic measurements. An additional limitation was the non-standardized cTnI analysis. Because of the timing of the study, two different analyzers were used which might have introduced measurement differences [43]. Although results from different analyzers ideally should not be compared, this situation likely did not have substantial impact on our results because most results were undetectable or low. Additionally, cardiac troponin has a short half-life thus single timepoint data is of lower interpretative value, and it is possible certain animals had higher peak cTnI concentrations than detected, which could have been missed on a single timepoint. Urgent surgical intervention in some cases (dystocia and urolithiasis) prevented us from being able to place 24-h Holter monitors immediately on admission in some goats. The post-operative 24-h Holter recording might have prevented us from detecting arrhythmias that were present when the goat was the most critically ill, although we attempted to mitigate this uncertainty by placing the Holter monitor within 24 h of admission. Unfortunately, our sample size was small and additional studies are needed to further investigate the prevalence of ventricular arrhythmias in both healthy and medically ill goats. Finally, goats enrolled in our study were not representative of the most critically ill goats. Although some of the goats were very ill, some were medically stable on arrival. Our study included a heterogeneous population in regard to the wide age range of goats, variety of small and large breeds of goats, and numerous diseases included. A homogeneous population of very ill goats could yield different results regarding the prevalence of ventricular arrhythmias.
Despite these limitations, we demonstrated that 24-h Holter monitor recordings were well-tolerated with minimal adverse effects. Ventricular arrhythmias occurred in both healthy and medically ill goats. Future studies should be conducted to determine the clinical relevance of arrhythmias in healthy and medically ill goats. Additional studies with a homogenous population of critically ill goats and the ability to detect both supraventricular and ventricular arrhythmias could improve the ability of veterinarians to identify and treat arrhythmias, thereby providing more specialized critical care medicine for goats.
Disclosure
Authors declare no off-label use of antimicrobials.
Ethics Statement
Approved by the Institutional Animal Care and Use Committee of the University of Florida, protocol 202011285. Authors declare human ethics approval was not needed.
Conflicts of Interest
The authors declare no conflicts of interest.




