Safety, pharmacokinetics, and pharmacodynamics of a next-generation subcutaneously administered coagulation factor IX variant, dalcinonacog alfa, in previously treated hemophilia B patients
Manuscript handled by: Flora Peyvandi
Final decision: Flora Peyvandi, 23 December 2020
Abstract
Background
Dalcinonacog alfa (DalcA), a next-generation, recombinant human factor IX (FIX) variant, was developed using a rational design approach for increased procoagulant activity and longer duration of action to be administered subcutaneously (SC) for prophylaxis of hemophilia B bleeding episodes.
Objectives
To investigate the safety, pharmacokinetics (PK), and pharmacodynamics (PD) of DalcA.
Methods
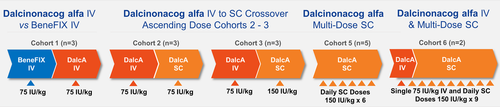
This multicenter, phase 1/2a study (NCT03186677) was conducted in 11 males aged 12 to 65 years with severe hemophilia B. In cohort 1, subjects received intravenous (IV) 75 IU/kg BeneFIX and DalcA. Cohorts 2 and 3 had DalcA IV 75 IU/kg and SC 75 IU/kg or 150 IU/kg. Cohort 4 was omitted. Cohort 5 received daily SC 150 IU/kg DalcA for 6 days and cohort 6 received IV 75 IU/kg and daily SC 150 IU/kg DalcA for 9 days. Blood sampling was performed for chemistry, hematology, PK, PD, and anti-drug antibody measurement. Subjects were monitored for safety endpoints for 30 days postdosing.
Results
DalcA demonstrated a 24-fold greater potency over BeneFIX and longer mean residence time (33.8 h). SC bioavailability 8.2% to 20.3%, beta half-life 53.9 to 106.9 h and Tmax 24 to 48 h. A median 15.7% FIX activity level (interquartile range, 14.9%-16.6%) was reached after 6 daily doses. Neutralizing antibodies to ISU304, but not wild-type FIX, occurred in two cousins.
Conclusions
The data demonstrated that DalcA achieved protective FIX activity levels between 11% and 18%, corresponding to a reduced chance of spontaneous bleeds. Based on the results, a phase 2b trial to assess the safety and efficacy of 28 daily SC doses of DalcA was performed.
Essentials
- Subcutaneous (SC) factor IX (FIX) dosing has been limited by low bioavailability and potency.
- Dalcinonacog alfa (DalcA) has 24-fold increased potency compared with wild-type FIX.
- Daily SC doses of DalcA reached activity levels of 11–18% and terminal t1/2 of 64.6 h.
- DalcA addresses the unmet need of SC prophylaxis for patients with hemophilia B.
1 BACKGROUND
Coagulation factor IX (FIX) is essential for hemostasis. Hemophilia B is a genetic disorder caused by missing or defective FIX clotting protein.1 The prevalence of hemophilia B is 5.3 cases per 100,000 male individuals,2 with approximately 44% of these cases having severe disease (those who bleed spontaneously or as a result of trivial trauma).3 The standard of care is replacement therapy with plasma-derived or recombinant FIX (rFIX).4-7 However, rapid in vivo clearance of FIX necessitates frequent intravenous (IV) dosing to achieve effective prophylaxis.8-12 In addition, the development of an inhibitor against FIX is the most serious complication that may occur in patients with hemophilia B, and is associated with significant morbidity related to the bleeding risk and the frequent occurrence of nephrotic syndrome and allergic/anaphylactic reactions.13, 14 Extended half-life (t1/2) agents reduce the number of injections per bleeding episode and improve patient care. They require weekly IV infusion and may have reduced extravascular distribution with a prolonged period of low activity levels that increase the risk of spontaneous bleeding before the next dose is administered prophylactically.15, 16 An rFIX agent with favorable pharmacokinetics (PK) and hemostatic properties, including increased potency and prolonged duration of effect, that allows subcutaneous (SC) administration, may help address an existing unmet medical need in patients with hemophilia B.
Dalcinonacog alfa (DalcA, also known as ISU304 and CB 2679d), was designed to be the next-generation coagulation FIX for SC prophylactic therapy for the prevention of hemophilia B bleeding episodes. DalcA was developed using a rational design approach with three amino acid substitutions in two loops (Figure S1) within the FIX protein to significantly enhance affinity to the cofactor VIII and stabilize activated FIX (FIXa). These mutations in the molecular structure enable DalcA to demonstrate three times the catalytic efficiency to FX (substrate), 10 times higher affinity to cofactor FVIIIa, and 15 times the resistivity to inhibitor antithrombin when compared with wild-type (WT) FIX. This enables DalcA to have 20 times the in vitro coagulation activity, as well as eight times’ increased duration of activated partial thromboplastin time (APTT) activity in vivo compared with recombinant WT-FIX dosed at the same mass. FIX activity was measured using a one-stage clotting assay using ACL TOP 700 and Instrumentation Laboratories (Bedford, MA) reagents.17, 18
Here, we describe the first-in-human clinical study to investigate the safety, PK, and pharmacodynamics (PD) of a longer acting rFIX in patients with hemophilia B.
2 METHODS
This phase 1/2a open-label, multicenter, dose-escalation study was designed to investigate the safety, PK, and PD of DalcA in patients with hemophilia B (ClinicalTrials.gov Registration Number: NCT03186677). This study was conducted at the following investigator sites: Eulji University Hospital, Yonsei University College of Medicine Severance Hospital, and Pusan National University Hospital.
This study was conducted in accordance with the US Code of Federal Regulations, International Conference on Harmonisation Guidelines on Good Clinical Practices, and South Korean national laws and regulations. The study protocol and all amendments were reviewed and approved by the institutional review board or independent ethics committees at each participating study site. Before any testing, all subjects or their legally authorized representative were required to provide written informed consent prior to participation in the study.
2.1 Study population
Subjects aged 12 to 65 years with a history of moderate or severe hemophilia B (defined as documented FIX activity ≤2% at screening) pretreated with any approved FIX product for a minimum of 150 exposure days were eligible for enrollment. Subjects with HIV were required to have a CD4 count >200 μl before study entry. All subjects were also required, and be willing, to comply with study washout requirements for screening and before dosing. Individuals with FIX inhibitors, known hypersensitivity to hamster protein or FIX products, history of thromboembolic events, use of regular concomitant therapy with immunomodulating drugs, or bleeding disorder other than hemophilia B were excluded. A complete list of inclusion and exclusion criteria can be found in Table S1. All subjects were in a nonbleeding state at the time of screening and drug administration.
2.2 Study design
Under the original study protocol, the study comprised five cohorts (Figure 1); however, the original cohort 4 was later dropped and two patients continued into an additional cohort 6 to receive a single IV dose of DalcA 75 IU/kg followed by daily SC doses of DalcA 150 IU/kg for 9 days. Agreement was obtained from regulatory authorities that cohort 4 was not needed. Three to five eligible subjects within each of the cohorts were administered IV 75 IU/kg, with single doses of DalcA administered subcutaneously at either 75 IU/kg or 150 IU/kg. A washout of all hemostatic treatments at least 96 h before dosing was required (no extended t1/2 products were approved in South Korea at the time of this study). Each dose escalation was approved only after review of the safety, PK, and PD data by the sponsor, Data Safety Monitoring Board, and Drug Monitoring Committee. Escalation to the next higher dose proceeded only if no safety and tolerability concerns were identified. Abnormal laboratory, electrocardiogram (ECG), or vital sign findings meeting protocol-defined quantitative criteria for stopping rules required additional assessment by the respective study investigator for clinical significance. Two or more similar treatment-emergent, clinically significant events, meeting the prespecified quantitative criteria, in a given cohort would trigger a stop in dose escalation. Baseline for all cohorts was defined as the level of FIX during a wash-out/pretreatment period. In cohorts 1 to 3, the maximum allowed intervals between the first and second dosing, were no more than 14 days. If bleeding occurred after 72 h of the first dosing in cohorts 1 to 3, a FIX product was to be administered as rescue therapy. A 3-day washout period was planned after the last infusion of any rescue FIX product.
2.3 Investigation product
DalcA, a rFIX variant, is produced by a recombinant Chinese hamster ovary cell clone in suspension culture that coexpresses rFIX and recombinant human WT-furin, which has been extensively characterized. The stored cell banks are free of human blood or plasma products. No additives of animal or human origin are used during the cell culture, purification, and formulation processes. The rFIX is purified by a four-step chromatography purification process. DalcA is formulated as a sterile, nonpyrogenic, lyophilized powder preparation intended to be reconstituted for administration by IV infusion or SC injection.
2.3.1 Intravenous dose
The initial IV dose of 75 IU/kg corresponds to the clinical dose of BeneFIX (coagulation factor IX [recombinant]). After the IV dose, blood was sampled for PK analysis over the subsequent 72 h.19 In a preclinical PK study, both DalcA (75 IU/kg) and BeneFIX (75 IU/kg) showed a similar PK profile in hemophilia B mice.17 Based on these unpublished PK data, we predicted that the PK profile of both DalcA and BeneFIX in animal models might be comparable in humans. Subjects enrolled in cohort 1 (n = 3) received 75 IU/kg of IV BeneFIX followed by 75 IU/kg of IV DalcA in a crossover fashion.
2.3.2 Subcutaneous dose
To date, no commercial SC administered rFIX protein product has been approved for use in hemophilia B. With this in mind, we selected the initial SC doses of DalcA at 75, 150, and 300 IU/kg based on nonclinical data obtained from normal and hemophilia B mice, hemophilia B dogs, and normal minipigs (the 300 IU/kg dose [cohort 4] was later found not to be needed). When administering up to 2 ml volume via SC bolus injection, 28G/6-mm needles were recommended.
2.4 Outcome measures
The primary objective was to assess the safety of DalcA, measuring the incidence of adverse events (AE) after the administration of DalcA through study completion, for an average of 8 days. Safety was evaluated by physical examination, vital signs, ECG, development of AE, binding antibody and neutralizing antibody development, and laboratory changes over time. Assessments included bleeding events; thromboembolic complications; local and systemic injection reactions; and hypersensitivity reactions, including anaphylaxis, nephrotic syndrome, development of anti-drug antibodies (ADA), and FIX neutralizing antibodies. The secondary objective was to evaluate the PK parameters of DalcA at doses ranging from 75 IU/kg to 150 IU/kg, including measurement of maximum plasma concentration (Cmax) and t1/2. Other objectives were to evaluate PD parameters, including levels of D-dimer, APTT, prothrombin fragment 1 + 2 (F1+2), fibrinogen, and thrombin-antithrombin (TAT) complex.
2.5 Laboratory analysis
Hematology, blood chemistry, serology, and urinalysis were conducted at the time of screening and at designated time points after dosing. ADA test, neutralizing antibody, and Bethesda assay when positive were conducted. ADAs to DalcA and BeneFIX were detected through a direct binding enzyme-linked immunosorbent assay. Inhibitors to DalcA and BeneFIX were sought through the Nijmegen method. When there was a positive result (>0.6 Bethesda units), a confirmatory test was carried out by two central laboratories.
2.6 PK and PD analysis
Parameters included Cmax, time to achieve that concentration (Tmax), terminal phase elimination t1/2, total plasma clearance (CL), volume of distribution at steady state, area under the FIX activity time curve from zero to the last time of observation (AUC0-t) and area under the FIX activity time curve from time zero to infinity (AUC0-inf), mean residence time (MRT), and incremental recovery. To select the blood sampling times after SC injection of DalcA, we estimated the primary PK parameters (Tmax, Cmax, t1/2) and predicted plasma drug concentration at each time period. Blood coagulation assessments included levels of D-dimer, APTT, F1+2, fibrinogen, and TAT.
2.7 Statistical analysis
The safety analysis set was defined as all subjects who received at least one dose of the investigational product. The PK set was defined as subjects who completed all procedures planned for the cohort and for whom PK samples were obtained from at least four post-dose points (IV and SC administration). For each cohort, descriptive statistics were given for individual parameters using SAS software (SAS Institute Inc., Cary, NC). The AEs were categorized according to seriousness, severity, measures performed related to the investigational product, causal relationship with the investigational product, and the number of occurrences and percentage were calculated. All AEs were analyzed according to system organ class and preferred term (PT) using MedDRA (Medical Dictionary for Regulatory Activities) Version 20.1. The PK and PD analyses were conducted via standard noncompartmental methods using WinNonlin software (Pharsight) and was carried out by the central laboratory of Seoul National University Hospital. The bioavailability of DalcA at SC dosing was also calculated. Bioanalysis was conducted by the US Haemtech Biopharma Service.
3 RESULTS
3.1 Subjects
This clinical study was initiated by recruiting the first subject in May 2017. A total of 13 unique subjects were screened, and 11 unique subjects completed the study across 16 slots, as five participated in two cohorts each, and were included in the Safety Analysis Set. Demographics, including patient age, height, and weight, are shown in Table S2. The mean age and weight of treated patients were 41.6 years and 72.3 kg, respectively. During the course of the study, all subjects received the investigational product according to the planned study procedures and predetermined doses. Dosing was per the nominal activity of DalcA vial; actual dosing per measured activity was 93% of nominal. The largest SC dose was 1.46 ml. No subjects received rescue FIX.
3.2 Safety
3.2.1 Adverse events
AEs and adverse drug reactions (Tables 1 and 2) were mild or moderate in severity. There were no serious AE and no premature withdrawal of participation because of AE. ADA of low titer to DalcA were detectable in a subject in cohort 3 and at high titer in both subjects in cohort 6 (Table 2). No abnormal laboratory values, clinically significant vital signs, or abnormal ECG findings were observed.
| Group |
Cohort 1 n = 3 |
Cohort 2 n = 3 |
Cohort 3 n = 3 |
Cohort 5 n = 5 |
Cohort 6 n = 2 |
All N = 16 |
|---|---|---|---|---|---|---|
| AE, n (%) | 1 (33.3) | 3 (100) | 3 (100) | 5 (100) | 2 (100) | 14 (87.5) |
| SAE, n (%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Discontinuation | 0 | 0 | 0 | 0 | 0 | 0 |
| Systemic AE related to dalcinonacog alfa administrationa | ||||||
| Fatigue/malaise, n (%) | 0 | 1 (33.3) | 0 | 0 | 0 | 1 (6.3) |
| Headache, n (%) | 0 | 1 (33.3) | 0 | 0 | 0 | 1 (6.3) |
| Dizziness, n (%) | 0 | 1 (33.3) | 0 | 0 | 0 | 1 (6.3) |
- Abbreviations: AE, adverse event; SAE, serious adverse event.
- a All three events occurred in the same subject.
|
Subjects n; SC injections |
Cohort 1 n = 3; SC = 0 |
Cohort 2 n = 3; SC = 3 |
Cohort 3 n = 3; SC = 3 |
Cohort 5 n = 5; SC = 30 |
Cohort 6 n = 2; SC = 18 |
All N = 16; SC = 54 |
|---|---|---|---|---|---|---|
| General disorders and administration site conditions; SC injections; subjects (%) | ||||||
| Injection site pain | 0 | 2 (66.7) | 3 (100) | 34c; 5 (100) | 11; 2 (100) | 50; 12 (75) |
| Injection site erythema | 0 | 2; 1 (33.3) | 3 (100) | 32c; 2 (40) | 15; 2 (100) | 52; 8 (50) |
| Injection site papule | 0 | 0 | 2 (66.7) | 0 | 9; 2 (100) | 11; 4 (25) |
| Injection site pruritus | 0 | 1 (33.3) | 0 | 0 | 2; 1 (50) | 3; 2 (12.5) |
| Injection site discomfort | 0 | 1 (33.3) | 0 | 0 | 0 | 1 (6.3) |
| Pain | 0 | 1 (33.3) | 0 | 0 | 0 | 1 (6.3) |
| Investigations | ||||||
| Anti-ISU304 antibody positive | 1 (33.3) | 0 | 1 (33.3) | 2 (40) | 2 (100)d | 6 (37.5) |
| Metabolism and nutrition disorders | ||||||
| Decreased appetite | 0 | 0 | 0 | 0 | 2 (100) | 2 (12.5) |
| Gastrointestinal disorders | ||||||
| Diarrhea | 0 | 0 | 0 | 1 (20) | 0 | 1 (6.3) |
| Musculoskeletal and connective tissue disorders | ||||||
| Musculoskeletal stiffness | 0 | 0 | 0 | 0 | 1 (50) | 1 (6.3) |
| Skin and subcutaneous tissue disorders | ||||||
| Erythema | 0 | 0 | 0 | 0 | 1 (50) | 1 (6.3) |
| Pruritus | 0 | 0 | 0 | 0 | 1 (50) | 1 (6.3) |
- Abbreviations: DalcA, dalcinonacog alfa; MedDRA, Medical Dictionary for Regulatory Activities; SC, subcutaneous, WT-FIX, wild-type factor IX.
- a Preferred term as defined by MedDRA V20.1.
- b Number of events; subjects with events (% subjects with event).
- c Split injections were administered to some subjects.
- d Neutralizing antibody to DalcA but not WT-FIX.
3.2.2 Injection site reactions
No immediate injection site reactions (ISRs) were observed from the subjects enrolled in any cohort, although one subject in cohort 2 experienced transient malaise, headache, and dizziness (Table 1). Mild to moderate SC ISRs were pain, erythema, papules, pruritis, or discomfort; all resolved without sequelae (Table 2). Subjects reported these AE as mild, or moderately severe for the initial injection and mild for subsequent injections. These ISRs typically did not occur for later injections for those who took six or nine doses (cohorts 5 and 6). Injection site bruising was seen with initial SC injections and did not occur with subsequent SC injections when FIX activity levels increased to the mild hemophilia range.
3.2.3 Neutralizing antibodies
Two subjects, who were cousins, developed neutralizing antibodies to DalcA (one transient low-titer) that did not cross-react with WT-FIX and, therefore, are not referred to as inhibitors of WT-FIX. The patients were able to successfully resume use of their prior FIX therapy. Subsequently, a comprehensive investigation concluded that the immunogenic potential of DalcA was low, similar to that of commercial WT-FIX products, and that drug product quality was also comparable to commercial FIX products.20
3.3 Pharmacokinetics
3.3.1 Cohort 1
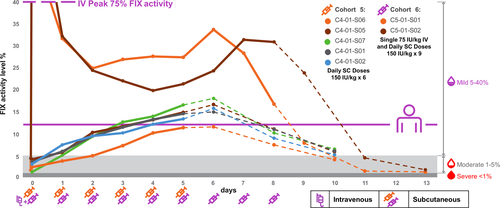
This cohort (n = 3) involved the crossover administration of 75 IU/kg of IV BeneFIX followed by 75 IU/kg of IV DalcA (although the actual dose given was 70 IU/kg because of use of nominal dose rather than actual potency assay result of the drug product). The mean injection volume for DalcA was 0.66 ml compared with 27.67 ml infusion for BeneFIX. The formal PK analyses (using a semiparametric compartmental model suggested by Lee et al [1990] and a noncompartmental approach21) are given in Table 3. DalcA had a higher Cmax and AUC, and longer MRT and t1/2 when compared with BeneFIX. The FIX activity for BeneFIX and DalcA are shown in Figure S2.
| Group | Intravenous | Subcutaneous | ||||
|---|---|---|---|---|---|---|
| BeneFIX | DalcA Cohorts 1, 2, and 3 | DalcA Cohort 2 | DalcA Cohort 3 | DalcA Cohort 5a DalcA Cohort 6 | a | |
| Mean injection volume, ml | 27.67 | 0.61 | 0.56 | 1.24 | 1.19 | 1.32 |
| t1/2 alpha, h | 5.3 ± 0.8 | 8.5 ± 4.0 | 3.4 | |||
| t1/2 beta, h | 21.0 ± 1.1 | 27.0 ± 2.2 | 53.9 ± 17.1 | 106.9 ± 52.7 | 64.6 ± 5.2 | |
| MRT, h | 25.1 ± 1.5 | 35.8 ± 2.5 | 93.2 | 166.6 ± 85.0 | ||
| Cmax, IU/dl | 70.2 ± 16.0 | 70.0 ± 46.9 | 5.3 ± 3.7 | 5.3 ± 1.2 | 15.3 ± 2.4 | 23.8 ± 10.0 |
| AUC0-inf, IU/dL × h | 933 ± 177 | 1148 ± 334 | 48.5 | 664 ± 420 | ||
| AUC0-t, IU/dl × h | 855 ± 163 | 973 ± 274 | 77 ± 50 | 287 ± 120 | 963 ± 144 | |
| Tmax, h | 24 | 48 | ||||
| Bioavailability, % | 8.2 ± 6.9 | 20.3 ± 7.1 | ||||
- Abbreviations: AUC0-t, area under the factor IX activity time curve from zero to a definite time t; AUC0-inf, area under the factor IX activity time curve from time zero to infinity; Cmax, peak serum concentration after test drug administration; MRT, mean residence time; t1/2, half-life; Tmax, time to maximal concentration after administration.
- a There are no summary statistics for some measures in cohorts 5 and 6 because daily injections were given. Maximum SC injection volume dosed was 1.46 ml.
3.3.2 Cohort 2
This cohort (n = 3) had sequential administration of DalcA IV (at 75 IU/kg as a nominal dose) and then DalcA SC (also at a 75 IU/kg nominal dose). As with cohort 1, the actual doses were 70 IU/kg. The mean injection volume for DalcA was 0.56 ml. The PK results from the activity data for the SC infusion for the three patients in this cohort are given in Table 3. DalcA had a beta t1/2 of 53.9 ± 17.1 h, MRT of 93.2 h, and Tmax of 24 h.
3.3.3 Cohort 3
Patients in this cohort (n = 3) were given an IV infusion of 75 IU/kg (nominal dose, but actually received 70 IU/kg) of DalcA followed by an SC dose of 150 IU/kg (nominal dose, actual dose 140 IU/kg). The mean injection volume for DalcA SC was 1.24 ml. The PK results for the activity data are given in Table 3. DalcA administered subcutaneously had a longer t1/2 of 106.9 ± 52.7 h, MRT of 166.6 ± 85.0 h, and Tmax of 48 h. The bioavailability was 20.3%.
3.3.4 Cohort 5 and 6
In cohort 5 (n = 5), each of the subjects received a nominal dose of 150 IU/kg (140 IU/kg actual) DalcA SC for 6 consecutive days. In cohort 6 (n = 2), each subject received a single IV dose of DalcA 75 IU/kg (nominal dose, actual dose 70 IU/kg) followed by daily SC doses of DalcA 150 IU/kg (140 IU/kg actual) for 9 days. The mean injection volume for DalcA was 1.19 ml in cohort 5 and 1.32 ml in cohort 6. Specific PK parameters (terminal t1/2 and AUC0-t) were determined for both the activity and antigen data using data from the end of the infusion period (hour 144) through the end of the data collection period (at least hour 240) (Table 3). Cohort 5 demonstrated that a median 15.7% FIX activity level (interquartile range [IQR], 14.9% –16.6%) was reached after six daily doses (Figure 2). Cohort 6 activity levels were greater than 30% before neutralizing antibody emergence reduced DalcA activity.
3.4 Pharmacodynamics
In all subjects, the change from the baseline in mean PD parameter levels at the end of each study cohort was analyzed as follows. The APTT levels were decreased in line with the expected pharmacology of increased FIX levels. The international normalized ratio (INR) was minimally changed in line with the expected pharmacology of FIX levels that have a small effect on PT and INR. There were no significant increases in TAT and in fibrinogen levels throughout the study. There were only minimal increases in D-dimer and in F1+2 levels throughout the study.
4 DISCUSSION
Subcutaneous administration confers a major advantage over IV infusion with the potential for simple and rapid injection, improved quality of life, and reduction in health care burden. Because of the characteristics of the administration route, SC products are limited by volume (typically 1–2 ml). Currently developed FIX products have low activity levels making them unsuitable for SC administration because of volume limitations. For example, a 50 IU/kg dose of WT-FIX to a patient who weighs 60 kg would require a total dose of 3000 IU, which translates to 5 ml in administration volume. DalcA provides benefits over currently marketed IV FIX products with simple SC administration, small volume injection (the mean volume in this study was 1.12 ml), prolonged t1/2, with SC injection and the potential to maintain continuous protective levels.
Extended t1/2 FIX products were not available in Korea at the time of this study. These products have a half-life of 86 to 97 h22, 23 and require approximately weekly IV infusions, and some have reduced extravascular distribution that has been associated with reduced prevention of bleeding.16, 24
PK and activity of DalcA demonstrate the 24-fold greater potency over BeneFIX, with a slightly longer t1/2 (25.3 vs 21.0 h) and longer MRT (Table 3). The bioavailability of DalcA was 8.2% to 20.3%, Tmax was 24 to 48 h and the SC beta t1/2 for a single dose was 53.9 to 106.9 h (cohorts 2 and 3; Table 3). Cohort 4 was dropped as it was considered not needed because of the low plasma levels resulting from single dose SC injection precluded accurate measurement of PK. Cohort 5 showed that a median 15.7% FIX activity level (IQR, 14.9-16.6) was reached with a mean terminal t1/2 of 64.6 h (IQR, 59.2-73.2 h) after 6 days of dosing. Blood coagulation assessments demonstrated that APTT was shortened, INRs were minimally changed in line with the expected pharmacology of FIX levels that have a small effect on PT and INR. There were no significant increases in TAT, fibrinogen, D-dimer, and F1+2 levels throughout the study. There were no serious systemic AEs or adverse drug reactions and no premature withdrawal of participation because of AEs. Injection site reactions were mild to moderate and did not prevent the adherence to daily dosing. Two subjects, who were cousins with the same genotype and similar human leukocyte antigen profile, developed neutralizing antibodies to DalcA (one transient low-titer) that did not cross-react with WT-FIX and, therefore, are not referred to as inhibitors of WT-FIX. The presence of ADA without neutralizing potential was not associated with decreased efficacy. No cases of anaphylaxis or hypersensitivity were reported in ADA-positive patients. Patients who tested positive for ADA did not have a safety profile that differed from that of the overall population, specifically, there was no trend towards increased frequency or severity of injection site reactions after patients tested positive for ADA. DalcA is a novel therapeutic agent, and the patients were able to successfully resume use of their prior FIX therapy, indicating that these DalcA neutralizing antibodies were not inhibitors to the class of FIX. Importantly, the presence of ADA was not associated with an effect on safety. A subsequent comprehensive investigation concluded that the immunogenic potential of DalcA was low, similar to that of commercial FIX products, and that drug product quality was also comparable to commercial FIX products.20 Based on these analyses, the rate of ADA with risk of clinical consequences in patients treated with DalcA is very low. Furthermore, results suggest that long-term dosing of DalcA has the potential to maintain FIX activity in the mild hemophilia to normal range. Collagen saturation by an IV administration before SC dosing may increase the bioavailability and result in shortening of the time required to reach the target activity levels. Subcutaneous dosing of DalcA may provide superior prophylaxis to IV extended half-life agents because extravascular distribution and binding to collagen is the same as for WT-FIX.
Although this study presents important data pertaining to the clinical PK, PD, and safety profile of DalcA, there are some limitations. The study analysis was derived from a small number of patients (11 unique subjects completed the study). Although this study initially used a chromogenic activity assay, we subsequently learned that the activity of dalcinonacog alfa is best determined by a one-stage assay.25, 26 Last, only patients aged 12 to 65 years were enrolled in this study.
5 CONCLUSION
This phase 1/2a (NCT03186677) proof-of-concept study evaluated the safety, PK, and PD of daily SC administration of DalcA in individuals with severe hemophilia B. The study objective was to assess the ability of DalcA to increase trough FIX activity from approximately 1% to >12%, essentially converting patients from severe to mild hemophilia levels. FIX levels of at least 12% are required to eliminate spontaneous hemarthrosis.27, 28 These data demonstrated that DalcA may be efficacious for the treatment of hemophilia B (with the absence of bleeding) and SC DalcA prophylaxis achieved continuously protective FIX activity levels between 12% and 30%, corresponding to a reduced chance of spontaneous bleeds. In general, DalcA was well tolerated. The prolonged t1/2 and stable high levels of FIX activity have the potential to allow for less frequent dosing. Based on the results of the evaluation and discussions with clinicians and regulatory experts, we are continuing development of DalcA and initiated a phase 2b trial to assess the safety and efficacy of 28 days of daily SC dosing in six subjects (NCT03995784). Data presented at the 2020 Annual Congress of the European Association for Haemophilia Allied Disorders showed that 28 days of daily SC dosing of DalcA achieved protective target FIX levels of >12%, with steady-state FIX levels of up to 27% after 14 days of dosing and no bleeds occurred during the entire study period, demonstrating effective prophylaxis and the potential for lower or less frequent dosing. In addition, no serious AEs or neutralizing antibodies were reported by trial subjects.29
ACKNOWLEDGMENTS
This study was supported by Catalyst Biosciences, Inc., and ISU Abxis. Medical writing support was provided by Bronwyn Boyes and sponsored by Catalyst Biosciences.
CONFLICT OF INTEREST
Drs. You, Shin, J. S. Kim, Han, S.-J. Kim, and D. Y. Kim have nothing to disclose. Drs. Hong and Kim report personal fees from ISU Abxis during the conduct of the study. Dr. Lee reports personal fees from Catalyst Bioscience during the conduct of the study. Dr. Levy reports personal fees from Catalyst Biosciences during the conduct of the study.
AUTHOR CONTRIBUTIONS
Chur Woo You was involved in investigation and supervision. Seung-Beom Hong was involved in the methodology. Suyeong Kim was involved in the methodology. Ho-Jin Shin was involved in investigation. Jin Seok Kim was involved in investigation. Jung Woo Han was involved in investigation. Soo-Jeong Kim was involved in investigation. Do Young Kim was involved in investigation. Martin Lee was involved in the formal analysis and interpretation. Howard Levy was involved in conceptualization, methodology, and interpretation.