Apoptosis Inhibitor of Macrophages in Cats: A Potential Link Between an Exon 3 Variant Allele and Progression of Naturally Occurring Chronic Kidney Disease
Funding: Gabriela C. L. Evangelista was supported in part by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brasília, DF, Brazil—#200552/2022-8), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brasília, DF, Brazil—Financial code 001), and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG, Belo Horizonte, MG, Brazil). Dr. Villarino kidney research program is supported by the Washington State University College of Veterinary Medicine Kay Yarborough Nelson Distinguished Professor in Feline Health Endowment, a generous gift from Mary Kay Fowler, Washington State University intramural grants program and Washington Research Foundation.
ABSTRACT
Background
The protein apoptosis inhibitor of macrophages (AIM) is involved in kidney repair. An AIM (fAIM) genetic variant in cats resulting in a domain duplication might abrogate fAIM's protective effect on kidney function.
Objectives
To confirm that the domain duplication previously described in fAIM results from an exon duplication at the genomic level and to determine if cats with chronic kidney disease (CKD) harboring the variant fAIM allele are at higher risk for a decline in renal function relative to fAIM wild-type cats.
Animals
Medical records (n = 172) and genomic DNA samples (n = 100) from cats presented to Washington State University and having a diagnosis of CKD were analyzed.
Methods
Sequencing and PCR were used to determine fAIM genotype. Based on serum creatinine (SCr) concentrations, cats were phenotyped according to IRIS CKD staging. The phenotype–genotype association was tested using Fisher's exact test.
Results
The 4-domain variant of fAIM was confirmed to be a result of exon 3 duplication and is present in 62% of the DNA samples from cats. Medical records of cats (n = 50) with CKD met the inclusion criteria. Cats homozygous for the exon 3 fAIM variant allele are more likely to have worse IRIS stage (p = 0.01) and more likely to have experienced renal function deterioration (increasing SCr concentration) than fAIM wild-type cats (p = 0.03).
Conclusions and Clinical Importance
The fAIM homozygous variant genotype is associated with declining kidney function in cats with CKD, presumably from deficient fAIM-mediated renal tubular repair.
Abbreviations
-
- AIM
-
- apoptosis inhibitor of macrophages
-
- BUN
-
- blood urea nitrogen
-
- CKD
-
- chronic kidney disease
-
- CNV
-
- copy number variation
-
- ddPCR
-
- droplet digital PCR
-
- E3×2
-
- 2 copies of exon 3 of fAIM
-
- E3×3
-
- 3 copies of Exon 3
-
- E3×4
-
- 4 copies of Exon 3
-
- fAIM
-
- feline apoptosis inhibitor of macrophages
-
- gDNA
-
- genomic DNA
-
- KIM-1
-
- kidney injury molecule 1
-
- SCr
-
- serum creatinine concentration
-
- SDMA
-
- symmetric dimethylarginine
-
- SPB
-
- systemic blood pressure
-
- SRCR
-
- scavenger receptor cysteine-rich
-
- T4
-
- serum T4 concentration
-
- UPC
-
- urine protein to creatinine ratio
-
- USG
-
- urine specific gravity
1 Introduction
Chronic kidney disease (CKD) in cats is characterized by progressive loss of multiple functions within the kidney [1-3]. Chronic kidney disease is caused by multiple insulting factors and initiated in different renal structures such as the renal tubules. Renal repair mechanisms are initiated almost immediately within the kidney after a renal insult. Tubular damage results in the accumulation of intratubular cellular debris [4, 5]. This debris is subsequently and rapidly cleared by phagocytic tubular epithelial cells as part of the normal renal repair response to ensure the continued flow of filtrate [5]. Failure to remove debris leads to the death of the associated nephron and irreversible, insidious interstitial fibrosis (maladaptive repair) with associated progressive decline in kidney function [5]. Renal fibrosis is inversely correlated with glomerular filtration rate (GFR); as interstitial fibrosis increases, GFR decreases [6]. One of the hallmarks of kidney disease is a decrease in GFR. Serum creatinine concentration (SCr) is routinely used as a proxy for GFR for assessing renal function [7].
The clinical course and long-term prognosis of CKD in cats are highly variable among individual cats [8, 9]. After a renal insult and initial decrease in GFR, filtration function might improve in some cats or at least remain stable. In contrast, it might continue to deteriorate in other cats over time, resulting in progressive CKD [8, 9]. The reason why renal filtration function improves in some animals with CKD but not in others remains unclear, but it is increasingly apparent that genetics influences the outcome of several kidney lesion repair processes and, thus, the pathogenesis of CKD [10, 11]. However, studies evaluating the intersection between genotype and CKD in cats remain largely unexplored.
Apoptosis inhibitor of macrophages (AIM) facilitates kidney repair in humans and mice [5, 12, 13]. Mice that are AIM-deficient (AIM−) fail to repair normal renal parenchyma and exhibit progressive renal dysfunction [13]. Cats can synthesize a wild-type or variant feline AIM (fAIM) protein or both. The wild-type fAIM protein contains three scavenger receptor cysteine-rich (SRCR) protein domains, whereas the variant contains 4-SRCR domains, resulting from duplication of the SRCR1 domain [12, 13].
Genes are organized in exons and introns (Figure 1). The DNA sequence is transcribed into messenger RNA (mRNA), a process that requires splicing out the introns. The resulting mRNA contains a sequence of exons carrying the nucleotide sequence that codes for amino acids and proteins. In humans and mice, the fAIM mRNA sequence consists of six exons that code for a 37 kDa three-domain AIMa protein. Cats uniquely express the three-domain 37 kDa and a 45 kDa four-domain fAIM [5, 12]. The SRCR1 protein domain is coded by exon 3. Based on the mRNA structure in cats [5], the duplicated SRCR1 can result from duplication of exon 3 at the gene level, but this possibility remains to be confirmed.
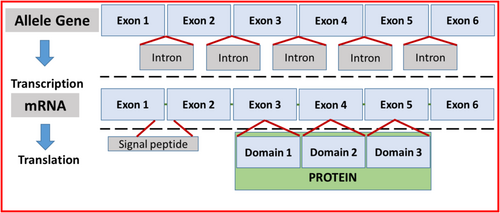
Whether the additional SRCR1 domain alters any or all of the core functions of AIM (e.g., attachment to debris, engulfment by phagocytes and association with or dissociation from IgM) remains to be determined, but it is reasonable to postulate that the predicted structural difference between wild-type AIM and the variant would cause functional differences. Thus, considering the role of wild-type fAIM in normal kidney repair and the marked alteration in the variant fAIM protein structure, we hypothesized that cats variant for the exon 3 allele of fAIM might experience impaired kidney lesion repair, which could be reflected in a progressive decline of kidney function. Our objectives were to confirm that the domain duplication previously described in fAIM results from exon duplication at the genomic level and to determine if cats with CKD harboring the variant fAIM allele are at higher risk for decline in renal function relative to fAIM wild-type cats.
2 Materials and Methods
2.1 Study Design
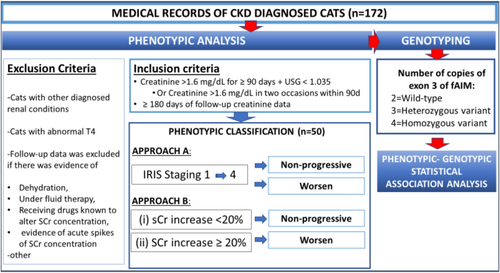
The study was carried out in four stages. First, DNA samples from cats collected from a study completed at Washington State University (WSU; protocol #4915) were screened to identify those with each fAIM exon 3 variant: homozygous wild-type, homozygous variant, or heterozygous (results not shown). Then, the fAIM gene in samples from cats identified as carrying 2, 3, and 4 copies of exon 3 were sequenced as described below. Second, medical records from client-owned cats admitted to the Veterinary Teaching Hospital at WSU were classified phenotypically following the inclusion and exclusion criteria described below (Figure 2). Third, archived (PrIMe DNA Bank-IACUC protocol #6493) genomic DNA from those cats meeting the inclusion criteria were classified as homozygous for the wild-type (2 copies of exon 3) and variant (3 and 4 copies of exon 3, respectively) alleles of the fAIM gene using droplet digital PCR (ddPCR). One of the researchers (NFV) determined the final list of CKD cases included in the study and remained blinded to the genotype. Lastly, a subset of archived genomic DNA from the PrIMe DNA Bank feline samples (IACUC protocol #6493) was genotyped using ddPCR to obtain preliminary information about the frequency of the variant in the cat population.
2.2 Genomic DNA Isolation
Genomic DNA from feline samples was isolated from cheek swabs using Quick-DNA miniprep kit (Zymo Research, Irvine, WA, USA) following the manufacturer's protocol. After extraction, the eluted DNA concentration was determined using Qubit fluorometric quantification following the manufacturers' instructions (Invitrogen, Carlsbad, CA, USA). The DNA samples were stored at −80°C until used.
2.3 Sequencing of Feline Apoptosis Inhibitor of Macrophage Gene (fAIM) Variants
Genomic DNA from archived feline samples (IACUC protocol #4915) was used for sequence studies. The complete DNA sequence of fAIM was amplified for PacBio sequencing, employing the following PCR parameters: a single cycle at 94°C for 30 s, followed by 30 cycles at 94°C for 30 s and 65°C for 750 s, with a final extension at 65°C for 10 min. In each PCR reaction, approximately 50 ng of genomic DNA template, 1× LongAmp Taq reaction buffer, 0.4 μM of each primer (Fa2 5′ CTGCAGACAAGACCTCTGTC 3′ and Ra1 5′ CAAGGCGTAGGCAGAGTTCAG 3′), 0.3 mM dNTPs, 5% dimethylsulfoxide (DMSO; Sigma–Aldrich, St. Louis, MO, USA), 2.5 U of LongAmp Taq DNA polymerase (New England Biosciences, Ipswich, MA, USA), and 10 μL of nuclease-free water were added for a final reaction volume of 25 μL.
Post-PCR amplification, the product was visualized on a 0.8% agarose/Tris acetate-EDTA (TAE) gel containing 0.5 μg/mL of ethidium bromide. Upon observing a PCR band of the expected size (approximately 11 kb), the PCR product was purified using 0.8× Ampure XP magnetic beads (Agencourt Biosciences, Beverly, MA, USA); product quality was assessed on the Agilent 5200 Fragment Analyzer (Agilent, Santa Clara, CA, USA) and subjected to PacBio sequencing at the WSU Laboratory for Biotechnology and Bioanalysis.
For the preparation of the PacBio SMRTbell library, the PCR products of 10–15 kb were employed by utilizing the Pacific Biosciences SMRTbell template preparation kit 1.0 (Pacific Biosciences, CA, USA) according to the manufacturer's instructions. A separate library was created for each amplicon, and purification was done using 0.8× Ampure XP magnetic beads (Agencourt Biosciences, Beverly, MA, USA). These purified libraries subsequently were sequenced using the P6C4 sequencing chemistry on the RS II platform, with a 240-min movie time and one SMRT cell per library.
The sequence analysis was carried out using the Pacific Biosciences SMRT Analysis software suite for long amplicon analysis and hierarchical genome assembly process (HGAP), resulting in polished amplicon sequences. Further sequence analysis was performed using Lasergene v. 14 (DNAstar, WI, USA).
2.4 Simulation of the Molecular Structure of 3-SRCR and 4-SRCR Domain Variants of fAIM and Estimation of Their Physicochemical Parameters
The nucleotide sequence for both the 3-SRCR and 4-SRCR domain variants of fAIM was converted to amino acid sequence using EditSeq in Lasergene v. 14 (DNAstar Madison, WI, USA). The corresponding amino acid sequences can be found in Supplemental information (Table S1). Initially, the amino acid sequence's signal was determined using deep neural networks as implemented through SignalP 5.0 (http://www.cbs.dtu.dk/services/SignalP/index.php) [14]. Utilizing this signal sequence information, the mature protein sequences and the predicted tertiary structures for both fAIM proteins were modeled using deep learning algorithms on the RaptorX server (http://raptorx.uchicago.edu/) [15, 16]. The predicted tertiary structure images were regenerated using UCSF Chimera v1.14 (https://www.cgl.ucsf.edu/chimera/download.html) [17, 18].
2.5 Case Selection
Medical records of cats presented to the Veterinary Teaching Hospital at WSU and diagnosed with CKD between 2011 and 2022 were screened for inclusion in the study (Figure 2). Records that indicated both a diagnosis of CKD and archived DNA were pre-selected. The following data were extracted from medical records: age at diagnosis of CKD, sex, reproductive status, breed, body weight, SCr, blood urea nitrogen concentration (BUN), urine specific gravity (USG), and serum thyroxine concentration (T4).
Cases were eligible for inclusion if they met the following criteria: (1) SCr ≥ 1.6 mg/dL together with a low USG (< 1.035) [19], or SCr ≥ 1.6 mg/dL on two occasions a minimum of 3 months apart [19], and (2) follow-up data for more than 6 months. The follow-up period was defined as the interval between the date of CKD diagnosis (designated baseline) and the date of the last available SCr (designated endpoint).
Cases were excluded if follow-up data was available for less than 6 months. Cats with other diagnosed renal conditions (e.g., ureteral obstruction, pyelonephritis, renal lymphoma, polycystic kidney disease, and subcutaneous ureteral bypass placement) were excluded. Cats with diagnosed hyperthyroidism were ineligible if T4 concentration was outside of the reference concentration range (1–4 μg/dL) during the follow-up period. Cats under treatment for hyperthyroidism with stable T4 concentrations within the reference concentration range (1–4 μg/dL) were not excluded. Follow-up data was not included if dehydration was reported. Serum creatinine concentration data from cats receiving fluid therapy or drugs suspected to alter SCr (e.g., doxorubicin, carboplatin, nonsteroidal anti-inflammatory drugs, gentamicin), or with a history compatible with an episode of acute disease in a patient with CKD, were excluded from the analysis.
2.6 Classification of Cases Based on Renal Function
Assessment of renal function was based on SCr reported in the medical records during the follow-up period. We used two approaches for phenotypic classification and analyzed them independently (Figure 2).
2.6.1 Approach A: International Renal Interest Society (IRIS) Staging
Cats were categorized based on the 1–4 International Renal Interest Society (IRIS) staging criteria [19]. Cats were assigned an IRIS stage at the beginning and end of the follow-up period. Kidney function was considered worse if there was an increase in stage severity at the end of the follow-up period compared with the baseline. Otherwise, a CKD cat was considered non-progressive.
2.6.2 Approach B: Percentage Change in SCr From Baseline
The percentage SCr change was estimated based on a ratio of endpoint and baseline SCr. If cats had an increase in SCr > 20% at the endpoint relative to the SCr at baseline, cats were classified as having declining kidney function (worsening). Otherwise, cats were classified as non-progressive.
2.7 Determination of fAIM Exon 3 Copy Number
After assessment of kidney function, fAIM exon 3 copy number variation was determined using archived (PrIMe DNA Bank (IACUC protocol #6493)) genomic DNA samples from CKD cats that met the inclusion criteria.
Exon 3 copy number variation (CNV) was determined using ddPCR. Approximately 100 ng of DNA was combined with a reaction mixture composed of 2× Bio-Rad ddPCR mix and 20× primer and TaqMan probe mixes for fAIM exon 3 as the region of interest (HEX) and an internal (exon 5 of fAIM; exon 5) and external (exon 3 of feline telomerase reverse transcriptase; TERT) reference gene (FAM). Then, droplets were generated in a droplet generator (QX200, Bio-Rad, USA) by loading the reaction mixture containing the genomic DNA (gDNA) sample into DG8 reaction cartridges (Bio-Rad, USA) with 70 μL droplet generation oil (Bio-Rad, USA). After droplet generation, droplets were transferred to a 96-well PCR plate (Bio-Rad, USA). The PCR amplification was performed in a T100 Touch thermal deep-well thermocycler (Bio-Rad, USA) with the following parameters: 96°C for 10 min followed by 45 cycles of 96°C for 15 s and 60°C for 2 min, and finally 96°C for 10 min and a 4°C infinite hold, all with a 2°C/s ramp rate. The PCR plate was cooled to room temperature before loading the plate in the QX200 digital droplet reader (Bio-Rad, USA).
Fluorescence data were acquired and analyzed using QuantaSoft Analysis Pro Software version 1.05.596 (Bio-Rad, USA) following manufacturer recommendations. Copy number variation determination involves quantification of target and reference loci (TERT or exon 5 for our study). The ddPCR software counts positive and negative droplets for each of the targets (exon 3 in fAIM gene and each of the reference genes), estimating the average CNV of exon 3 per droplet. The software was set to calculate the copy number automatically based on input data. Each study sample was run in combination with both of the reference loci once.
2.8 Frequency of fAIM Exon 3 Genotypes From Archival DNA
The frequency of cats with 2, 3, and 4 copies of exon 3 of fAIM was determined using 100 archived DNA samples from the PrIMe DNA Bank. A subset of samples was selected from > 1500 banked samples by simple randomization. Genotyping for fAIM exon 3 CNV was determined using ddPCR as described above.
2.9 Statistical Analysis
Age at diagnosis, duration of follow-up period, SCr at baseline and endpoint, percentage change in SCr from baseline, BUN, and body weight for the cats were evaluated descriptively and compared statistically. Shapiro–Wilk and Brown–Forsythe tests were used to verify normality and the homogeneity of variances, respectively. An unpaired t-test was used to compare variables at the baseline or endpoint for cats within progressive and non-progressive groups. Mann–Whitney's test was performed if the data was not normally distributed. A paired t-test was used to compare body weight and SCr at baseline and endpoint within each group. Spearman's correlation coefficient was used to investigate the correlations between body weight and SCr. The strength of the association between fAIM exon 3 CNV and phenotype (IRIS stage progression and SCr worsening) was determined by calculating the odds ratio (OR) and tested statistically using Fisher's exact test. The OR and 95% confidence interval (CI) were calculated as described previously [20]. Statistical analyses were performed using Microsoft Excel and SigmaPlot version 14.0 (Systat Software Inc., California). For all tests, p ≤ 0.05 was considered significant.
3 Results
3.1 Sequencing of Feline Apoptosis Inhibitor of Macrophage (fAIM) Gene
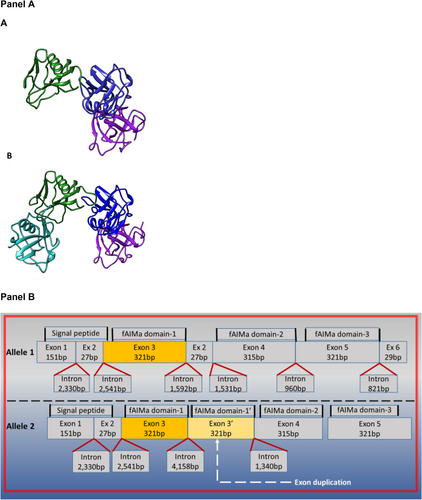
To ascertain the genomic status and provide a comprehensive understanding of exon 3 structure, three feline gDNA samples were subjected to long-read sequencing. These samples, previously identified with 2, 3, and 4 copies of exon 3 by qPCR screening (data not shown), underwent precise examination. A summary of the fragment analyzer run is included in the Supporting Information (Figure S1). Fragment analysis of the fAIM PCR product indicates three amplicon sizes between approximately 10 and 12.5 kb pairs (Figure S2). In the sample with approximately 10.4 kb pairs, each allele contained an exon 3 of 321 base pairs, indicating a homozygous wild-type gene (Figure S2, Panel A). For the sample with approximately 12.5 kb pairs amplicon, both alleles contained 2 copies of exon 3 of 321 base pairs, reflecting a homozygous state for the mutant allele (Figure S2, Panel B). The sample with both size amplicons contains an allele with an exon 3 and the other allele with 2 copies of exon 3, indicative of a heterozygous state for the mutant allele (Figure S2, Panel C). Exon 3 copies exhibited a homology range of 96.2%–100%. The altered fAIM gene consists of a large novel intragenic tandem exon 3 and flanking intron duplication of the fAIM gene (Figure S2, Panel A, the intron size is approximately 2.5 kb pairs). The wild-type fAIM gene is composed of six exons (exons 1–6; Figure S2, Panel A). Detailed maps of fAIM introns and exons for both wild-type and mutant alleles are provided in Figure S2, Panel B.
3.2 Simulation of the Molecular Structure of 3-SRCR and 4-SRCR Domain Variants of fAIM and Estimation of Their Physicochemical Parameters
The copy number variation of feline exon 3 results in an additional SRCR domain 1 in the protein structure (Figure 3A,B). Thus, affected cats express a 4-SRCR domain variant of fAIM. To explore the potential impact of the extra protein domain on the protein's physicochemical features, we modeled in silico the molecular structure of 3-SRCR and 4-SRCR domain variants of fAIM and estimated their physicochemical parameters. A protein simulation model for both protein variants is shown in Figure 3, Panel A and B. Amino acid sequences for 3-SRCR and 4-SRCR domain fAIM variants are presented in Table S1. The 4-SRCR domain protein variant results in a duplication of the first domain, resulting in a larger, more negatively charged, and less water-soluble protein than the wild-type 3-SRCR domain fAIM protein (Table S2).
3.3 Phenotypic Case Selection
Initially, 172 medical records of cats diagnosed with CKD during the designated time period were identified. Ninety-one of these also had archived DNA, and thus, only those medical records were reviewed. Fifty cases met the inclusion criteria (Table S3). Twenty cases had SCr ≥ 1.6 mg/dL and USG < 1.035 at diagnosis, and 30 had SCr ≥ 1.6 mg/dL at least twice ≥ 3 months apart. Forty-one cases were excluded because follow-up data duration was < 6 months (n = 24), other renal conditions were present (n = 6), T4 concentrations were abnormal (n = 6) or clinical information was incomplete (n = 5, Table S4).
The most common breeds represented were domestic short hair (29/50; 58%), domestic long hair (7/50; 14%), and Siamese or Siamese-mix (6/50; 12%). Other breeds were Bengal (2/50; 4%), Ragdoll (2/50; 4%), Bombay (1/50; 2%), domestic medium hair (1/50; 2%), Egyptian Mau (1/50; 2%), and Persian (1/50; 2%; Table S3). Of 50 cats, 28 (56%) were neutered males, 21 (42%) were spayed females, and 1 (2%) was an intact male (Table 1). Age of cats at CKD diagnosis ranged from 8 to 15 years for all cats, and no significant difference was found in age at diagnosis between cats with progressively increased SCr versus those that remained non-progressive (p = 0.90, Table 1).
| Cats classified as worsen CKD | Cats classified as non-progressive CKD | p | |
|---|---|---|---|
| n | 24 | 26 | |
| Sex |
7 FS 16 MC 1 M |
14 FS 12 MC |
p = 0.08 |
| Age on diagnosis (years) | 13 (8.0–15) | 12 (9.0–15) | p = 0.90 |
| Follow-up period (months) | 39 (18–70) | 28 (16–46) | p = 0.35 |
| Weight on baseline (kg) | 4.6 (4.0–5.3) | 5.0 (3.9–6) | p = 0.28 |
| Weight on endpoint (kg) | 3.9 (3.5–4.8) | 4.1 (3.4–5.3) | p = 0.63 |
| sCr on baseline (mg/dL) | 1.9 (1.7–2.1) | 2.1 (1.9–2.7) | p = 0.06 |
| sCr on endpoint (mg/dL) | 2.8 (2.6–3.7) | 1.8 (1.6–2.5) | p < 0.001* |
| Change of sCr (%) | 58 (29–91) | −12 (−23–1.5) | p < 0.001* |
| BUN on baseline (mg/dL) | 31 (26–44) | 28 (24–37) | p = 0.26 |
| BUN on endpoint (mg/dL) | 52 (45–81) | 37 (27–42) | p < 0.001* |
- Abbreviations: FS, females spayed; M, male intact; MC, males castrated.
- * Significantly different (p < 0.05).
3.4 Classification of Patients According to Renal Function
3.4.1 Approach A: IRIS Staging
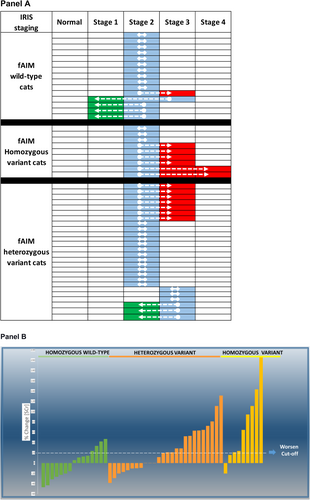
Cats were classified as IRIS stage 2 (n = 43) and stage 3 (n = 7) at baseline. Most of the cats (n = 29) remained in the same IRIS stage at the end of the follow-up period. Twelve (24%) cats progressed from stage 2 to 3 and two cats from stage 2 to 4. Four cats changed from IRIS stage 2 to 1 and two cats from IRIS stage 3 to 2 (Figure 4A and Table S3).
3.4.2 Approach B: Percentage Change in SCr From Baseline
Twenty-four cats had progressively worsened in the SCr, whereas the remaining cats remained non-progressive (n = 26; Figure 4B and Table S3). No correlation was found between body weight and SCr (p > 0.05). Results for SCr and BUN changes are presented in Tables 1 and S3.
3.5 Number of Copies of fAIM Exon 3
Fifteen of 50 (30%) cats were homozygous for the wild-type allele of fAIM, and 35 of 50 (70%) cats were variant-type (> 2 copies of exon 3, Table S3). Within the fAIM variant group, 26 of 50 (52%) cats were heterozygous for the variant allele of fAIM (3 copies of exon 3), whereas 9 (18%) cats were homozygous for the variant allele of fAIM (4 copies of exon 3, Table S3).
3.6 Association Between Copies of fAIM Exon 3 and Change in SCr
A significant association was found between fAIM exon 3 copy number variation and IRIS staging change (Figure 4, Panels A and B, Table S2). Cats with 4 copies of exon 3 of fAIM had higher odds of worsening (p = 0.01) IRIS stage (approach A) and SCr than cats with wild-type fAIM exon 3 (approach B, p = 0.03, Table 2).
| Genotype | Phenotype | |
|---|---|---|
| Worsen IRIS stage vs. non-progressive IRIS stage | Worsen Serum creatinine concentration vs. non-progressive SCr | |
| Homozygous + heterozygous variant vs. wild-type |
7.3 (0.85 to 62) p = 0.07 |
3.66 (0.97 to 13) p = 0.05 |
|
Homozygous variant vs. wild-type |
17 (1.55 to 196) p = 0.01 |
9.6 (1.38 to 67) p = 0.03 |
3.7 Frequency of fAIM Exon 3 Genotypes in a Subset of DNA Samples From WSU DNA Bank
Of the selected 100 banked feline DNA samples, 38% were homozygous for the wild-type fAIM exon 3 allele, 20% of the cats were homozygous for the variant fAIM exon 3 allele, and the remaining 42% were heterozygous.
4 Discussion
We determined that some cats harbor an altered fAIM gene consisting of a large intragenic tandem exon 3 and flanking intron duplication of the fAIM gene (Figures 3, Panel B and S2). Duplication of exon 3 can occur in one or both alleles of fAIM, resulting in three genotypes: those homozygous for the wild-type fAIM exon 3 allele, those homozygous for the variant fAIM exon 3 allele, and heterozygotes. The wild-type fAIM gene codes for a protein consisting of three cysteine-rich domains referred to as scavenger receptor cysteine-rich domains (SRCR1, 2, 3, Figure 3, Panel B) [5]. On the other hand, the duplication of exon 3 leads to the duplication of the SRCR protein subunit 1 (Figure 3, Panels A and B).
The most clinically relevant result of our study is that the fAIM genotype is associated with deteriorating renal function (Table 2). Most of the cats with CKD (83%, 20/24, Table S3) that showed a progressive decline in kidney function were cats in the population with the variant fAIM genotype (Table 2 and Figures 3 and S2). The altered structure of the fAIM variant protein might affect its function in vivo. In a previous study [5], one of many functions of fAIM was investigated [21] and it was shown that both the wild-type and mutant fAIM variants support comparable macrophage phagocytic capability in vitro. However, the authors did not investigate the differences in pro-repair renal function of the wild-type and variant fAIM proteins in vivo.
Based on computer simulations, we identified potential physicochemical differences between the fAIM protein variants (Table S2). The 4-SRCR domain variant of the fAIM protein (variant protein resulting from an exon 3 duplication) is not only larger than the wild-type 3-SRCR domain protein, but the extra domain also increases the overall negative charge. Because negative charge hinders the renal filtration of molecules [22], a larger and more negatively charged variant fAIM might not be filtered into the renal tubules (its site of action) as efficiently as wild-type fAIM, thereby compromising its pro-repair function in fAIM variant cats. Comparing fAIM urine concentrations of genotyped cats would provide valuable insight into potential glomerular filtration impairment. Alternatively, the larger fAIM variant might reach its sites of action in the tubular lumen, but the additional SRCR domain might sterically hinder its interaction with ligands (e.g., kidney injury molecule-1 or cluster differentiation 36) in vivo [5, 23-25]. Although these possibilities have yet to be investigated, they offer plausible mechanisms consistent with our findings.
In our study, 50% of the heterozygous cats showed a ≥ 20% increase in SCr at the end of the follow-up period with respect to baseline. This finding could be a result of differential gene expression leading to the synthesis of the wild-type fAIM protein only or higher proportional quantities of wild-type fAIM than variant fAIM protein. Considering the complexity of renal disease, other explanations not involving fAIM could impact our results, but the specific explanations have yet to be identified. Notably, findings from a recent study provide a possible explanation for the worsening of IRIS stage in wild-type cats [26]. The study showed that a high salt diet prevents AIM activation, precluding its renal function [26]. Our study was not designed to evaluate the impact of diet on the effect of wild-type and variant fAIM on CKD cats, but this aspect deserves attention in future research.
Our research suggests a role for the fAIM genetic variant with respect to renal function (as estimated by SCr) in cats with CKD. The potential clinical utility of a gene variant as a marker to identify cats at a higher risk of progressive decline in kidney function would be limited if it were harbored only by a few cat breeds and present in a small fraction of the population. However, the fAIM variant reported here is present in several breeds (Table S3) and was identified in 60% of the cats screened. This finding emphasizes the potential clinical impact of this genetic marker and should prompt additional research efforts.
Our study has some limitations, some of which are inherent to its retrospective design and the complexity of kidney disease. Most of the cases had comorbidities such as hypertension and were receiving various treatments (Table S3) that might have confounded the SCr and study results. Some of these factors were controlled, in part, by the inclusion and exclusion criteria applied, but inclusion and exclusion criteria also might have biased the study results. The other important limitation regards constraints of SCr as a maker of kidney disease. We used two different criteria for phenotyping cats to improve the rigor of the results, but both approaches rely on SCr. Unfortunately, there were no other makers routinely used in clinical practice that we could have applied consistently in our study, but future research would benefit from information provided by other biomarkers.
A larger genetic population study also would help us understand which other breeds carry this genetic change and whether any specific breeds are more likely to harbor the fAIM exon 3 variant genotypes. Also, our study simulated wild-type and variant fAIM protein (Figure 3, Panel B) in silico based on the DNA sequence but did not study the protein structure of fAIM protein isolated from genotyped cats. Therefore, interpretations of the results assume that the altered fAIM gene would produce an altered fAIM protein. All of these limitations emphasize the need for more studies to confirm our findings and clarify the limitations of fAIM genetic testing as a risk factor for progressive CKD or perhaps even CKD itself.
Our study confirms that the previously described 4-domain variant of fAIM resulted from an exon duplication at the genomic level. This finding supports noninvasive sampling methods for identifying cats harboring this fAIM genetic alteration. The exon 3 duplication in the fAIM gene is present in multiple breeds. Our study also found a significant association between exon 3 duplication in the fAIM gene and a decline in kidney function in a group of client-owned cats from the Pacific Northwest. This discovery can potentially change strategies for diagnosing, treating, and preventing kidney disease, creating new opportunities to improve the standard of care for cats. Although results of our study suggest that fAIM variants are associated with CKD progression, supporting experimental evidence would be necessary to determine if fAIM variants are biologically detrimental or functionally inferior to the wild-type fAIM. A large multicenter prospective study is needed to confirm our findings, evaluate the predictive value of fAIM exon 3 variants on progressive CKD, and to understand the global impact of this genetic alteration on CKD cats using a genetic epidemiology study.
Acknowledgments
Gabriela C.L. Evangelista was supported in part by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brasília, DF, Brazil—#200552/2022-8), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brasília, DF, Brazil—Financial code 001), and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG, Belo Horizonte, MG, Brazil). Dr. Villarino kidney research program is supported by the Washington State University College of Veterinary Medicine Kay Yarborough Nelson Distinguished Professor in Feline Health Endowment, a generous gift from Mary Kay Fowler, Washington State University intramural grants program and Washington Research Foundation.
Disclosure
Authors declare no off-label use of antimicrobials.
Ethics Statement
Approved by the Institutional Animal Care and Use Committee of Washington State University (protocols #6493 and 4915).
Conflicts of Interest
Dr. Villarino might establish a service center at Washington State University that offers genetic testing. The other authors declare no conflicts of interest.




