Thoracic Ultrasonography Findings and Their Association With Respiratory Pathogens in 221 Young Beef Cattle at Fattening Farms: A Cross-Sectional Study
Funding: This research was funded by the SEPTIME project (grant from the Carnot institute France Futur Élevage) and by MSD Animal Health (serology and qPCR funding). F. Meurens is supported by the Natural Science and Engineering Research Council of Canada (NSERC, grant RGPIN-2024-04212).
Gilles Foucras, François Meurens, and Sébastien Assié contributed equally.
ABSTRACT
Background
Thoracic ultrasonography (TUS) could improve antibiotic treatment selection in cattle with respiratory diseases.
Hypothesis/Objectives
Evaluate the association between respiratory pathogens and consolidations on TUS in feedlot cattle, at both individual and group levels.
Animals
A total of 221 bulls, aged 8.8 months and weighing 322.5 ± 160 kg, from nine farms.
Methods
Cross-sectional study including all data from clinical examinations and TUS collected weekly during the first month on feed. Pathogens were assessed by seroconversion (all animals) and qPCR on nasal swabs (sick animals). At the individual level, the association between pathogen detection and TUS consolidation was investigated using univariate logistic regression, and the ability of consolidation size to differentiate bacterial from non-bacterial pneumonia was assessed using receiver operating characteristic curves. Principal component analysis identified clusters at the group level based on pathogen detection and TUS results.
Results
At the individual level, bulls infected with multiple pathogens (odds ratio [OR], 8.1; 95% confidence interval [CI], 2.21–29.8) or a single virus (OR, 5.49; 95% CI, 1.42–21.3) were more likely to have consolidations than those not infected. A total consolidation size > 14 cm2 in the scanned thoracic region differentiated bacterial from non-bacterial pneumonia with a sensitivity of 47.8% (95% CI, 36.4–83.3) and specificity of 94.1% (95% CI, 60.0–100.0). These results were consistent at the group level; clustering based on bacterial versus non-bacterial etiology correlated with the number and size of consolidations.
Conclusions and Clinical Importance
Consolidation size could help differentiate bacterial from non-bacterial pneumonia, guiding treatment at both individual and group levels.
Abbreviations
-
- AUC
-
- area under the receiver operating characteristic curves
-
- BCoV
-
- bovine coronavirus (betacoronavirus gravedinis)
-
- BoHV-1
-
- bovine herpesvirus type 1 (varicellovirus bovinealpha1)
-
- BPI3V
-
- bovine parainfluenza virus type 3 (respirovirus bovis)
-
- BRD
-
- bovine respiratory diseases
-
- BRSV
-
- bovine respiratory syncytial virus (orthopneumovirus bovis)
-
- BTUS
-
- bilateral thoracic ultrasonography
-
- BVDV
-
- bovine viral diarrhea virus (pestivirus bovis)
-
- CI
-
- confidence interval
-
- ICS
-
- intercostal spaces
-
- IDV
-
- influenza D virus (deltainfluenzavirus influenzae)
-
- KV
-
- killed virus
-
- LTUS
-
- left thoracic ultrasonography
-
- MaxAreaC
-
- maximum value of the total consolidation area
-
- MaxNbC
-
- maximum number of regions of interest with a consolidation
-
- MLV
-
- modified-live virus
-
- OR
-
- odds ratio
-
- PABAK
-
- prevalence-adjusted and bias-adjusted kappa
-
- PCA
-
- principal component analysis
-
- ROC
-
- receiver operating characteristics
-
- ROI
-
- region of interest
-
- RTUS
-
- right thoracic ultrasonography
-
- SD
-
- standard deviation
-
- Se
-
- sensitivity
-
- Sp
-
- specificity
-
- TUS
-
- thoracic ultrasonography
1 Introduction
Bovine respiratory diseases (BRD) pose important medical and economic challenges in beef and dairy cattle [1, 2]. They encompass inflammatory and infectious conditions affecting the upper respiratory tract and the lungs. Bovine respiratory diseases often necessitate antibiotic treatment because of the high likelihood of bacterial infection at diagnosis or shortly thereafter [3]. With the growing need to decrease antibiotic use to combat resistance [4], careful selection of animals for treatment is crucial. A laboratory test can guide therapeutic decisions by identifying primary or secondary BRD pathogens, including viruses such as Varicellovirus bovinealpha1 (BoHV-1), Orthopneumovirus bovis (BRSV), Pestivirus bovis (BVDV), Respirovirus bovis (BPI3V), Betacoronavirus gravedinis (BCoV), or bacteria such as Pasteurellaceae and Mycoplasmopsis bovis [5]. However, because treatment often is started before common laboratory test results are available, particularly when the morbidity rate is high, improving on-site techniques such as thoracic ultrasonography (TUS) for selecting which animals to treat is essential [6]. This approach also could be valuable at the group level because it might help refine the criteria for initiating metaphylaxis, which lack consensus [7].
Most studies on TUS, including its ability to distinguish between upper and lower respiratory tract infections, were conducted in pre-weaned dairy or veal calves [8-10], or in beef calves younger than 9 months [11]. Studies involving larger animals, such as feedlot cattle, are less common [12, 13], and their conclusions regarding the utility of TUS for diagnosis are inconsistent [14]. More research is needed to refine TUS performance in these animals. Thoracic ultrasonography has shown promise in identifying the cause of respiratory diseases, as demonstrated by its ability to differentiate between viral and bacterial pneumonia in human pediatric patients based on the size, number, and location of consolidations [15, 16]. This ability raises the question of whether similar applications could be effective in bovine medicine. Indeed, pulmonary lesions such as pleuritis, and the pattern, nature, and intensity of lung lesions at necropsy can vary, depending on the pathogens [17]. However, studies linking TUS findings to specific pathogens in natural BRD outbreaks are scarce [6, 18, 19]. Two studies demonstrated a promising link between bacterial etiology and presence [18] or extension [19] of consolidations on TUS in dairy calves. Another study found no association between lung bacteria and TUS-detected lesions in pre-weaned and weaned calves, instead indicating a high prevalence of non-infectious inflammatory respiratory diseases [6].
Given the increased exposure to BRD pathogens after arrival at fattening farms, in conjunction with stress and impaired immunity, it is critical to further investigate the link between TUS and BRD etiology, because it could help clinicians determine which animals require treatment [20, 21]. Our main objective was to test the association between consolidations on TUS and infections by respiratory pathogens. Our second objective was to determine whether results obtained at the individual level align with those observed at the group level.
2 Materials and Methods
All experiments were conducted in compliance with the French regulation on experimental animal care under the authorization of Oniris Ethics Committee for Clinical and Epidemiological Veterinary Research (authorization on living animals No. CERVO-2022-7-V).
2.1 Study Design and Animals
Following STROBE (STrengthening the Reporting of Observational studies in Epidemiology) guidelines (see STROBE checklist in Supporting Information Materials), a cross-sectional study was conducted on nine fattening farms in Western France from January to December 2023. The recruitment criteria included weaned, intact young beef bulls, purchased from multiple cow-calf farms across France. Fattening farms were selected based on a minimum production of three batches of at least eight bulls and the availability of a handling chute with lateral access and a head gate for TUS exams. Recruitment was organized by the Institut de l'Élevage (Beaucouzé, France) with support from two cooperatives (Bovineo and Terrena, La Ferrière and Mésanger, France).
Young bulls were retrieved from cow-calf farms on Mondays or Tuesdays, and grouped in a sorting facility according to their weight and age, alongside other animals from different origins. At this time, they received vaccines and deworming (with macrocyclic lactones), as decided by the fattener, before being delivered to fattening farms on Thursdays or Fridays. The vaccination program was the same for all animals within a farm but differed among the farms. Two types of vaccines were used: an intranasal vaccine containing modified live virus (MLV) BRSV and BPI3V (Rispoval RS + PI3 Intranasal, Zoetis, Malakoff, France), and a SC vaccine containing killed virus (KV) BRSV, BPI3V, and M. haemolytica (either Bovalto respi 4, Boehringer Ingelheim, Lyon, France or Bovilis Bovigrip, Intervet, Beaucouzé, France). Different protocols were applied: KV vaccine alone (7/9), MLV intranasal vaccine then KV vaccine 10 days later (1/9), or no vaccine (1/9).
2.2 Procedures
On each arrival (Day 0, D0), three batches of 8–12 bulls from the same pen, corresponding to all of the animals arriving at the farm that day or a subset of them (randomly chosen), were examined. For subsequent procedures, to minimize animal handling and time spent by the farmer on restraint, one batch was selected based on the prevalence of sick animals or consolidations on D0, with further examinations on Days 5, 14, 21, and 28 (±1 or 2 days, depending on the farmer's availability). All examinations were carried out by the same veterinarian.
2.2.1 Clinical Examination and TUS
The clinical examinations included rectal temperature (as soon as the bull was caught in the head gate, to prevent stress-induced hyperthermia) and visual BRD signs (e.g., nasal or ocular discharge, coughing, increased respiratory rate, dyspnea, decreased rumen fill, and lethargy). An animal was considered sick if rectal temperature was ≥ 39.7°C, and at least one clinical BRD sign was present.
Thoracic ultrasonography was performed using a 7 MHz linear probe (Draminski iScan Mini, Draminski S.A., Sząbruk, Poland), with a maximal depth of 8 cm. Animals were clipped, and 70% isopropyl alcohol was sprayed as a transducing agent. Depending on restraint conditions on the farm, animals underwent bilateral (BTUS) or unilateral (LTUS and RTUS, respectively left and right) examinations. Because pneumonia lesions typically progress from cranial to caudal regions, TUS was focused only on the most cranially accessible intercostal spaces (ICS). In animals of this age [22], this region corresponds to the fourth and fifth ICS. Each ICS was divided into ventral and caudal portions, separated by the shoulder line, resulting in four regions of interest (ROI) scanned per hemithorax. The largest consolidation (≥ 1 cm2, as described earlier [22]) in each ROI was recorded, along with the number of ROI with consolidation, which correlated with prognosis in a previous study [8]. The total consolidation area for the entire scanned region also was calculated, because it has been used as a prognostic factor [13]. For the entire follow-up period, the maximum value for the number of ROI with a consolidation (MaxNbC), and the maximum value for the total consolidation area (MaxAreaC) were recorded for each animal.
Clinical examination and TUS were performed on at least one-third of the animals (the first to enter the handling chute) in all three batches on D0, providing a uniform overview of the different batches within a limited period. From D5 onwards, procedures were conducted on all animals in the chosen batch, selected based either on clinical signs or consolidations detected by TUS on D0. Otherwise, animals easiest to restrain (based on temperament or location on the farm) were chosen.
2.2.2 BRD Etiology: Infection and Immune Statuses
Two nasal swabs (one in each nostril, using 175 mm long sterile nasal swabs) were collected from one-third of the animals on D0 (the same animals that underwent clinical examination and TUS) to determine nasal carriage of BRD pathogens, being representative of the batch and in order to decrease handling. After collection, the nasal swabs were immediately placed on ice, transported to the laboratory within 3 h, and suspended in 400–600 μL of phosphate-buffered saline, and then frozen at −80°C until analysis. All nasal swabs collected on D0 were analyzed by quantitative polymerase chain reaction (qPCR). Subsequently, if the animal was considered sick (per the study definition) on a subsequent examination day, nasal swabs were repeated and qPCR analyses were performed on swabs taken on the first day the animal was considered sick. The qPCR analyses were conducted within 6 months after freezing at the BIOEPAR laboratory using various commercial kits (BIOTK051, BIOTK052, BIOTK053, BIOTK054, BioSellal, Dardilly, France) to detect Mannheimia haemolytica (M. haemolytica), Pasteurella multocida (P. multocida), Histophilus somni (H. somni), Mycoplasmopsis bovis (M. bovis), BRSV, BPI3V, BCoV, and Deltainfluenzavirus influenzae (Influenza D Virus [IDV]).
On D0 and D28, blood samples were collected by coccygeal venipuncture, allowed to clot at room temperature and then centrifuged at 1000g for 10 min at 4°C. Serum was frozen at −20°C until analysis. Serologies were conducted within 6 months by Intervet International B.V (Boxmeer, Netherlands) using two commercial ELISA kits (BIO K 392/2, BIO K 369/2, BioX Diagnostics, Rochefort, Belgium) to evaluate seroconversion for M. haemolytica, M. bovis, BRSV, BPI3V, and BCoV. As per manufacturer recommendations, seroconversion was defined as a twofold or more increase in the test result for the same animal between D0 and D28. Antibody concentrations of four or five crosses at D0, with five crosses being the maximum value reported by the kits used, were interpreted as “high antibody concentrations at D0.”
The infection status of the animal regarding a BRD pathogen was determined by evaluating qPCR results, antibody concentrations at D0, and the vaccination protocol practiced on each farm (which could influence the seroconversion or qPCR results) [23]. The animals thus were considered either “immune,” “infected,” or “neither immune, nor infected” as defined in Figure 1.
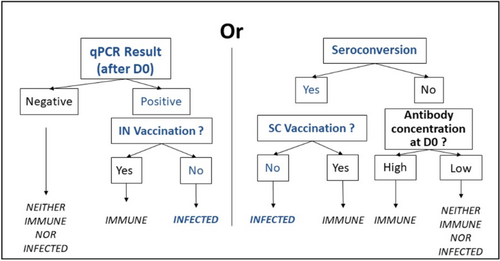
The summarized infection status based on pathogen combinations of each bull was determined by considering the infection status for each of the five pathogens. These combinations then were grouped into four categories to facilitate statistical analyses: no pathogens detected, a single bacterial species detected, a virus detected alone, and multiple pathogens detected.
2.3 Statistical Analysis
The sample size was based on a 30% expected difference in TUS consolidation prevalence between clinically sick (50%) and non-clinical animals (20%), using data from previous studies on animals of similar weight, age, and breed [12, 14, 24]. The α and β errors were set at 0.05 and 0.20, respectively, requiring 38 animals per category. Considering the estimated incidence of BRD in similar animals in the same location according to a previous study [25], the total number of animals to be included was 205.
Statistical analyses were performed using RStudio (RStudio: Integrated Development Environment for R. Posit Software, PBC, Boston, MA, version 2024.4.2.764). The experimental unit was the animal or the batch. Results were considered significant if p < 0.05.
At the individual animal level, three populations were studied: animals that underwent bilateral TUS (BTUS); left TUS (LTUS, including left side data from BTUS animals); and animals that underwent right TUS (RTUS, including right side data from BTUS animals).
All variables included in the statistical analyses were summarized at month level, as mentioned above (cross-sectional study). Descriptive statistics were performed for binary variables (clinical status, treatment, qPCR results on D0, consolidations at least once during the month), ordinal categorical variables (MaxNbC: maximum value of the number of ROI with a consolidation; MaxAreaC: maximum value of the total area of consolidation), and nominal categorical variables (Infection status: “immune,” “neither immune nor infected,” “infected”; Summarized infection status based on pathogen combinations: “no pathogens,” “bacteria alone,” “virus alone,” “multiple pathogens”). The MaxAreaC was divided into four categories, with the boundaries varying depending on whether the animal underwent bilateral (0 cm2; 1–8 cm2; 8–16 cm2; > 16 cm2) or unilateral TUS (0 cm2; 1–4 cm2; 4–8 cm2; > 8 cm2). The thresholds between categories were chosen to ensure homogeneity in group sizes.
To evaluate the impact of performing unilateral TUS, agreement between left and right side consolidations was assessed in BTUS bulls using Cohen's κ test with the prevalence-adjusted and bias-adjusted kappa (PABAK) calculated to account for prevalence bias. Kappa agreement levels were categorized as slight (0 ≤ κ ≤ 0.20), fair (0.21 ≤ κ ≤ 0.40), moderate (0.41 ≤ κ ≤ 0.60), substantial (0.61 ≤ κ ≤ 0.80), and almost perfect (0.81 ≤ κ ≤ 1) [8].
Association between MaxNbC and MaxAreaC, and the clinical status or treatment was assessed using Fisher's exact test.
A univariate logistic regression model including farm as a random effect was fit to assess the effect of the summarized infection status based on pathogen combinations on the risk of having consolidation detected at least once during the month. Multivariate logistic regression models previously attempted, using the infection statuses of the five pathogens as independent predictors, failed because of excessive collinearity. Receiver operating characteristics (ROC) curves and areas under the ROC curves (AUC) were determined to assess the performance of MaxAreaCc (MaxAreaC used as a continuous variable) for differentiating bacterial (at least one bacterial species) from non-bacterial pneumonia (no pathogen detected or only viruses). The optimal threshold, maximizing both sensitivity (Se) and specificity (Sp), was determined and the 95% confidence intervals (CI) were estimated.
Given the contagious nature of BRD pathogens and the mixing of bulls in batches on feedlots, we considered analyzing the data at the batch level. Principal component analysis (PCA) was conducted to identify clusters among the batches, taking into account the percentage of animals in each batch corresponding to different infection statuses for the five pathogens, the percentage corresponding to different MaxNbC values, and the percentage corresponding to different MaxAreaC values.
Animals without serological data or missing information on the results of the clinical examination and TUS at more than one visit, because of sickness (e.g., recumbency) or safety concerns (e.g., aggressiveness) were excluded. Animals with missing swab results while they were sick were not excluded because of the availability of serology results.
3 Results
3.1 Population
Of the 224 animals initially enrolled, three were excluded: two because of death and one for missing serology data on D28.
Thus, 221 young bulls were assessed for the study, with partial data missing for two bulls (one missing clinical and TUS data, and one missing swabbing results). The bulls were Charolais (83%), Limousin (10%), or crossbreeds, with a mean ± SD age of 8.8 ± 1.9 months (range, 4–14 months) and a mean ± SD weight of 322.5 ± 44.1 kg (range, 224–482 kg). Among them, 159 bulls underwent bilateral TUS (BTUS), 24 bulls had unilateral right TUS (183 RTUS bulls in total), and 38 bulls had unilateral left TUS (197 LTUS bulls in total).
3.2 Incidence of BRD and TUS Results in Beef Cattle at Fattening
During the first month of fattening, 47.5% (105/221) of bulls were reported clinically sick at least once, with 29.4% (65/221) sick once and 18.0% (40/221) sick twice or more.
On D0, 3.6% (4/110) of bulls that underwent TUS exhibited at least one consolidation ≥ 1 cm2. Between D5 and D28, their proportion increased to 27.6% (61/221). The maximum area of consolidation (MaxAreaC) and the maximum number of ROI with a consolidation (MaxNbC) observed during the month in BTUS bulls are presented in Table 1, with results for RTUS and LTUS animals in Tables S1 and S2, respectively. Briefly, consolidations were found in 25.2% (40/159) of the BTUS bulls, 23.9% (47/197) of LTUS bulls, and 19.1% (35/183) of RTUS bulls.
| MaxNbC | MaxAreaC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 0 | 1–8 | 8–16 | > 16 | ||
| Health status |
Clinical (n = 71) |
44 | 8 | 12 | 3 | 3 | 1 | 44 | 9 | 10 | 8 |
|
Non-clinical (n = 88) |
75 | 10 | 1 | 2 | 0 | 0 | 75 | 11 | 0 | 2 | |
| Treatment |
Treated (n = 33) |
16 | 4 | 7 | 4 | 1 | 1 | 16 | 4 | 5 | 8 |
|
Untreated (n = 126) |
103 | 14 | 6 | 1 | 2 | 0 | 103 | 16 | 5 | 2 | |
| Total | n = 159 | 119 | 18 | 13 | 5 | 3 | 1 | 119 | 20 | 10 | 10 |
Location of TUS consolidations observed on the left, right, or both sides of the thorax in BTUS animals is given in Table 2. Agreement between sides was substantial (Cohen's κ = 0.62, 95% CI, moderate to substantial, 0.47–0.77). The PABAK value, which accounts for prevalence bias, was higher at 0.76 (95% CI, substantial to almost perfect, 0.66–0.86).
| Right side | ||||
|---|---|---|---|---|
| No consolidation | Consolidation | Total | ||
| Left side | No consolidation | 119 | 3 | 122 |
| Consolidation | 16 | 21 | 37 | |
| Total | 135 | 24 | 159 | |
Clinically sick BTUS bulls had significantly more ROI with consolidation (p = 0.001) and a larger total area of consolidation (p < 0.001) than non-clinical animals. Similarly, the treated animals had significantly more ROI containing a consolidation (p < 0.001) and a larger total area of consolidation (p < 0.001) compared with non-treated animals. These findings were consistent for LTUS or RTUS animals (Tables S1 and S2).
3.3 Individual-Level Analysis of TUS Results and BRD Etiology
On D0, BCoV (67.1%, 55/82) and P. multocida (51.2%, 42/82) were the most common qPCR-detected BRD pathogens, followed by H. somni (8.5%, 7/82), M. haemolytica (6.1%, 5/82), BPI3V (6.1%, 5/82), M. bovis (2.4%, 2/82), and BRSV (2.4%, 2/82). Throughout the study, no animals tested positive for IDV by qPCR.
During the first month of fattening, the most frequent infections were BCoV (21.3%, 47/221), M. bovis (11.8%, 26/221), or their combination (19.5%, 43/221; Table 3). Thus, considering the summarized infection status based on pathogen combinations in BTUS animals, 25.8% (41/159) were negative for all pathogens during the first month, whereas 27.0% (43/159) were infected by a single virus, 13.2% (21/159) by a single bacterial species, and 34.0% (54/159) by any combination of several pathogens.
| Bacteria | |||||
|---|---|---|---|---|---|
| None | M. bovis | M. haemolytica | M. bovis and M. haemolytica | Total | |
| Viruses | |||||
| None |
20.8% (46/221) |
11.8% (26/221) |
1.8% (4/221) |
0.1% (2/221) |
35.3% (78/221) |
| BRSV |
0.1% (2/221) |
0.05% (1/221) |
1.4% (3/221) |
||
| BCoV |
21.3% (47/221) |
19.5% (43/221) |
4.5% (10/221) |
4.5% (10/221) |
49.8% (110/221) |
| BPI3V |
1.4% (3/221) |
2.3% (5/221) |
3.6% (8/221) |
||
| BCoV and BPI3V |
1.8% (4/221) |
2.7% (6/221) |
1.8% (4/221) |
6.3% (14/221) |
|
| BCoV and BRSV |
0.05% (1/221) |
1.4% (3/221) |
1.8% (4/221) |
||
| BPI3V and BRSV |
0.05% (1/221) |
0.05% (1/221) |
|||
| BPI3V and BRSV and BCoV |
1.4% (3/221) |
1.4% (3/221) |
|||
| Total |
45.7% (101/221) |
40.3% (89/221) |
6.8% (15/221) |
7.2% (16/221) |
|
Univariate logistic regression analysis with farm as a random effect showed that summarized infection status based on pathogen combinations was significantly associated with TUS consolidations (p = 0.01) in BTUS animals. Animals infected with multiple pathogens (odds ratio [OR], 8.1; 95% CI, 2.21–29.8) or with a virus alone (OR, 5.49; 95% CI, 1.42–21.3) were more likely to have consolidations compared to those not infected with any pathogens. Such was not the case for animals infected with a single species of bacteria (OR, 2.15; 95% CI, 0.39–11.9). Similar results were found for LTUS (Table S3) and RTUS (Table S4) animals.
The ROC curves (Figure 2 and Figure S1) showed that the optimal cut-off for the maximal area of consolidation to differentiate bacterial (n = 23) from non-bacterial (n = 17) pneumonia in BTUS animals with TUS consolidations (n = 40) was 14 cm2 (AUC, 0.70; 95% CI, 0.53–0.87) with Se = 47.8% (95% CI, 36.4–83.3) and Sp = 94.1% (95% CI, 60.0–100.0). For RTUS animals (n = 35), the cut-off was 8.5 cm2 (AUC, 0.70; 95% CI, 0.52–0.89) with Se = 60.0% (95% CI, 41.7–85.7) and Sp = 90.0% (95% CI, 58.3–100.0), whereas it was 9.5 cm2 (AUC, 0.78; 95% CI, 0.64–0.91) with Se = 64.5% (95% CI, 44.4–90.9) and Sp = 87.5% (95% CI, 61.0–100.0) for LTUS animals (n = 47).

3.4 Group-Level Analysis of TUS Results and BRD Etiology
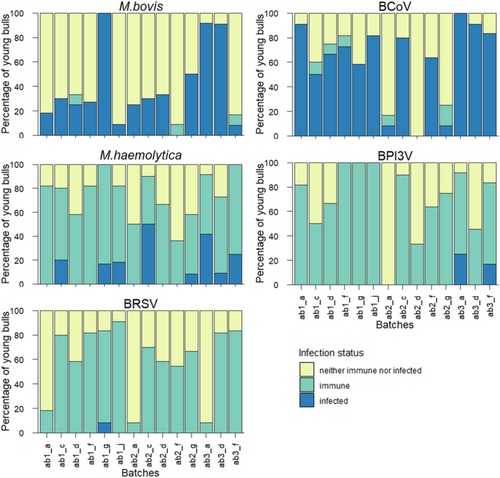
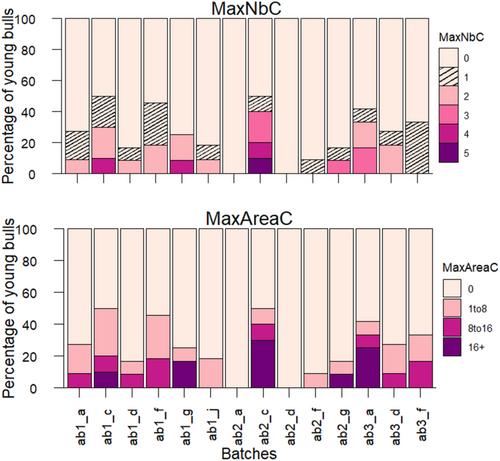
Figures 3 and 4 present the percentage of bulls in each batch corresponding to one of the three infection statuses, and the number and size of TUS consolidations in BTUS animals. Results for LTUS and RTUS animals are presented in Figures S2 and S3.
The PCA identified three clusters across batches based on the definitions and characteristics specified above. The first five principal component axes, each with an eigenvalue higher than one and explaining 82.0% of the total variance, were retained for the analysis. The two most contributing dimensions explained 32.59% and 17.93% of the total variance. Details of the variable contributions are provided in Tables S5 and S6.
The three clusters obtained from hierarchical clustering analysis, along with the variables and their contributive values of the two first dimensions are depicted graphically in Figure 5. Cluster 1 consisted of five batches (ab1_d, ab2_a, ab2_d, ab2_g, and ab2_f) from four different farms (d, a, g, and f). The variables that significantly contributed to defining this cluster were the “neither immune nor infected” infection statuses for three pathogens: M. haemolytica, BCoV, and BRSV, as well as the absence of consolidations on BTUS during the month after arrival. Cluster 2 consisted of seven batches (ab1_a, ab1_c, ab1_f, ab1_g, ab1_j, ab3_d, and ab3_f) from six farms (a, c, f, g, j, and d). The variables “immune” to M. haemolytica, MaxNbC equal to 2, and MaxAreaC between 1 and 8 cm2 contributed the most to the cluster definition. Finally, Cluster 3 contained two batches (ab2_c and ab3_a) from two farms (c and a) and was characterized by the variables “infected” with M. haemolytica, MaxNbC equal to 3, and MaxAreaC greater than 16 cm2. Table 4 summarizes the significant variables contributing to each cluster, along with their mean values.

| v.test | Mean in category | Overall mean | SD in category | Overall SD | p | |
|---|---|---|---|---|---|---|
| Cluster 1 | ||||||
| mh_neither immune nor infected | 3.10 | 46.06 | 25.03 | 10.25 | 18.26 | 0.002 |
| MaxAreaC_0 | 2.85 | 91.52 | 74.24 | 7.46 | 16.27 | 0.004 |
| MaxNbC_0 | 2.85 | 91.52 | 74.24 | 7.46 | 16.27 | 0.004 |
| bcov_neither immune nor infected | 2.64 | 63.94 | 35.18 | 28.55 | 29.31 | 0.01 |
| bpi3v_neither immune nor infected | 2.12 | 52.27 | 29.94 | 27.72 | 28.30 | 0.03 |
| Cluster 2 | ||||||
| MaxArea_1to8 | 2.78 | 19.55 | 12.92 | 6.64 | 8.60 | 0.01 |
| mh_immune | 2.77 | 72.75 | 61.47 | 9.34 | 14.70 | 0.01 |
| MaxNbC_2 | 2.14 | 13.03 | 8.30 | 6.72 | 7.96 | 0.03 |
| Cluster 3 | ||||||
| MacNbC_3 | 3.39 | 18.33 | 3.21 | 1.67 | 6.56 | < 0.001 |
| MaxAreaC_16+ | 3.11 | 27.50 | 6.43 | 2.50 | 9.98 | 0.002 |
| mh_infected | 3.02 | 45.83 | 13.50 | 4.17 | 15.75 | 0.003 |
- Abbreviation: SD, standard deviation.
The PCA comparisons across BTUS, LTUS, and RTUS animals (Figures S4 and S5, and Tables S7 and S8) identified consistent patterns. Briefly, Cluster 1 was characterized by the absence of TUS-consolidations and a “neither immune nor infected” status for the majority of pathogens. Cluster 3 was characterized by numerous and large consolidations, and an “infected” status for at least one of the following pathogens: M. haemolytica (alone for BTUS animals), with BPI3V and M. bovis for LTUS animals, and BPI3V and BRSV for RTUS animals. Even when these “infected” status variables did not significantly contribute to the definition of Cluster 3, they were located in the area of this cluster on the PCA biplots. Finally, Cluster 2 was more variable. For BTUS and LTUS bulls, it was characterized by a moderate number and size of consolidations. For BTUS animals, no significant association was found for any pathogen. For LTUS animals, this cluster also was characterized by an “infected” status for BCoV. For RTUS animals, no variable was significantly associated with this cluster.
4 Discussion
To the best of our knowledge, our study is the first to explore the relationships between TUS consolidations and BRD pathogen detection in young beef bulls. Bulls infected with multiple pathogens were more likely to have consolidations, both at the individual and group level; similar results were observed in animals infected with a single viral agent, at the individual level and in some cases at the group level. As reported in human medicine [15, 16], the size of consolidations, rather than their presence alone, showed promise in differentiating bacterial from non-bacterial pneumonia at both levels. The cut-off values for consolidation size identified in our study to make this distinction should be confirmed by including a larger number of animals exhibiting consolidations on TUS. Interestingly, these values (8.5–14 cm2) are close to those reported previously in a study that found that consolidations exceeding 8 cm2 could predict the relapse rate [13]. These findings align with the fact that bacterial infections in BRD, particularly those involving M. haemolytica and M. bovis, are known to be more difficult to manage than viral infections alone, leading to extensive lung lesions [7, 17-19].
Diagnostic efficacy of TUS has been reported, particularly in pre-weaned dairy calves, with sensitivity and specificity values for detecting animals with lung lesions surpassing those of clinical examination alone [19, 26, 27]. Our study confirmed the diagnostic value of TUS in feedlot cattle [12, 13], because consolidations in the fourth and fifth ICS during the first month of fattening were significantly more frequent in clinically sick or treated animals than in non-clinical animals. Consolidations were detected in 3.6% of the bulls that underwent TUS upon arrival. This finding is consistent with a previous study on young cattle at fattening farms, where no consolidations were detected at arrival [24]. However, it is lower than in other studies on younger and lighter animals under similar transport and grouping conditions, where the proportion of animals with consolidation areas at arrival was approximately 20% and 15% [28, 29]. Additionally, during the first month of fattening, approximately 15% of the “non-clinical” animals had consolidations on TUS, and 62% of the clinically sick animals did not have any consolidations detected. Once again, these results are similar to those observed in a previous study [24]. These results vary from those obtained in dairy calves, where more animals had consolidations and a negative clinical score (46%) and fewer animals had a positive clinical score without consolidations (40%) [8]. Some animals might be falsely considered sick because of isolated upper respiratory tract infections with no consolidation detected [22]. However, the animal format also might lead to false negative results of the TUS examination. In pre-weaned calves, the right cranial lobe, which is the site of early lung lesion development, especially for subclinical pneumonia, is accessible for TUS examination, through the 2nd and 3rd intercostal spaces (ICS) [22]. In contrast, cranial parts of the lungs (on both sides) are nearly impossible to visualize in beef cattle older than 6 months [22, 24]. Therefore, TUS might be a delayed diagnostic tool in heavily muscled animals compared to pre-weaned calves using the above-defined TUS conditions (both ultrasound equipment and accessibility), thus detecting more severe conditions. The association found between consolidation on TUS and infection by multiple pathogens supports this idea. This correlation was evident both at the individual and group levels with Cluster 3 characterized by larger and more numerous consolidation areas and infection by multiple BRD pathogens including M. haemolytica, M. bovis, BRSV, and BPI3V.
The association of a single viral agent such as BCoV and the detection of consolidation was less expected, but it was observed to a lesser extent than infections by multiple pathogens, and at both individual and group levels. Specifically, for LTUS animals, a cluster was significantly characterized by moderate-sized and moderate numbers of consolidations and infection by BCoV. For BTUS animals, this correlation was not significant, but still visible on the PCA biplots, whereas it was absent for RTUS animals. More marked differences were observed for RTUS, BTUS, and LTUS considered together, in terms of farm clusters and explanatory variables. These differences are likely related to the presence of a farm without BRD vaccination in the RTUS group. This farm had more sick animals, infected by more pathogens, and with more consolidations, forming a cluster on its own.
An association between BCoV infection and lung lesions at slaughter has been reported [30], but its role in BRD development remains unclear [5]. The contributing role of BCoV must be considered with caution based on our definition of infection status. Specifically, we considered that seroconversion was induced by vaccination with KV vaccines for BRSV, BPI3V, and M. haemolytica. Similarly, the detection of BRSV or BPI3V in the nasal cavity of bulls vaccinated with MLV was attributed to the vaccination. This strict definition was used to exclude false positive results and to be certain of an infection by a virulent pathogen in animals with positive qPCR results or seroconversion. However, the prevalence of the pathogens for which animals were predominantly vaccinated in our study is likely underestimated compared to the prevalence of the two agents for which vaccination was unavailable at that time, namely M. bovis and BCoV. Nevertheless, the total number of animals considered infected by M. haemolytica (14%), BRSV (5%), and BPI3V (12%), as well as those considered infected by M. bovis (48%) and BCoV (59%), were similar to the number of feedlot cattle positive by deep nasal swabbing 4 days after arrival in another study (M. haemolytica: 16%; BRSV: 9%, BPI3V 2%; M. bovis: 46%; BCoV: 65%) [21]. The choice to use nasal swabs instead of guarded nasopharyngeal swabs, bronchoalveolar lavages, or transtracheal washes was made for practicality, considering the handling and time constraints. This sampling method reflects what is commonly done in field conditions for such animals. However, this time, it might have led to an overestimation of the detection of Pasteurellaceae because these bacteria are commensal bacteria of the nasal cavity [31]. Nevertheless, a study in feedlot cattle showed that the detection of Pasteurellaceae by qPCR on nasal swabs was highly consistent with its detection on deep guarded nasal swabs, although its isolation on nasal swabs was more frequent [32]. Another study on sick dairy calves demonstrated an excellent correlation between the detection of M. haemolytica and M. bovis by culture in the nasal cavity and their detection in the lungs. The agreement between sample methods was less consistent for viruses because BRSV was isolated less frequently from the nasal cavity than in the lungs, whereas the opposite was observed for BCoV [33]. In our study, issues related to the underestimation of pathogen detection using nasal swabs might have been mitigated by including seroconversion data. The potential over-detection, particularly of BCoV, means its role should be interpreted with caution, as previously mentioned.
Another limitation might be the method used to perform TUS, focusing on the two most cranial accessible ICS (fourth and fifth ICS) to save time. As a result, more caudal consolidations might have been missed. However, a previous study concluded that focusing TUS on the fourth and fifth ICS is a good alternative to a complete TUS, particularly when the prevalence of consolidations is below 30%, which was the case in our study [34]. Similarly, results from farms where TUS could only be performed on one side because of restraint conditions are presented, even though the agreement between the two sides was not perfect (0.76). This choice was made to provide results that are applicable to the various conditions encountered on commercial farms.
Finally, animals were selected by convenience on D0 to perform TUS or nasal swabs, which is another limitation of the study, particularly regarding generalization of the results. However, we examined one-third of the animals from each of the three batches, that is, animals raised under the same conditions (same barn, same pen), which allows for a representative sample of the herd structure at that time [35]. Similarly, batch selection at D5, particularly when based on the presence of sick animals or TUS consolidations on D0, might have led to an increased number of sick animals or animals having consolidations on TUS during the study. However, these numbers are similar to those observed in other studies on comparable animals, as previously mentioned. This choice was made consistent with the study's objective, which was to explore the relationships between the consolidations on TUS and the respiratory pathogens, rather than to establish the prevalence of BRD or TUS consolidations in young French bulls at fattening farms.
In conclusion, TUS in young beef cattle during the first month of fattening could provide valuable insights into BRD etiology, particularly by considering consolidation size. In our study, larger consolidations, both at the individual and group levels, were indicative of bacterial involvement. This approach could guide the sampling strategy of sick animals for laboratory analyses (sample animals with small consolidations to identify the initiating pathogens for prevention, or those with large consolidations for bacteriology and antibiogram) or justify the use of antibiotic treatment, either individually or in batches. These results should be confirmed, and their application to younger animals remains to be investigated, primarily because access to more cranial ICS in these animals might alter the findings and allow detection of earlier BRD stages. Additionally, the farming context, including transport-related stress, impaired immunity, and the circulation of pathogens upon arrival at the fattening farm, is quite specific to this production sector. Although these findings might not be directly translated to all categories of animals (pre-weaned calves or adult cattle), further research is needed to explore their relevance in different age groups and farming contexts.
Acknowledgments
The authors acknowledge the participating farmers, the cooperatives Bovineo and Terrena, and the Institut de l'Élevage (Carole Toczé), as well as the seven veterinary students who assisted with data collection in the field (Louis Anthore, Alison Boffelli, Juliette Divet, Charlotte Giraud, Marianne Guy, Lucile Hannappe, and Emilie Rochet). The authors express their gratitude to Emmanuelle Blandin, Anne-Sophie Noël and Intervet International B.V laboratory for their valuable technical work, and to Nadine Brisseau and Anne Lehebel for their help with statistical analysis. This research was funded by the SEPTIME project (grant from the Carnot institute France Futur Élevage) and by MSD Animal Health (serology and qPCR funding). F. Meurens is supported by the Natural Science and Engineering Research Council of Canada (NSERC, grant RGPIN-2024-04212). No funder was involved in the scientific or editorial decisions made for this study.
Disclosure
Authors declare no off-label use of antimicrobials.
Ethics Statement
Authors declare no institutional animal care and use committee or other approval was needed. Authors declare human ethics approval was not needed.
Conflicts of Interest
The authors declare no conflicts of interest.




