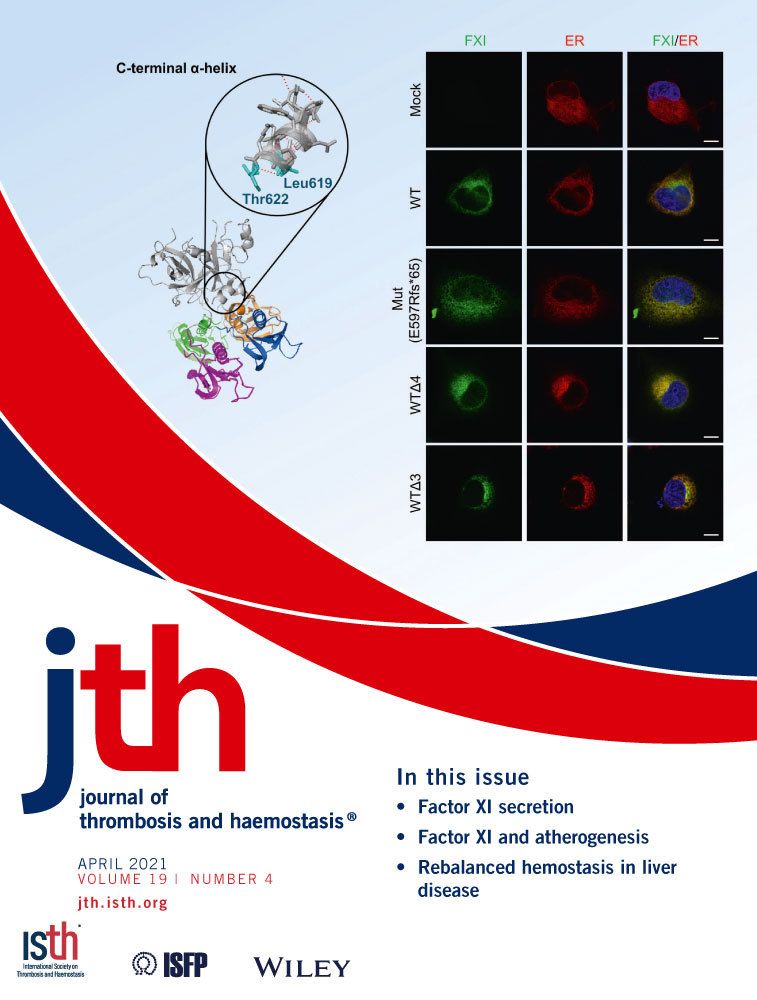
Essential role of a carboxyl-terminal α-helix motif in the secretion of coagulation factor XI
Manuscript handled by: Alan Mast
Final decision: Alan Mast, 4 January 2021
Abstract
Background
Coagulation factor XI (FXI) is a plasma serine protease zymogen that contributes to hemostasis. However, the mechanism of its secretion remains unclear.
Objective
To determine the molecular mechanism of FXI secretion by characterizing a novel FXI mutant identified in a FXI-deficient Japanese patient.
Patient/Methods
The FXI gene (F11) was analyzed by direct sequencing. Mutant recombinant FXI (rFXI) was overexpressed in HEK293 or COS-7 cells. Western blotting and enzyme-linked immunosorbent assay were performed to examine the FXI extracellular secretion profile. Immunofluorescence microscopy was used to investigate the subcellular localization of the rFXI mutant.
Results
We identified a novel homozygous frameshift mutation in F11 [c.1788dupC (p.E597Rfs*65)], resulting in a unique and extended carboxyl-terminal (C-terminal) structure in FXI. Although rFXI-E597Rfs*65 was intracellularly synthesized, its extracellular secretion was markedly reduced. Subcellular localization analysis revealed that rFXI-E597Rfs*65 was abnormally retained in the endoplasmic reticulum (ER). We generated a series of C-terminal–truncated rFXI mutants to further investigate the role of the C-terminal region in FXI secretion. Serial rFXI experiments revealed that a threonine at position 622, the fourth residue from the C-terminus, was essential for secretion. Notably, Thr622 engages in the formation of an α-helix motif, indicating the importance of the C-terminal α-helix in FXI intracellular behavior and secretion.
Conclusion
FXI E597Rfs*65 results in the pathogenesis of a severe secretory defect resulting from aberrant ER-to-Golgi trafficking caused by the lack of a C-terminal α-helix motif. This study demonstrates the impact of the C-terminal structure, especially the α-helix motif, on FXI secretion.
Essentials
- The role of the carboxyl-terminal (C-terminal) region in factor XI (FXI) remains unclear.
- We generated a panel of recombinant FXI mutants and examined their secreted and functional levels.
- Abnormal C-terminal structure of FXI leads to aberrant retention in the endoplasmic reticulum.
- The C-terminal α-helix motif is crucial for FXI extracellular secretion.
1 INTRODUCTION
Coagulation factor XI (FXI) is a plasma serine protease zymogen of activated FXI (FXIa) that contributes to hemostasis by triggering the activation of coagulation factor IX (FIX).1 The plasma antigen level of FXI is 3 to 7 µg/ml, and its activity (FXI: C) ranges from 70 to 150 IU/dl in normal human plasma.2 The FXI monomer contains four tandem apple domains (A1, A2, A3, and A4) and a carboxyl-terminal (C-terminal) serine protease catalytic domain. FXI forms a homodimer via a disulfide bond, and this dimeric structure is essential for its extracellular secretion and enzymatic function.3-5
FXI activation by activated factor XII (FXIIa) initiates fibrin formation in a cascade/waterfall model of blood coagulation. More recently, however, FXI/FXIa was considered as part of a feedback loop that sustained thrombin generation in a cell-based model.6 In this feedback loop, α-thrombin and the autoactivation of FXIa activate FXI, which consolidates the coagulation process.7-9 FXI/FXIa also interacts with activated platelets via GPIb and/or ApoER2, which may contribute to the localization of FXI/FXIa to the fibrin formation site.10, 11
The gene encoding FXI (F11), located at 4q35.2, is 23 kb long and consists of 15 exons and 14 introns. The F11 transcript is translated to 625 amino acids, including an 18-amino-acid signal peptide.12 Mutations within the F11 gene cause FXI deficiency, an inherited autosomal-recessive bleeding disorder characterized by abnormal bleeding events after trauma or surgery and rare instances of spontaneous bleeding. To date, 244 different F11 mutations, more than 50% of which are missense mutations, are recorded in the Human Gene Mutation Database (http://www.hgmd.org; last updated in 2019).
Coagulation serine proteases share extensive structural homology and possess an α-helix motif at their C-terminal region.13 In spite of their structural similarities, these proteases differ in length and amino acid composition of the C-terminal region, which could underlie their functional and/or molecular behavioral differences. Several studies have indicated that a lack or alteration of the C-terminal residue causes an extracellular secretory defect in factor VII (FVII),14, 15 FIX,16, 17 factor X (FX),18 and protein C (PC).19 On the other hand, the functional role of the C-terminal region in FXI remains unclear.
In this study, we conducted genetic analysis of a Japanese patient with FXI deficiency and identified a novel homozygous frameshift mutation, F11 c.1788dupC (p.E597Rfs*65), which seemed to be a deleterious mutation resulting in a unique elongated C-terminus of FXI. Subsequently, we investigated the molecular pathogenesis caused by FXI p.E597Rfs*65 and determined the essential role of the C-terminal region, especially the α-helix motif, in the intracellular behavior and secretion of FXI.
2 MATERIALS AND METHODS
2.1 Patient and DNA sample
A 75-year-old Japanese male patient was diagnosed with FXI deficiency (FXI: C < 3 IU/dl). Results of the coagulation laboratory test are shown in Supplementary Table S1. Neither the patient nor his family members reported a tendency for excessive bleeding. Blood samples were collected after the patient provided written informed consent. Genomic DNA (gDNA) was extracted from peripheral blood leukocytes, as previously described.20 This study was approved by the Ethics Committee of the Nagoya University Graduate School of Medicine (identification no. 2015-0391).
2.2 DNA sequencing and genetic copy number analysis
All 15 exons, including exon–intron boundaries of F11, were amplified by polymerase chain reaction (PCR) using KOD FX DNA polymerase (Toyobo, Osaka, Japan) and gene-specific primers (Supplementary Table S2). PCR products were separated via electrophoresis on an ethidium bromide stained 1.5% agarose gel. The identified mutation was described according to the nomenclature of the Human Genome Variation Society (http://www.hgvs.org/mutnomen/).
For copy number analysis, quantitative PCR (qPCR) was performed using SYBR Premix Ex Taq II in Thermal Cycler Dice Real-time System II (Takara Bio Inc., Shiga, Japan). The PCR conditions used were chosen according to the manufacturer's instructions. We designed a quantitative primer set for a neighboring region of the mutation in F11 exon 15. ZBTB37, located on chromosome 1, was used as the genomic reference gene in this qPCR. Each chosen quantitative primer set is listed in Supplementary Table S2.
2.3 Mismatch PCR-restriction fragment length polymorphism (PCR-RFLP) analysis
Mutant-specific mismatch PCR-restriction fragment length polymorphism analysis of F11 exon 15 was performed using a forward primer, 5′-AAGGAGATTGACTGGATGAACG-3′, and a mismatched reverse primer, 5′-CTCCCTTTGAGCACAGCCCGCG-3′ (mismatch bases are underlined), to introduce a KspI site in the amplicon derived from the mutant allele (Supplementary Figure S1A). The amplicons were digested using KspI, and the digested fragments were analyzed by electrophoresis on 3% agarose gel.
2.4 Thrombin generation assay
Thrombin generation assays were performed in accordance with our previous report.21 In brief, we monitored the reactions for 2 h using Fluoroskan Ascent FL (Thermo Labsystems, Philadelphia, PA, USA) calibrated automated thrombography (Thrombinoscope BV, Maastricht, the Netherlands) and Thrombinoscope software program (Thrombinoscope BV). The excitation and emission wavelengths used were 390 nm and 460 nm, respectively. Serial dilutions of normal pooled plasma in FXI-depleted plasma (Sysmex, Kobe, Japan) were used as references.
2.5 Construction of recombinant FXI expression vector
A complementary DNA (cDNA) fragment of human FXI (wild-type [WT] FXI) was obtained from a human liver cDNA library (Takara Bio). The FXI coding sequence was inserted into a pcDNA3.1 (+) vector (Invitrogen, Carlsbad, CA, USA). Each mutant FXI expression vector was generated using site-directed mutagenesis with specific primer sets (Supplementary Table S3). The sequences of all FXI expression vectors were confirmed by direct sequencing.
2.6 Transient expression of recombinant FXI
HEK293 cells were grown at 37°C under 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) (Wako, Osaka, Japan) supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO, USA) and penicillin/streptomycin/amphotericin B (Wako). A WT or individual mutant FXI expression vectors was transiently transfected into the cells using Lipofectamine 3000 reagent (Invitrogen) according to the manufacturer's instructions. Five hours after transfection, the medium was changed to DMEM containing 10% fetal bovine serum, and the cells were incubated for additional 19 h. Subsequently, the culture medium was changed to serum-free DMEM, and the cells were further incubated for 24 h.
2.7 Western blotting analysis
Twenty-four hours after the change to serum-free medium as described earlier, the transfected cells and culture media were harvested. Then, cells were lysed in sodium dodecyl sulfate (SDS) sample buffer (50 mM Tris-HCl, 2% SDS, and 10% glycerol; pH 6.8) to prepare the cell lysate. The culture media were centrifuged at 3000g for 10 min to remove debris, and the protein was concentrated by acetone precipitation.22 Thereafter, the precipitated pellet was dissolved in SDS sample buffer. The total protein concentration in each sample was measured using DC Protein Assay Kit (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer's instructions. Equal amounts of total protein in each sample were boiled for 5 min with or without 2-mercaptoethanol, then subjected to SDS–polyacrylamide gel electrophoresis (PAGE) (6% or 7.5%), and transferred onto Immobilon-P Transfer Membrane (Merck, Darmstadt, Germany). After blocking with 2.5% skim milk for 2 h at room temperature, the membranes were incubated overnight with monoclonal mouse anti-human FXI antibody (1:1,000 dilution, clone: G-2; Santa Cruz Biotechnology, Dallas, TX, USA) or monoclonal mouse anti-β-actin antibody (1:2,500 dilution, clone: BA3R; BioVision Inc., Milpitas, CA, USA) at room temperature. The membranes were subsequently incubated with horseradish peroxidase–conjugated goat anti-mouse immunoglobulin G (IgG) (1:5,000 dilution; Cell Signaling Technology, Danvers, MA, USA) for 90 min at room temperature. Next, the membranes were incubated with ECL Select Western Blotting Detection Reagent (GE Healthcare UK Ltd., Little Chalfont, UK) and the signals on them were detected using the Light Capture II system (Atto Corporation, Tokyo, Japan).
2.8 Measurement of FXI protein level
FXI protein levels in the plasma and culture media were measured by enzyme-linked immunosorbent assay (ELISA) using Human Factor XI ELISA Kit (product no. ab168547; Abcam, Cambridge, UK) according to the manufacturer's instructions. The antibodies used for capture and detection in this ELISA kit were anti-FXI polyclonal antibodies. The standard curve was created with a plasma-derived FXI, which was included in the ELISA kit (Abcam).
2.9 Coagulant activity of recombinant FXI
The coagulant activity of recombinant FXI (rFXI) mutants was measured by an activated partial thromboplastin time (APTT)-based one-stage clotting assay using C.K. Prest 2 (FUJIREBIO Inc., Tokyo, Japan). FXI-depleted plasma (George King Bio-Medical, Inc., Overland Park, KS, USA) was supplemented with rFXI-containing culture medium and subjected to the APTT-based assay. Coagulation times from serial dilutions of rFXI-WT were used to create a standard curve. The specific coagulant activity was calculated as the ratio between the coagulant activity (% of rFXI-WT) and the secreted protein level (% of rFXI-WT).
2.10 Immunofluorescence microscopy
COS-7 cells were grown on a cover glass measuring 15 mm in diameter in 12-well plates and transiently transfected with WT or individual mutant FXI expression vectors. Twenty-four hours after transfection, the cells were washed twice with phosphate buffered saline (PBS), fixed with 4% paraformaldehyde in PBS for 15 min, and permeabilized with 0.5% Triton X-100 in PBS for 10 min at room temperature. After washing again with PBS, the cells were incubated with an endoplasmic reticulum (ER) marker, Alexa Fluor 594-conjugated concanavalin A (25 µg/ml; Invitrogen), or a Golgi apparatus marker, Alexa Fluor 488-conjugated lectin Helix pomatia agglutinin (10 µg/ml; Invitrogen) for 30 min at room temperature. Subsequently, the cells were blocked with PBS containing 3% bovine serum albumin and 2% goat serum for 1 h at room temperature, and then incubated overnight with a monoclonal mouse anti-human FXI antibody (1:200 dilution, clone: G-2; Santa Cruz Biotechnology) at 4°C. The secondary antibody, Alexa Fluor 488-conjugated goat anti-mouse IgG or Alexa Fluor 555-conjugated goat anti-mouse IgG (Invitrogen), was 1:1000 diluted. Finally, the cover glass was mounted on a glass slide with VECTASHIELD Antifade Mounting Medium containing 4′, 6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA, USA) and observed using an inverted confocal laser microscope (A1Rsi; Nikon). The fluorescence intensity was analyzed using the ImageJ plot profile function (National Institutes of Health). FXI colocalizations with the ER or the Golgi apparatus were statistically evaluated using Pearson's correlation coefficient (PCC), relying on Fiji's plugin, Coloc2 (https://imagej.net/Coloc_2), for the ImageJ software program (National Institutes of Health). For quantitative analysis of FXI colocalization with subcellular organelles, we referred the method of Pignani et al.23 and Cohen et al.24
2.11 Statistics
All quantitative data are presented as mean ± standard deviation (SD) or standard error of the mean values. Comparisons involving multiple groups were performed using Tukey's one-way analysis of variance. Statistical analyses were carried out using GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA).
3 RESULTS
3.1 F11 c.1788dupC is a pathogenic mutation representing the FXI null phenotype
We herein analyzed F11 in a patient with FXI deficiency and identified a novel duplication mutation at exon 15, c.1788dupC (Figure 1A). Gene sequencing revealed a single peak of c.1788dupC, indicating a homo- or hemizygous mutation. Moreover, copy number analysis using qPCR showed that the gDNA of the patient retained a gene dosage of F11 exon 15 equal to those of gDNA from pooled normal individuals (Figure 1B). This finding suggests that the patient carried a homozygous F11 c.1788dupC. Next, to investigate whether F11 c.1788dupC was a polymorphism in the Japanese population, we designed a mismatch PCR-RFLP analysis (Supplementary Figure S1) and analyzed 104 alleles in normal Japanese individuals (n = 52 subjects); however, no mutant alleles were detected.
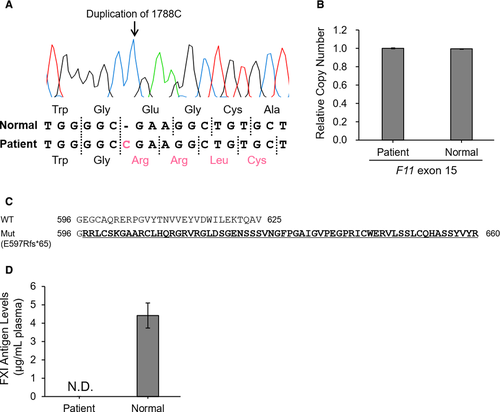
F11 c.1788dupC causes a unique FXI frameshift, p.E597Rfs*65, which is an extra–C-terminal extension (Figure 1C). In the mutant FXI, the glutamic acid residue located at position 597 is replaced by arginine and carries an abnormal 64-amino-acid alignment. Thrombin generation assays showed that the peak score (199.24) of the patient plasma was comparable with those of FXI 1.6% to 0% plasma, reconstituted by serial dilutions of normal pooled plasma with FXI-deficient plasma (Supplementary Figure S2). This result suggests that the F11 c.1788dupC (p.E597Rfs*65) represents the FXI null mutation. An assessment of plasma FXI levels using commercial ELISA kit revealed that the FXI level in the patient was undetectable (Figure 1D). Thus, we characterized F11 c.1788dupC as a pathogenic mutation causing the FXI-null phenotype.
3.2 Abnormal C-terminal structure causes a defect in FXI extracellular secretion
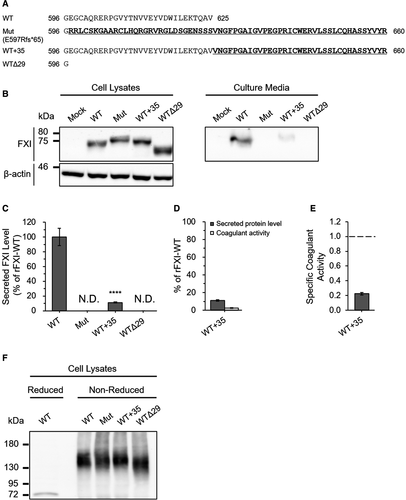
To discern the possible molecular pathogenesis caused by FXI E597Rfs*65, we constructed the following rFXI expression vectors: rFXI-Mut (E597Rfs*65), WT+35, and WTΔ29 (Figure 2A). rFXI-WT+35 is a C-terminal–extended rFXI mutant in which 35 amino acids of E597Rfs*65 are added to the C-terminus of WT FXI. rFXI-WTΔ29 is a C-terminal–deleted rFXI mutant in which 29 amino acids are deleted from the C-terminus of WT FXI. We transiently expressed WT or each of the rFXI mutants in HEK293 cells and analyzed the resultant rFXI production and extracellular secretion. Western blotting (WB) analysis of cell lysates showed no apparent quantitative differences among intracellular rFXIs (Figure 2B, left). In contrast, WB of culture media revealed a remarkable decrease in the secreted levels of rFXI-Mut, WT+35, and WTΔ29 (Figure 2B, right). In particular, the secreted levels of rFXI-Mut and rFXI-WTΔ29 were undetectable. To confirm this observation, we quantitatively assessed the secreted rFXI levels by ELISA (Figure 2C). The levels of secreted rFXI-WT and WT+35 were 697.3 ± 82.0 ng/ml and 77.2 ± 4.9 ng/ml (11.1 ± 0.7% of rFXI-WT), respectively. Consistent with the findings of the aforementioned WB analysis, the secreted levels of rFXI-Mut and rFXI-WTΔ29 were undetectable by ELISA (Figure 2C). In addition, to assess the coagulation function of the C-terminal–extended rFXI mutant, we measured the coagulant activity of rFXI-WT+35 by APTT-based one-stage assay. The coagulant activity and specific activity of rFXI-WT+35 were 2.5 ± 0.3% of rFXI-WT and 0.22 ± 0.02, respectively (Figure 2D,E). These results suggested that the addition of amino acids to the C-terminus severely affected the secretory behavior and functional activity of FXI. Furthermore, because FXI secretion was dependent on its homodimer structure with a disulfide cross-link,3-5 we analyzed whether these rFXI mutants intracellularly composed dimers using nonreduced WB (Figure 2F). rFXI-Mut, WT+35, and WTΔ29 were detected as dimers in cell lysates, suggesting that the C-terminal abnormality had no effect on FXI dimer formation.
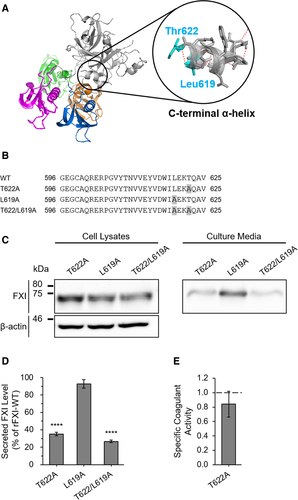
3.3 Threonine at position 622 is essential for FXI secretion
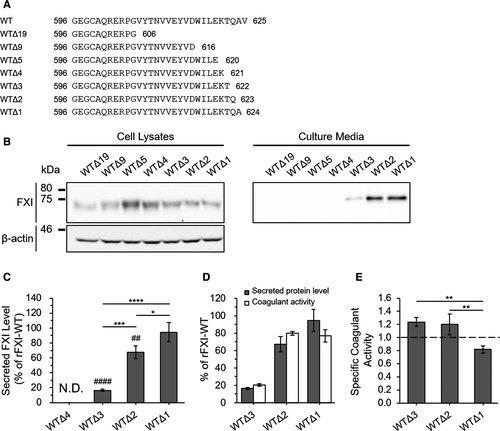
Given the observation that the C-terminus of FXI was associated with its extracellular secretion, we next investigated the amino acid(s) responsible for its secretion. To screen for functional alignment within the C-terminus, we designed the following series of C-terminal–truncated FXI mutants: rFXI-WTΔ19, WTΔ9, WTΔ5, WTΔ4, WTΔ3, WTΔ2, and WTΔ1 (Figure 3A). WB analysis detected all of these rFXI mutants in the cell lysate (Figure 3B, left). On the other hand, rFXI-WTΔ3, WTΔ2, and WTΔ1 were detected in the culture media, whereas further truncated mutants, specifically rFXI-WTΔ19, WTΔ9, WTΔ5, and WTΔ4, were undetectable (Figure 3B, right). ELISA of the culture media showed that the secreted levels of rFXI-WTΔ3, WTΔ2, and WTΔ1 were 16.5 ± 1.3%, 67.4 ± 8.7%, and 94.5 ± 12.7% of rFXI-WT, respectively (Figure 3C). The secreted level of rFXI-WTΔ4 was undetectable by ELISA, which was consistent with the WB data. To assess the coagulation function of C-terminal–truncated rFXI mutants, we measured the coagulant activity of the secreted rFXI deletion mutants, rFXI-WTΔ3, WTΔ2, and WTΔ1 (Figure 3D,E). The coagulant activity values of rFXI-WTΔ3, WTΔ2, and WTΔ1 were 20.4 ± 1.6%, 79.9 ± 2.2%, and 76.9 ± 7.1% of rFXI-WT respectively (Figure 3D). Moreover, the specific activity values of these rFXI mutants were 1.24 ± 0.07 (WTΔ3), 1.20 ± 0.16 (WTΔ2), and 0.86 ± 0.05 (WTΔ1) (Figure 3E). Although the specific activity of rFXI-WTΔ1 decreased with a level of statistical significance relative to rFXI-WTΔ2 or WTΔ3, we considered that the coagulant activities of rFXI-WTΔ3, WTΔ2, and WTΔ1 were all retained. The sequential C-terminal deletion of FXI, especially the second or third residue from the C-terminus, causes a decrease in their extracellular secretion level while maintaining relevant activity, which indicates a major contribution of these residues on FXI secretion. Meanwhile, the deletion of the fourth residue from the FXI C-terminus, threonine at position 622 (Thr622), causes a severe FXI secretion defect, which indicates an essential role of Thr622 in FXI secretion.
3.4 FXI C-terminal α-helix is a crucial motif for extracellular secretion
To investigate the characteristics of Thr622 in the context of FXI secretion, we conducted an in silico structure simulation. In silico analysis suggested that Thr622 was a component residue of the C-terminal α-helix (Figure 4A). In the α-helix motif, Thr622 is bound to leucine at position 619 (Leu619) via main-chain conjunction with a hydrogen bond, which is formed between a hydrogen atom of the amino group in Thr622 and an oxygen atom of the carboxy group in Leu619. This simulation suggested that the lack or amino acid substitutions of Thr622 led to the destruction of the FXI C-terminal α-helix motif. To verify the validity of the simulation, we generated rFXIs with alanine substitution of Thr622 (T622A) and/or Leu619 (L619A) (Figure 4B). WB analysis showed that these alanine-substituted rFXI mutants were all detected in the cell lysate and culture media (Figure 4C). ELISA of culture media showed that the secreted levels of rFXI-T622A, L619A, and T622/L619A were 35.2 ± 2.1%, 92.8 ± 4.9%, and 26.8 ± 1.3% of rFXI-WT, respectively (Figure 4D). To further assess the properties of Thr622, we measured the specific activity of rFXI-T622A, which was found to be 0.84 ± 0.18 (Figure 4E). These observations indicated that the alanine substitution at position 622 of FXI reduced its secretory capacity but retained the normal specific coagulant activity. The present results suggest that the α-helix at the C-terminus is an indispensable motif for extracellular secretion of FXI.
3.5 C-terminal disruption in FXI leads to its abnormal ER retention
To investigate the subcellular localization of C-terminal–disrupted rFXIs, we expressed rFXI-Mut (E597Rfs*65) or a C-terminal–truncated rFXI mutant (rFXI-WTΔ4 and rFXI-WTΔ3) in COS-7 cells and observed their colocalization with the ER (Figure 5) or the Golgi apparatus (Figure 6). Colocalization of rFXI with the ER or the Golgi apparatus was quantitatively examined by the calculation of PCC.
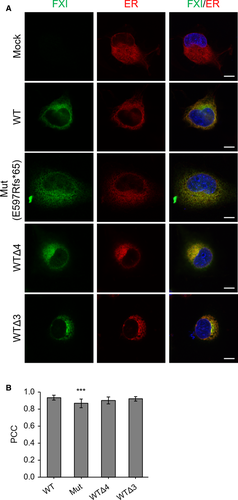
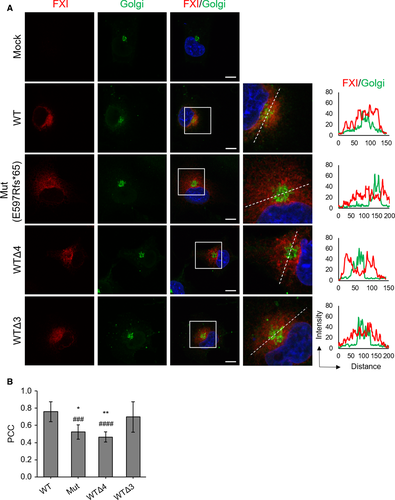
In double staining of FXI and the ER, all rFXIs were found to be highly colocalized with the ER (Figure 5A,B). Although the PCC value was >0.8 for all rFXIs, rFXI-Mut showed a statistically significant decrease in PCC when paired with the ER, indicating an intracellular distribution abnormality of rFXI-Mut. Double staining of FXI and the Golgi apparatus showed that secretory rFXIs (rFXI-WT and rFXI-WTΔ3) were colocalized with the Golgi apparatus (Figure 6A). In contrast, nonsecretory rFXIs (rFXI-Mut and rFXI-WTΔ4) were barely detectable in the Golgi apparatus. Fluorescence intensity analysis visualized the distribution difference of the secretory- and nonsecretory-rFXIs in the Golgi apparatus (Figure 6A, right graphs), and the PCC measurements further supported these findings (Figure 6B). The PCC values of the nonsecretory rFXIs (rFXI-Mut and rFXI-WTΔ4) were significantly lower than that of the secretory rFXIs (rFXI-WT and rFXI-WTΔ3). These observations indicated that rFXI mutants with disrupted C-terminus were abnormally retained in the ER and not transported into the Golgi apparatus. In fact, the lack of a C-terminal α-helix motif severely impaired ER-to-Golgi trafficking of FXI.
4 DISCUSSION
In the present study, we identified a novel duplication mutation, F11 c.1788dupC, in a 75-year-old Japanese male patient that resulted in a frameshift mutant protein with the C-terminal structural alteration, FXI p.E597Rfs*65. FXI E597Rfs*65 has a unique C-terminus, in which Glu597 is replaced by Arg and contains 64 abnormal amino acids. The major feature of this mutation is the lack of a C-terminal α-helix motif. This study revealed that C-terminal disruption resulted in extracellular secretion defect of FXI resulting from abnormal ER retention. In particular, it was demonstrated that the C-terminal α-helix was the key motif in ER-to-Golgi trafficking of FXI. Consequently, a severe secretory defect of FXI from aberrant ER-to-Golgi trafficking, which is driven by the lack of a C-terminal α-helix motif, can be considered as the molecular pathogenesis caused by FXI p.E597Rfs*65.
In addition to the absence of the C-terminal α-helix, FXI E597Rfs*65 has another molecular feature: the C-terminal extra tail, which is composed of 35 amino acids. To investigate the pathogenic potential of the extra tail, we generated rFXI-WT+35, in which amino acids were added to the C-terminus of FXI-WT (Figure 2). Interestingly, the extra tail significantly reduced the extracellular secretion of FXI, indicating a deleterious characteristic of the extra tail in FXI secretion. Hydrophobicity analysis indicated that the extra-tail alignment had a highly hydrophobic property (Supplementary Figure S3). Our group has previously identified a C-terminal–extended protein C, labeled as protein C Nagoya, which is retained and degraded within the ER.25, 26 Protein C Nagoya also has a high hydrophobic C-terminal tail, resulting in abnormal binding with ER-retention chaperones such as immunoglobulin heavy chain-binding protein (BiP, known as GRP78), which possesses an ER-retention signal (Lys-Asp-Glu-Leu, KDEL). BiP combines with misfolded or abnormally structured proteins and induces retention/degradation of the unconformable proteins in the ER.27 We consider that the extra tail of FXI E597Rfs*65 is also disposed of by ER-retention chaperones such as BiP.
Furthermore, the specific activity of rFXI-WT+35 was significantly reduced (Figure 2E), suggesting that the C-terminal extra tail inhibited the cleavage of FIX, the substrate of FXIa. FXIa mediates the conversion of FIX to the protease FIXaβ via an intermediate form of FIXaα.28, 29 In the FIX-to-FIXaβ conversion process, FIX is catalyzed in a small pocket formed by the interface between the FXIa A3 domain and the serine protease catalytic domain.30, 31 The crystal structure model in Figure 4A shows that the FXI(a) C-terminal α-helix motif faces the small pocket. Thus, we consider that the extra-tail of rFXI-WT+35 interfered with FIX cleavage within the small pocket.
C-terminal alignment in coagulation or anticoagulation factors has been discussed regarding their secretory behavior. Studies of mutant FVII,14, 15 FIX,16, 17 FX,18 and PC19 have revealed that the loss or alteration of a C-terminal residue causes extracellular secretory defects. Notably, the C-terminal structure of FXI resembles those of serine protease coagulation/anticoagulation factors (Supplementary Figure S4). In fact, the FXI C-terminal aberrance, especially the lack of a C-terminal α-helix motif, impairs its ER-to-Golgi trafficking. These previous and present findings together indicate that the C-terminal motif has an impact on coagulation/anticoagulation factor secretion. To date, several recombinant coagulation factors have been developed for use as replacement/supplemental therapies to treat bleeding disorders (e.g., hemophilia) or thrombosis (e.g., antithrombin deficiency). Among these, some recombinant factors are combined with an extra domain at their C-terminus to extend their half-life in circulation. However, in some molecules, the inclusion of an additional domain at the C-terminus presents an increased risk of lowering the secretion and/or functional activity of the molecule, as seen in the present findings concerning rFXI-WT+35. We believe that further investigations of the C-terminus in coagulation factors (mainly serine protease type coagulation factors) will contribute to enhanced productivity and/or function of therapeutic recombinant products.
In conclusion, we have disclosed here the molecular mechanism of FXI extracellular secretion through the investigation of FXI p.E597Rfs*65. The C-terminal structure, especially the α-helix, is a crucial motif for FXI ER-to-Golgi trafficking. Besides, the addition of the extra tail to the FXI C-terminus causes secretory and activity defects. In the clinical setting, FXI/FXIa has emerged as a novel target for antithrombotic therapy.32-35 The present findings regarding the molecular characteristics of FXI may provide fundamental knowledge for advancements in antithrombotic drug development.
ACKNOWLEDGMENTS
The authors thank Mrs. Wakamatsu for her excellent technical assistance. This study was supported in part by grants-in-aid provided by the Japanese Ministry of Education, Culture, Sports, Science, and Technology (grant no. 16K09825 to T. Kojima) and the Japanese Ministry of Health, Labor, and Welfare (Research on Measures for Intractable Diseases, grant no. 2017-012 to T. Kojima). We also thank Wiley Editing services for English language editing.
CONFLICT OF INTEREST
The authors declare no conflicts of interest exist that are associated with this manuscript.
AUTHORS CONTRIBUTIONS
Yuri Hayakawa and Shogo Tamura designed and performed the research, analyzed the data, and drafted the manuscript. Nobuaki Suzuki designed the project, and collected and analyzed the clinical data. Koya Odaira, Mahiru Tokoro, Fumika Kawashima, Fumihiko Hayakawa, and Akira Takagi conducted the research and analyzed the data. Akira Katsumi, Atsuo Suzuki, Shuichi Okamoto, Takeshi Kanematsu, and Tadashi Matsushita developed the project, and collected and analyzed the clinical data. Tetsuhito Kojima designed the project, analyzed the data, and drafted the manuscript.