Endothelial-derived von Willebrand factor accelerates fibrin clotting within engineered microvessels
Samuel G. Rayner and Zackary Scholl contributed equally to this work. Hongxia Fu and Ying Zheng are considered co-senior authors.
Manuscript handled by: Keith Neeves
Final decision: Jean Connors, 25 March 2022
Funding information
This work was supported by funding from National Institutes of Health (NIH) R61HL154250 and R01AI141602 to Y.Z.; F32 HL143949, Institutional National Research Service Award T32675551, and Institutional KL2 training award 5KL2TR002317-04 to S.G.R.; NIH K25 HL135432, American Society of Hematology Scholar Award to H. F.; NIH 5T32HL007093-44 to Z.S.; and NIH R35HL145262 to J.A.L.
Abstract
Background
Von Willebrand factor (VWF) is classically associated with primary hemostasis and platelet-rich arterial thromboses, but recently has also been implicated in fibrin clotting and venous thrombosis. Direct interaction between fibrin and VWF may mediate these processes, although prior reports are conflicting.
Objectives
We combined two complementary platforms to characterize VWF-fibrin(ogen) interactions and identify their potential physiologic significance.
Methods
Engineered microvessels were lined with human endothelial cells, cultured under flow, and activated to release VWF and form transluminal VWF fibers. Fibrinogen, fibrin monomers, or polymerizing fibrin were then perfused, and interactions with VWF evaluated. Thrombin and fibrinogen were perfused into living versus paraformeldahyde-fixed microvessels and the pressure drop across microvessels monitored. Separately, protein binding to tethered VWF was assessed on a single-molecule level using total internal reflection fluorescence (TIRF) microscopy.
Results
Within microvessels, VWF fibers colocalized with polymerizing fibrin, but not fibrinogen. TIRF microscopy showed no colocalization between VWF and fibrinogen or fibrin monomers in a microfluidic flow chamber across a range of shear rates and protein concentrations. Thrombin-mediated fibrin polymerization within living microvessels triggered endothelial VWF release, increasing the rate and amount of microvessel obstruction compared to fixed vessels with an inert endothelium.
Conclusions
We did not identify specific binding between fibrin(ogen) and VWF at a single-molecule level. Despite this, our results suggest that rapid release of endothelial VWF during clotting may provide a physical support for fibrin polymerization and accelerate thrombosis. This interaction may be of fundamental importance for the understanding and treatment of human thrombotic disease.
ESSENTIALS
- Von Willebrand factor (VWF) interactions with fibrin(ogen) may contribute to thrombosis.
- VWF association with fibrin(ogen) was evaluated via two complementary bioengineering techniques.
- VWF did not bind fibrin or fibrinogen under flow at the single-molecule level.
- In microvessels, endothelial-derived VWF formed a scaffolding accelerating fibrin clot formation.
1 INTRODUCTION
Thrombosis is a leading cause of mortality worldwide through its mechanistic role in numerous diseases including myocardial infarction, stroke, pulmonary embolism, thrombotic microangiopathic disorders, and even infectious and rheumatologic processes.1-3 Despite this immense importance, many potentially targetable interactions involved in thrombosis remain incompletely understood, at least partially due to the complex and interrelated nature of human coagulation.4 Thromboses are generally classified as either “white” thrombi—rich in platelets and von Willebrand factor (VWF) and associated with high-shear arterial thrombosis, or “red” thrombi—rich in fibrin and trapped red blood cells and often associated with the venous system. This dichotomous view, however, has been challenged by recent observations. For example, VWF is necessary for murine deep venous thrombosis,5 consistent with observations that platelet accumulation is an important early step in the formation of both red and white thrombi.6 VWF absence leads to reduced thrombin generation,7, 8 and VWF appears to recruit platelets to fibrin networks.9, 10 Together with other work, these findings support a role for VWF in fibrin clot formation.
Von Willebrand factor is a large multimeric blood protein that is primarily secreted from endothelial cells, either constitutively or in a regulated fashion from storage granules known as Weibel-Palade bodies (WPBs) following chemical or mechanical stimulation.11-14 Following WPB degranulation, VWF is released in an ultra-large multimeric form from endothelial cells and may remain tethered to the activated endothelium until being rapidly cleaved into lower molecular weight multimers by circulating ADAMTS-13 (A Disintegrin And Metalloprotease with ThrombSpondin type 1 motif, member 13).15 Under high fluid shear stress, individual domains of tethered VWF partially unfold under tension, exposing binding sites and leading to increased affinity for platelets.16 Failure to remove endothelium-bound VWF often leads to VWF self-association,17 forming remarkably long VWF fibers and webs that have been shown to reach over several hundred microns in vitro.18, 19 These pathologic VWF fibers interact with many other binding partners,20 including other coagulation proteins, and likely play a fundamental role in thrombotic microangiopathic disorders.21
One intriguing potential binding partner for VWF is fibrin(ogen). Fibrinogen is the most abundant coagulation protein in blood and is cleaved by thrombin to form fibrin—the final product of the coagulation cascade.22 A direct interaction between VWF and fibrinogen could contribute to the findings above and support a role for VWF in fibrin clotting. However, prior studies of VWF and fibrin(ogen) interactions have yielded conflicting results. Some studies have reported interactions between VWF and fibrin of varying strength, with equilibrium dissociation constants (Kd) ranging from 2.223 to 15 µg/ml,10 but others have not identified a direct interaction.9 Increased binding to larger VWF multimers was noted in one study,24 but not replicated in subsequent work.23 Binding of VWF to fibrin but not fibrinogen has been reported, through a binding site exposed when fibrinopeptides are removed from fibrinogen following the actions of thrombin.25 Binding of fibrin to both the C domain23, 25 and the A2 domain26 of VWF has been reported. The variability in these results may be related to differences in protein preparation, VWF multimer size, the presence of additional plasma proteins, assay conditions including shear stress, and the readout used to measure binding. Importantly, these studies have primarily used VWF in human plasma or in purified form, which may differ in behavior from ultra-large multimers directly released from the endothelium during thrombosis.
In the current study, we used two complementary bioengineering approaches to study the interaction of VWF with fibrin(ogen) and explore the potential physiologic consequences of this interaction. Building on a three-dimensional endothelialized microvessel system previously used to study VWF fiber formation,18 we have developed a higher throughput microvessel system to study the interaction of fibrin(ogen) with endothelial-derived VWF fibers. We then directly measured binding characteristics at the single molecule/multimer level using total internal reflection microscopy to visualize the colocalization of fluorophore-labeled recombinant proteins within a microfluidic flow chamber under varied protein concentration and shear rates. We further explored the ramifications of VWF--fibrin interactions using a simplified model of fibrin clot formation within engineered microvessels. Defining the role that VWF plays in fibrin clot formation may have important ramifications for the basic understanding and future treatment of human thrombotic disease, ranging from microangiopathy to cardiovascular and cerebrovascular disease.
2 METHODS
2.1 Protein purification and labeling
Human fibrinogen (FIB3, Enzyme Research Lab) was diluted to 2.5 mg/ml in phosphate buffered saline (PBS) at pH 6.4 and precipitated by the addition of solid glycine to 2M. The fibrinogen was kept on a reciprocal tumbler at 4°C for 30 min, and the precipitated fibrinogen was sedimented at 13 000 g for 10 min at 4°C. The precipitated fibrinogen was dissolved in PBS, pH 6.4 and insoluble material was removed by centrifugation. The soluble fibrinogen was repeatedly precipitated in 2M glycine four times. After the last precipitation, the fibrinogen was dissolved in 2 ml of 140 mM NaCl and 10 mM HEPES pH 7.4. The concentration was determined by absorption at 280 nm (extinction coefficient 1.5 for a solution of 1 mg/ml). Purity was determined first using a sodium dodecyl sulfide (SDS)-gel to ensure that fibrinogen had not been converted to fibrin (>97% purity, gel not shown) and then by measuring VWF content by ELISA after each glycine precipitation stage, with which the final concentration was measured to be 0.9 µg VWF/mg fibrinogen. This purified protein was subsequently labeled using Alexa Fluor 647 NHS Ester (Thermo Fisher Scientific) in 150 mM NaCl, 20 mM HEPES (pH 7.4), and 130 mM NaHCO3 for 1 h at room temperature. Free dye was then removed by dialyzing in 150 mM NaCl and 20 mM HEPES (pH 7.4) at 4°C. The final protein concentration was measured to be 3.4 mg/ml using A280 absorbance measurements. The final degree of labeling was determined to be 4.94 fluorophores/molecule using measurements of absorbance at A650. Recombinant human VWF and recombinant human platelet glycoprotein Ib alpha chain (GPIbα) were prepared and fluorescently labeled as described by Fu et al.27 Detailed methods are available in the Method S1 in supporting information.
2.2 VWF fiber formation and immunofluorescence within humanized microvessels
We designed a dual-grid microvessel system in 7.5 mg/ml collagen, manufactured through a combination of lithographic and injection molding techniques as previously described.18, 28-30 Microvessels consist of perfusable three-dimensional channels created in collagen, lined with human umbilical vein endothelial cells (HUVECs), and cultured under flow (Figure 1). The dual-grid geometry utilized herein encompasses two 8 × 8 grid networks formed by intersecting 120 µm-diameter channels. Computational fluid dynamic modeling was performed to simulate flowing fluid within this grid geometry during initial gravity-driven perfusion (Figure 1a, Figure S1 in supporting information). Detailed information regarding microvessel fabrication and computational fluid dynamic modeling is available in Method S1.
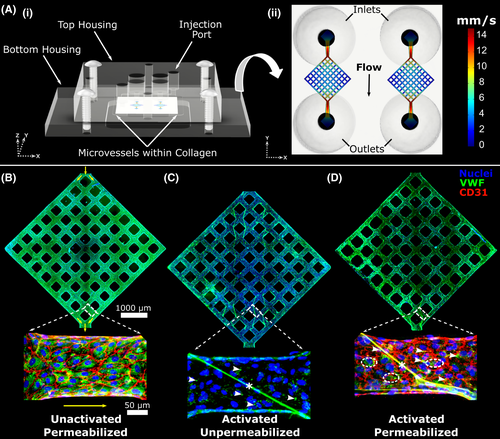
Microvessels were seeded with HUVECs and cultured for 5–7 days. Phorbol 12-myristate 13-acetate (PMA) perfusion was then used to provoke VWF release from endothelial cells, leading to intraluminal VWF fiber formation.18 Vessels were washed with serum-free endothelial basal media (EBM; Lonza) three times for 5 min each, followed by perfusion with PMA at 100 ng/ml in EBM for 40 min. PMA perfusion was done via gravity by filling the inlet with 200 µl of solution and removing liquid from the outlet. After 40 min, vessels were washed once with EBM. At this stage, perfusion of potential VWF fiber binding partners could be performed, or vessels could be fixed for imaging. For fixation, 4% paraformaldehyde in PBS was perfused for 20 min, followed by three washes with PBS. For comparison, three different staining conditions were used. First, unactivated (no PMA perfusion) control vessels were permeabilized for 1 h in 0.1% triton in 2% BSA followed by staining with anti-VWF and anti-CD31 antibodies and Hoechst (Figure 1b). Second, vessels activated with PMA as above were stained for VWF without permeabilizing, to visualize only intraluminal VWF released by the endothelium (Figure 1c). Finally, vessels activated with PMA were permeabilized and stained to visualize both intraluminal and residual intracellular VWF as well as endothelial cell adhesion molecule, CD31 (Figure 1d). Immunostaining was performed in situ within microvessel platforms, by perfusing antibody solutions from inlet to outlet via gravity. Staining with primary antibody was done overnight at 4°C, and secondary antibody staining was performed at room temperature for 1 h, with six 5-min PBS washes performed after each antibody staining. Antibodies used were rabbit anti-CD31 (1:30, Abcam ab28364, lot GR3176844-6), fluorescein isothiocyanate-conjugated sheep anti-VWF (1:100, Abcam ab8822, lot GR3197920-2), goat anti-rabbit 568 (Invitrogen A11011, lot 1842719), and Hoechst (1:250, Thermo Fisher) for nuclear staining. Imaging was performed without disassembly, through the glass coverslip at the bottom of the device, using a Nikon Ti microscope attached to a Yokogawa W1 spinning disk system.
2.3 Evaluating fibrin(ogen) association with VWF within activated microvessels
Following PMA perfusion and vessel activation, blocking of nonspecific binding was done by perfusing 2% BSA in EBM for 10 min. Perfusion of three different fibrin(ogen) solutions was then performed to assay for association with VWF, using purified and fluorescently labeled fibrin(ogen) as described above. Perfusion was done via gravity, with a pressure differential of ~100 Pa established from inlet to outlet. To test fibrinogen association with VWF, fibrinogen-Alexa647 was diluted to 500 µg/ml in EBM and perfused for 20 min followed by washing with EBM and fixation. To assay for the association of polymerizing fibrin with VWF, fibrinogen-Alexa647 was diluted to 500 µg/ml in EBM within a separate Eppendorf tube, thrombin (Sigma) was rapidly added to a final concentration of 1 U/ml and the solution immediately perfused through the microvessels for 20 min followed by fixation. Albumin perfusion was done as a control for nonspecific binding. Rhodamine-labeled human serum albumin (Abcam, Lot GR3244745-1) was diluted to a concentration of 1.5 µM and perfused through PMA-activated microvessels for 20 min. Platelets were used as a positive binding control.18 Blood was drawn from healthy volunteers following University of Washington Institutional Review Board (IRB) approval (IRB study#00012120) into 3.8% sodium citrate. This was then centrifuged at 200 g for 20 min at room temperature without braking on the centrifuge. The platelet-rich plasma layer was then transferred to another tube and centrifuged at 1200 g for 10 min, again without braking used on the centrifuge. The recovered pellet was resuspended into 4 ml of CGS buffer (120 mM sodium chloride, 13 mM sodium citrate, and 30 mM glucose) with 1 µM prostaglandin E1 added. This solution was centrifuged again at 1200 g for 10 min, resuspended in 200 µl Tyrode’s buffer and labeled with fluorescent anti CD-41a antibody (BD Biosciences 555467, lot 12603) for 20 min. The solution was then centrifuged and resuspended in PBS at 3 × 106 platelets/ml. The labeled platelet solution was perfused through PMA-activated microvessels for 20 min prior to fixation. For additional controls, unactivated vessels (not PMA treated) were perfused with fibrinogen 500 µg/ml in EBM or fibrinogen 500 µg/ml +3 U/ml thrombin.
For quantification of protein or platelet binding to VWF, all vessels were perfused with fluorescently labeled antibodies for VWF as above, without permeabilization, to visualize only intraluminal VWF. Cross-sectional imaging through fibers within vessels was done using a Nikon A1R confocal microscope. Images were imported into the Fiji distribution of ImageJ software (US National Institutes of Health) for analysis.31, 32 Fibers were manually traced within each image of interest. Using a custom script written within Fiji, each traced fiber was separated from the remainder of the image, and a z-stack of each fiber done to create a planar image. Color channels were separated to yield individual images of VWF staining and staining for the binding partner of interest: fibrin(ogen), albumin, or platelets. Images were thresholded manually to create a binary image intended to capture all positive staining. The total area of overlapping signal (VWF AND binding partner) was divided by the area of VWF staining to approximate the percentage of VWF fiber coverage by the item of interest (Figure S2 in supporting information). Analysis of variance (ANOVA) was performed using GraphPad Prism software (GraphPad Software) with Tukey’s post hoc analysis performed for multiple comparisons. The samples were thresholded and analyzed independently by one of the authors (SGR) as well as separately by a third party who was blinded to the identity of each binding partner, to reduce bias. At least three technical replicates of vessels were performed for each condition. Finally, we estimated the total amount of intraluminal VWF present, including both fibrillar VWF and punctate VWF on the vessel walls, and compared this across conditions via ANOVA testing. The percentage of luminal area covered by VWF antibody signal was measured in Z-projection images for a 1 mm × 1 mm section of the inlet (n ≥ 3 vessels for each condition).
2.4 Single-molecule imaging of VWF and fibrin(ogen)
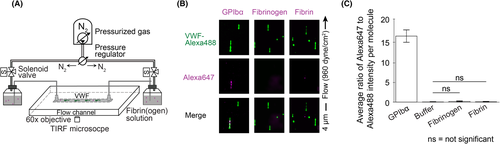
To further evaluate fibrin(ogen) interactions with VWF we combined a microfluidic flow chamber with total internal reflection fluorescence (TIRF) microscopy and used fluorophore-labeled recombinant proteins for direct visualization of binding between single molecules of VWF and fibrinogen or fibrin (see Figure 3a). Methods for controlling shear rate, registering dual channels, labeling molecules, and preparing the experimental channel with tethered VWF for dual-color TIRF imaging have been described in detail previously and are discussed in Method S1.27 We tethered recombinant VWF labeled with biotin (~0.3 per monomer) and Alexa Fluor 488 (0.61 fluorophore/monomer) to a low-density biotin-PEG surface via traptavidin inside a microfluidic flow channel. In the same channel, we then perfused either Alexa Fluor 647-labeled fibrinogen (1.52 fluorophore/fibrinogen) or Alexa Fluor 647-labeled fibrin monomers. These Alexa Fluor 647-labeled fibrin monomers were prepared by incubating fibrinogen-Alexa647 in 1xPBS with 2.5 mM Gly-Pro-Arg-Pro (GPRP, Sigma-Aldrich), a peptide inhibitor of fibrin polymerization, for 5 min and then adding 1 U/ml thrombin (Sigma-Aldrich) and incubating the mixture for 40 min. The final concentration of fibrinogen-Alexa647 or fibrin-Alexa647 was diluted to 5–500 µg/ml prior to perfusion in EBM. GPIbα was perfused as a positive control, as described by Fu et al.27 We measured co-localization between Alexa Fluor 488 and 647 fluorescence, which would indicate a specific interaction between VWF and the perfused molecule. All experiments were analyzed using custom MATLAB software by computing a region of interest (ROI) box around each tethered VWF molecule and identifying co-localized fluorescence intensity signals within it as described previously.27
A large range of wall shear rates was tested, from 125 up to 128 000 s−1, including values both within and beyond physiological or pathophysiological shear rates for human blood flow. We included such supraphysiological shear rates as VWF can exhibit tension-dependent binding interactions as we have shown previously.27 For a tethered VWF multimer, the tension at each point along the molecule is approximately proportional to the total number of monomers downstream, meaning that longer VWF multimers have higher tension at the tether point than shorter multimers. Because VWF molecules used in our experiment (average ~40 monomers per multimer) are generally shorter than newly secreted VWF or VWF strings on endothelial cells, we tested shear rates up to 128 000 s−1 to ensure any tension-dependent binding would be captured.
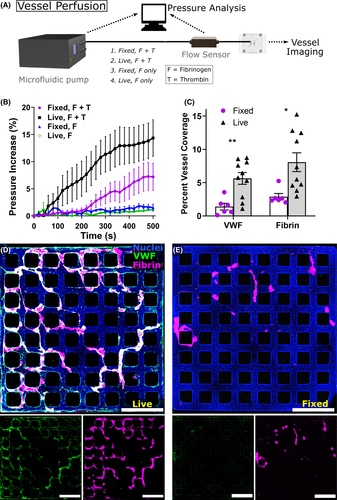
2.5 Microfluidic pump perfusion of HUVEC microvessels for in situ thrombus formation
Dual-grid HUVEC microvessels cultured for 5–7 days were used for pump perfusion experiments, attached to pump tubing with a custom adaptor printed on a Form 2 3D printer (Formlabs). An Ob1 MK3+ microfluidic pump controller (Elveflow) was set to adjust driving pressure to perfuse solutions at 10 µl/min, measured with a flow sensor (Elveflow) just prior to entering the microvessels. Fluid was passed through a small microfluidic polydimethylsiloxane (PMDS) channel prior to entering the microvessels to increase resistance into a stable range for measurement, and the pressure required to drive flow was continuously recorded by an attached computer. Commercially obtained human fibrinogen-Alexa647 (Sigma) was used rather than purified protein (as above) for pump-based experiments given the high volume of protein needed and the low likelihood that trace impurities would significantly affect this experiment. Fibrinogen was diluted to 50 µg/ml in EBM, and this was used as the solution for continuous perfusion. Thrombin was added to 200 µl of fibrinogen to a concentration of 2 U/ml and this solution immediately added to fill the inlet. The microfluidic pump tubing was rapidly attached, and perfusion done for 15 min. Fluid was continuously aspirated from the outlet well, to avoid changes in pressure related to hydrostatic forces. After, perfusion vessels were washed three times with PBS and fixed for 20 min in 4% paraformaldehyde. This same experiment was performed in vessels which were instead fixed in paraformaldehyde just prior to perfusion. Finally, as a control fibrinogen alone (without thrombin) was perfused in the same manner through both fixed and living microvessels.
Following the above experiment, vessels were stained with VWF antibody and Hoechst without permeabilization, as above, and imaged on a Nikon Ti microscope attached to a Yokogawa W1 spinning disk system. For all imaging experiments the same laser intensity, gain multiplier, and exposure were used. Images were then analyzed in ImageJ software to ascertain the extent of vessel coverage with VWF and fibrinogen. Binary thresholding was automated by using the same multiple of background signal as the threshold for a positive signal for all vessels. A two-tailed Student’s t-test was performed to compare fixed and live vessels. For pressure data, a linear regression was performed for each dataset comparing change in pressure over time across 500 s of data for both fixed and live microvessels, and F testing performed in GraphPad to obtain a P value for whether the slope differs between data sets.
3 RESULTS
3.1 Dual-grid microvessel creation and VWF fiber formation
Experiments were done within a dual-grid microvessel system, consisting of two separately perfusable grids within a single acrylic housing to increase experimental throughput. Compared to prior platforms,18 computational models showed that this new geometry reduced low-flow regions within the grids while maintaining regions of high flow and flow acceleration near the entry and exit regions of the vessel network—features designed to enhance VWF fiber formation (Figure 1a and Figure S1). Cultured microvessels demonstrated patent lumens lined with a confluent HUVEC monolayer, with intact intercellular junctions and robust expression of intracellular VWF (Figure 1b). When microvessels were perfused with PMA, a known secretagogue for VWF, endothelial cells became activated and secreted large amounts of VWF into the lumen under flow. This led to formation of large interconnected VWF fibers spanning the vessel lumen and smaller punctate VWF globules and strands along the vessel wall (Figure 1c). After activation, endothelial cells displayed disrupted intercellular (CD31) junctions with only small amounts of residual intracellular VWF (Figure 1d).
3.2 Fibrin(ogen) association with VWF fibers inside humanized microvessels
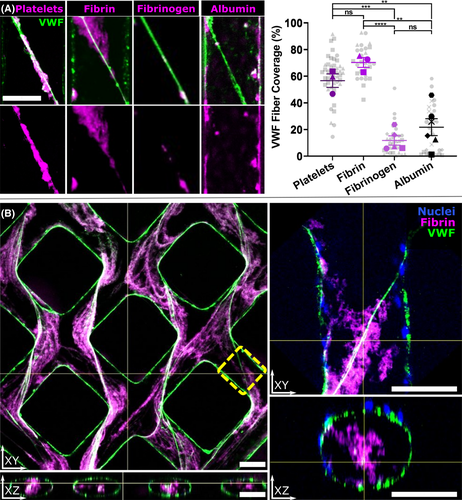
We next examined the interactions of fibrin(ogen) with VWF fibers by perfusing fluorescently labeled proteins through activated microvessels. Four substances were perfused through PMA-activated HUVEC microvessels: (1) purified fibrinogen-Alexa647, (2) polymerizing fibrin-Alexa647, (3) platelets labeled with fluorescent CD41a antibody (positive control for VWF binding), and (4) albumin-rhodamine (control for nonspecific binding). Following staining and confocal imaging, the percentage of VWF fiber signal that colocalized with that of the perfused substance of interest was quantified (Figure 2; methods displayed in Figure S2). Platelets (n = 3 vessels containing 46 VWF fibers) colocalized strongly with VWF, as expected. Fibrin-Alexa647 (n = 3 vessels containing 29 VWF fibers) colocalized similarly to platelets, and significantly more than fibrinogen (P < .0001) or albumin (P < .01). Fibrinogen-Alexa647 (n = 5 vessels containing 32 VWF fibers) colocalized significantly less than fibrin or platelets (P < .0001 and P < .001, respectively), and was similar to the nonspecific binding control, albumin-rhodamine (n = 6 vessels containing 32 fibers). While polymerizing fibrin appeared to coat the larger transluminal VWF fibers, extending outward from them in three dimensions (Figure 2b), very little colocalization was noted with the VWF found along vessel walls as punctate globules or small fibers adhering only to the vessel surface (Figure S3 in supporting information). To reduce bias, blinded independent analysis of all colocalization experiments was performed by a third party not involved in this project, and confirmed the above findings (Figure S4 in supporting information). Significance values above are for analysis done at the level of vessels (technical replicates), comparing mean fiber coverage between each vessel. Analysis at the level of individual fibers led to similar results with increased significance (Figure S5 in supporting information). Luminal coverage by VWF was not statistically different across conditions (mean coverage 9.4%), though polymerizing fibrin had a non-statistically higher mean coverage of 11.4%, possibly related to the combinatorial effects of PMA and thrombin on endothelial cells. Taken together, our data showed significant interactions between polymerizing fibrin, but not fibrinogen, with membrane-bound VWF released from the endothelium.
3.3 Single-molecule imaging of fibrin(ogen) interactions with individual VWF multimers
As prior reports suggest that fibrin monomers bind VWF, but that fibrinogen does not bind VWF,23, 25 we next used a combination of a microfluidic flow chamber and TIRF microscopy to evaluate whether our findings in microvessels above were due to specific binding of fibrin(ogen) to tethered VWF multimers. As shown in Figure 3b, tethered VWF multimers extended when flow was applied but no fluorescence specifically overlapped between VWF and fibrinogen or VWF and fibrin monomers, indicating no specific binding of fibrinogen to VWF or fibrin monomers to VWF. Within the time window of our measurement (15 min at 125 s−1, down to 10 s at 128 000 s−1), we did not observe co-localization between VWF and fibrinogen or fibrin monomers at any wall shear rate (Figure 3c). In contrast, as a positive control, we used Alexa Fluor 647-labeled GPIbα, which showed a high degree of co-localization with tethered VWF (Figure 3b,c). Perfusion of buffer alone was used as a negative control, with results similar to those seen with perfused fibrin or fibrinogen (Figure 3c). Varying perfused protein concentration from 5–500 µg/ml did not influence the results. In summary, there were no specific interactions between individual VWF multimers and fibrinogen or fibrin monomers based on our single-molecule imaging experiments.
3.4 Endothelial-released VWF and fibrin clot formation
While we used PMA as a VWF secretagogue for the above microvessel experiments, VWF release in vivo is rapidly triggered by substances present during coagulation including thrombin and fibrin itself.11, 33 When we perfused fibrinogen and thrombin into endothelialized microvessels without PMA pre-activation, we observed VWF fiber formation and association with polymerizing fibrin similar to that seen with PMA pre-activation, which was not observed when fibrinogen alone (without thrombin) was perfused as a control (Figure S6 in supporting information). To further evaluate the contribution of living endothelium to fibrin clotting under flow, we perfused fibrinogen with or without thrombin through both living and previously paraformaldehyde-fixed microvessels in the absence of serum (Figure 4), similar to prior approaches.34 We monitored the pressure drop between the inlet and outlet of microvessels required to drive flow at 10 µl/min throughout perfusion and evaluated the amount of intraluminal fibrinogen and VWF after perfusion via immunostaining (Figure 4a). Vessels with a living endothelium (n = 9) displayed a significantly higher increase in pressure over time compared to fixed vessels (n = 6, P < .0001; Figure 4b). Image quantification demonstrated significantly increased levels of intraluminal VWF and fibrin following perfusion, with fluorescent VWF antibody signal covering 5.6% of living vessels and 1.4% of fixed vessels (P < .01) and fluorescent fibrin signal covering 8.1% versus 2.9% (P < .05; Figure 4c). With living endothelium, but not fixed, fibrillar VWF structures were noted to provide a scaffolding for fibrin clotting (Figure 4d,e). These results suggest that VWF released rapidly following endothelial activation by thrombin35 and/or fibrin33 provides a nidus for fibrin clot formation and accelerates microvascular occlusion.
4 DISCUSSION
A possible interaction between fibrin(ogen) and VWF has drawn significant attention given its potential ramifications for understanding human thrombotic disease, although prior reports on this interaction are conflicting. Our work presents two major findings obtained using the complementary bioengineering approaches of microvessel models and single-molecule imaging under flow. First we showed that neither fibrinogen nor fibrin monomers specifically bind VWF multimers under flow. Second, despite not identifying a specific interaction between non-polymerizing fibrin and VWF, we found that polymerizing fibrin interacted significantly with VWF fibers and appeared to modulate thrombosis within human microvessels. These findings raise the intriguing possibility that fibrin polymerization may occur rapidly along a VWF multimer scaffolding following VWF release from activated endothelium.
Our complementary approaches provide evidence that neither non-polymerizing fibrin monomers nor fibrinogen appear to bind VWF multimers under flow. Most prior reports concur with our findings that fibrinogen does not specifically bind VWF.9, 10, 25 Regarding fibrin interactions with VWF, prior studies have produced variable results. Agreeing with our findings, Endenburg et al.9 found no interaction between fibrin-coated glass and VWF-containing plasma perfused at a wall shear rate of 340 s−1 or 1600 s−1. Other investigators, however, have described a specific interaction between fibrin monomers and VWF, contradicting our results. Loscalzo et al.10 reported non-covalent binding of bead-bound fibrin monomers to VWF with a Kd of 15 µg/ml. Ribes and Francis24 showed size-dependent incorporation of high molecular weight VWF multimers into formed fibrin clot. Keuren et al.23 showed binding of VWF to already-polymerized fibrin within a filter cup that was specific and saturable (Kd 2.2 µg/ml), may involve the VWF C1C2 domain, but was not dependent on VWF multimer size. In a more recent work, Miszta et al.25 described fibrin binding to the C1C2 domain of VWF using ellipsometry but, contrary to Keuren et al., they observed that VWF could only bind fibrin during fibrin polymerization and did not bind already-polymerized fibrin.
These variable results may be due to differences in the protein preparations or experimental methods employed. The presence of additional coagulation proteins within fibrinogen preparations could influence binding results, especially given that VWF is known to bind numerous compounds.20, 36 Prior approaches also vary widely in the experimental methods used, amount of shear applied, assay readouts, and the presence or absence of other plasma components. We incorporated additional purification steps in preparing fibrinogen and in our single-molecule experiments we tested a range of shear rates and protein concentrations without identifying specific binding of purified VWF protein with fibrinogen or fibrin monomers. In our experiments, fibrin and VWF did not demonstrate specific binding on a single-molecule level. Our results instead suggest that fibrin can polymerize around a scaffolding of VWF fibers, perhaps aided by non-covalent interactions, with potentially important pathophysiologic ramifications. However, we cannot completely rule out a specific covalent interaction under alternative reaction conditions including those found in vivo.
Importantly, we have demonstrated novel interactions between polymerizing fibrin and endothelial-released VWF. When endothelium-lined vessels were exposed to a mixture of thrombin and fibrinogen, significant release of VWF was observed, leading to increased intraluminal fibrin clot compared to fixed vessels where endothelial cells are unable to release VWF. We also found a significantly increased rise over time in the perfusion pressure required to drive a constant flow within living vessels compared to fixed ones, suggesting increased early obstructive thrombosis. The time course of this pressure rise is consistent with the kinetics of VWF release from endothelial cells following thrombin stimulation where WPB degranulation is rapid, and nearly complete within 5 min.35 These results suggest that thrombin and fibrin present during local thrombosis lead to endothelial VWF release. Subsequently, endothelial-derived VWF fibers rapidly modulate fibrin clotting by acting as a scaffold for fibrin polymerization and clot extension. This paradigm is intriguing. If rapid release of ultra-large VWF multimers upon initiation of thrombosis provides a scaffold for the polymerization of fibrin—along with platelet binding and attachment of red blood cells37 and other clotting components20—this may have important ramifications for the understanding and treatment of human thrombosis. The observation of strand-like areas of VWF staining within “red” regions of venous thromboembolism, and the importance of VWF for the formation of both red and white thrombi in vivo,38-40 further support a potential role for endothelial-derived VWF as a scaffold for fibrin clotting; however, further study is necessary.
We recognize several limitations to this study. We performed experiments in the absence of human serum, a reductionist approach that allowed us to evaluate individual hemostatic interactions but does not recapitulate the complexity of human thrombosis. Whether and how other hemostatic proteins may modulate the interactions we observed is not informed by our results. Additionally, while VWF fibers are believed to play a role in thrombosis when ADAMTS-13 levels are depleted, as in disorders including thrombotic thrombocytopenic purpura15, 41 or disseminated intravascular coagulation,42 the role of VWF fibers during thrombosis under normal ADAMTS-13 plasma levels is unclear.21 Other research suggests that under the right conditions VWF strands/fibers can form in the presence of ADAMTS-13 and persist for several minutes,43 presumably long enough to modulate the dynamics of early clotting, but further study is necessary. We observed fibrin colocalization with VWF in both the low- and high-shear regions of microvessels, where shear rates ranged from ~50–500 s−1 (Figure S1). Shear rates around tortuous 3D VWF fibers and webs are difficult to model, with shear also varying along the length of fibers from a maximum at the vessel wall toward zero at the center of the lumen. Given these complexities, and the fact that shear stress affects VWF fiber formation itself,18 precise evaluation of how shear stress affects VWF-fibrin interactions is not feasible within our microvessel model. This remains an important area for future study.
Overall, our work revealed no specific interaction between VWF and fibrinogen or fibrin monomers, but demonstrated significant interaction between endothelial-derived VWF fibers and polymerizing fibrin. Our results show that VWF can act as a scaffolding for fibrin clotting and that VWF multimers released from the endothelium early in thrombosis can rapidly increase the speed and extent of intraluminal fibrin clot formation within microvessel models. Our engineered microvessel system will enable future work evaluating the individual effects of other circulating hemostatic components on VWF-fibrin interaction. If this potentially fundamental mechanism of thrombosis is confirmed in vivo, future therapeutic targeting may be possible, in hopes of lessening the tremendous burden of human thrombotic disease.
ACKNOWLEDGMENTS
The authors would like to acknowledge the Lynn and Mike Garvey Imaging Laboratory in the Institute of Stem Cell and Regenerative Medicine, the Washington Nanofabrication Facility, and the Physics Machine Shop, all located at the University of Washington. Support from the NSF award NNCI-2025489 to the Washington Nanofabrication Facility is appreciated as well. The authors would like to thank Mohammed Talib for assistance with the computer-aided design of the dual-grid microvessel geometry.
CONFLICTS OF INTEREST
The authors have declared that no conflicts of interest exist.
AUTHOR CONTRIBUTIONS
S.G. Rayner and Z. Scholl contributed equally to this work. Y. Zheng and H. Fu also contributed equally as co-senior authors. S.G. Rayner, Z. Scholl, J.A. López, H. Fu, and Y. Zheng designed the experiments. S.G. Rayner and Z. Scholl performed experiments with the assistance of C.J. Mandrycky, J. Chen, K.N. LaValley, and D.W. Chung. S.G. Rayner and Z. Scholl interpreted data with the assistance of P.J. Leary, W.A. Altemeier, W.C. Liles, D.W. Chung, J.A. López, H. Fu, and Y. Zheng. S.G. Rayner, Z. Scholl, H. Fu, and Y. Zheng wrote the manuscript. The manuscript has been read and approved for submission to JTH™ by all authors.