Monitoring of anticoagulation in thrombotic antiphospholipid syndrome
Abstract
Anticoagulation is central to the management of thrombotic antiphospholipid syndrome (APS). The standard anticoagulant treatment for thrombotic APS is life-long warfarin or an alternative vitamin K antagonist. The role of direct oral anticoagulants for thrombotic APS is not established due to the lack of definitive evidence and has recently been addressed in international guidance. Other anticoagulant options include low molecular weight heparin, unfractionated heparin, and fondaparinux. In APS patients, lupus anticoagulant can affect phospholipid-dependent coagulation monitoring tests, so that they may not reflect true anticoagulation intensity. Accurate assessment of anticoagulation intensity is essential, to optimize anticoagulant dosing and facilitate thrombus resolution; minimize the risk of recurrent thrombosis or bleeding; inform assessment of whether recurrent thrombosis is related to breakthrough thrombosis while on therapeutic anticoagulation, subtherapeutic anticoagulation, non-adherence, or spurious results; and guide the management of bleeding. Knowledge of anticoagulant intensity also informs assessment and comparison of anticoagulation regimens in clinical studies. Considerations regarding anticoagulation dosing and/or monitoring of thrombotic APS patients underpin appropriate management in special situations, notably APS-related severe renal impairment, which can occur in APS or APS/systemic lupus erythematosus-related nephropathy or catastrophic APS; and APS-related thrombocytopenia. Anticoagulant dosing and monitoring in thrombotic APS patients also require consideration in anticoagulant-refractory APS and during pregnancy. In this review, we summarize the tests generally used in monitoring anticoagulant therapy, use of the main anticoagulants considered for thrombotic APS, lupus anticoagulant effects on anticoagulation monitoring tests, and strategies for appropriate anticoagulant monitoring in thrombotic APS.
1 INTRODUCTION
Antiphospholipid syndrome (APS) is an acquired autoimmune disorder characterized by thrombosis and/or pregnancy morbidity in association with persistent antiphospholipid antibodies (aPL; lupus anticoagulant [LA], anticardiolipin [aCL], and/or anti-beta-2-glycoprotein I [aβ2GPI]).1, 2 Thrombosis may be venous, arterial, and/or microvascular.1 Catastrophic APS (CAPS), the most severe form of APS, which occurs in approximately 1% of APS patients and has a mortality rate of ~ 30%, is associated with mainly multiple small vessel thromboses leading to a disseminated microangiopathic syndrome.3, 4 In a prospective study, thrombotic APS, noted in ~ 15% of patients with systemic lupus erythematosus (SLE), was a major predictor of irreversible organ damage and death.5
The main thrombotic manifestations of APS are lower limb deep venous thromboses (DVT) and pulmonary emboli, or strokes and transient ischemic attacks (TIAs), accounting for approximately 50% and 30%, respectively.6 A systematic review estimated that 10% of patients with DVT have aPL,7 with the prevalence of thrombotic APS in individuals with a first unprovoked venous thromboembolism (VTE) episode 9%, both in a prospective study in 290 patients8 and a cross-sectional study in 491 patients < 50 years.9 Systematic reviews suggest that ~ 14% of patients with ischemic strokes and 17% of those under the age of 50 years have aPL.7, 10 These figures, as well as the estimated prevalence of APS, 50 per 100 000 of the population, with approximately 80% of patients having DVT or pulmonary embolism, and 45% stroke or TIA,11 suggest possible underdiagnosis of thrombotic APS.
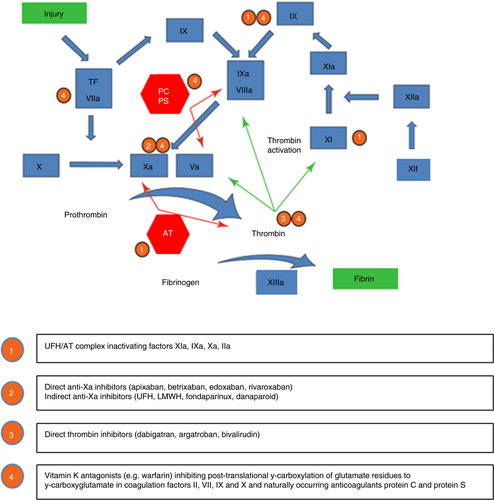
Anticoagulation is central to the management of thrombotic APS. The standard anticoagulant treatment for thrombotic APS is life-long warfarin or an alternative vitamin K antagonist (VKA). The role of direct oral anticoagulants (DOACs) for thrombotic APS has not been established due to the lack of definitive evidence, and has been addressed in International Society on Thrombosis and Haemostasis (ISTH) and 16th International Congress on Antiphospholipid Antibodies Task Force Report on APS Treatment Trends guidance.12, 13 Parenteral anticoagulant options in APS include low molecular weight heparin (LMWH), unfractionated heparin (UFH), and fondaparinux. LMWH, in combination with low dose aspirin (LDA), is standard treatment for thrombotic APS during pregnancy.14 Figure 1 summarizes coagulation mechanisms and targets for actions of different anticoagulants.15 Anticoagulation, in combination with corticosteroids, plasma exchange, and/or intravenous immunoglobulin (IVIG), is standard treatment for CAPS.3, 4, 16 Non-criteria APS manifestations, which include livedo reticularis, thrombocytopenia, hemolytic anemia, cardiac valve disease, nephropathy, skin ulcers, and cognitive dysfunction,1 are generally anticoagulant-refractory and require consideration of further therapeutic approaches.
2 WHAT IS PARTICULAR ABOUT ANTICOAGULATION MONITORING IN THROMBOTIC APS?
Accurate assessment of anticoagulation intensity is essential, to optimize anticoagulant dosing and facilitate thrombus resolution; minimize the risk of recurrent thrombosis or bleeding; inform assessment of whether recurrent thrombosis is related to breakthrough thrombosis while on therapeutic anticoagulation, subtherapeutic anticoagulation, non-adherence, or spurious results; and guide the management of bleeding. Knowledge of anticoagulant intensity also informs assessment and comparison of anticoagulation regimens in clinical studies. Considerations regarding anticoagulant dosing and/or monitoring of thrombotic APS patients underpin appropriate management in special situations, notably APS-related severe renal impairment, that can occur in APS or APS/SLE-related nephropathy or CAPS; and APS-related thrombocytopenia. Anticoagulant dosing and monitoring in thrombotic APS patients also require consideration in anticoagulant-refractory APS patients and during pregnancy. Monitoring of anticoagulation may be hampered by the presence of aPL. In LA-positive APS patients, LA can affect phospholipid-dependent coagulation monitoring tests, so that they may not reflect true anticoagulation intensity.17-19
As in non-APS patients, anticoagulation monitoring should be accompanied by ongoing assessment of venous and arterial thrombotic risk factors and their optimization, and proactive management of potential bleeding risk factors. In addition, patients should be counselled about anticoagulation, with points addressed including the dose regimen, what to do in the event of bleeding or symptoms suggestive of recurrent thrombosis, and potential interacting drugs. In this review, we summarize tests generally used in monitoring anticoagulant therapy, use of the main anticoagulants considered for thrombotic APS, LA effects on anticoagulation monitoring tests, and strategies for appropriate anticoagulant monitoring in thrombotic APS.
3 TESTS USED IN MONITORING ANTICOAGULANT THERAPY
3.1 Prothrombin time-international normalized ratio (PT-INR)
The INR is derived from the PT screening coagulation test (sensitive to reduced activity of factors (F)I (fibrinogen), II (prothrombin), V, VII, and X, according to the formula: INR=(patient's PT/MNPT)ISI, where the MNPT is the mean normal PT and ISI is the international sensitivity index, a correction factor applied to adjust for differences in instrument and reagent sensitivity. Accurate INR determination is critically dependent upon accurate definition of the MNPT and ISI for the reagent/instrument combination. Guidance is available for appropriate ISI verification/validation, to help reduce inter-laboratory variability.20 A European Committee for External Quality Assurance Programmes in Laboratory Medicine study demonstrated that between-laboratory INR variation was lower after local INR calibration (coefficient of variation [CV] 6.7% vs. 8.6), which is strongly recommended.21
3.2 Point-of-care PT-INR testing
Several point-of-care (POC) devices have been shown to give reliable and accurate INR values when compared to traditional laboratory methods.22 The World Health Organisation (WHO) recommended inclusion of plasmas from patients with INRs 1.5-4.5 for calibration of a thromboplastin's ISI.23 Thus, the INR system is strictly valid up to an INR of 4.5. However, higher INRs are measured to assess bleeding risk and guide appropriate management.24 A study of 312 patients on warfarin showed that INRs > 4.5 with the CoaguChek XS Plus were comparable to laboratory INRs.25 Patient self-management (PSM) reduces the risk of mortality and VTE, but probably has no influence on the risk of major bleeding, and patient self-testing (PST) possibly reduces the risk of VTE and major bleeding.26 Accordingly, the American Society of Hematology (ASH) recommends PSM over any other management approach, and suggests PST over any other INR testing approach except for PSM, for suitable patients on maintenance VKA for VTE.26 British Society for Haematology (BSH) guidance for health-care professionals exists on PST/PSM.27
3.3 Activated partial thromboplastin time (aPTT)
The aPTT is sensitive to the activity of coagulation FI, II, V, VIII, IX, X, XI, and XII. It can thus be used to monitor the anticoagulant effect of agents that reduce the activity of these factors, if a therapeutic range can be established. Baseline aPTT prolongation in an acute situation should prompt assessment of fibrinogen to investigate acute phase, and together with assessment of D-dimer, to check for disseminated intravascular coagulation. C-reactive protein (CRP) interferes with the aPTT, prolonging clotting times proportional to CRP concentration and depending on the type of the aPTT reagent.28 Raised levels of FVIII, an acute phase reactant, lead to aPTT shortening.29
3.4 Thrombin time (TT) modifications
The TT measures the conversion of fibrinogen to fibrin following addition of thrombin. The protamine sulphate neutralization assay, a TT-based assay, can be used to calculate the plasma concentration of UFH. Protamine titration produces reliable and reproducible results, but is not generally undertaken in modern hemostasis laboratories, as it is not readily automated30 and has been superseded by anti-Xa assays. In the dilute TT (dTT), a simple, rapid, sensitive, quantitative method, patient sample is diluted with normal plasma prior to the addition of thrombin. The dilution eliminates most interferences on the assay including factor deficiencies, D-dimer, and LA.
3.5 Chromogenic factor X (CFX)
The CFX activity is an alternative assay to the PT-INR. There is an inverse linear relationship between CFX and INR18, 19; a CFX range of 23.5% to 35.5% is suggested to be therapeutic (INR range 2.0-3.0), with the lower CFX limit corresponding to an INR of 3.0.31 CFX assays showed poor utility for INR values ≥ 3.5 in non-APS patients, possibly due to interference by acarboxy-FX when the level of fully functional FX becomes very low, ≤12.0%.18 Therapeutic ranges for CFX are not established, and the assay is not well standardized, nor widely available or practicable for routine use.
3.6 Clotting factor II and X (FII and FX)
PT-based clotting assays for FII and X have been proposed as alternatives to the INR. Plasma concentrations of FII and X were proportional to increases in INR in patients on warfarin, for INRs 2.0 to 3.5. However, for INRs > 3.5, a non-proportional relationship was found between INR and both factors. The authors suggested that below critical clotting factor concentrations (20.6% and 15.6% for factors II and X activity, respectively), the time required for clot formation becomes non-proportional and hemostasis will be jeopardized.32
3.7 Chromogenic anti-Xa or anti-IIa activity
Measurement of anti-Xa or anti-IIa activity is the preferred method when quantitative information is useful. Reference ranges for anti-Xa levels depend on the anticoagulant in use, the type, dose, schedule, and indication. Monitoring is based on peak levels, with the timing of these depending on the half-life of the anticoagulant, and/or trough levels.33 The anti-Xa assay has important advantages, including that it is unaffected by raised CRP or FVIII related to an acute phase reaction; however, it has limitations, including considerable inter-laboratory variation34 and inter-assay variability.35, 36
3.8 Ecarin based assays
Ecarin is a metalloprotease from the saw-scaled viper, Echis carinatus, that converts prothrombin to meizothrombin, which can be inhibited by direct thrombin inhibitors, but not heparin.37 The accuracy of the ecarin clotting time (ECT) may be impacted by fibrinogen and prothrombin deficiencies. The ecarin chromogenic assay (ECA) pre-dilutes the patient sample with a buffer containing prothrombin to minimize the prothrombin factor limitation; and, as it not a clot-based assay, it is not influenced by fibrinogen levels.37
3.9 Thrombin generation (TG)
TG assesses overall functional coagulation status of plasma and the dynamic processes of thrombin generation. The TG curve is quantified in terms of the lag time, time to peak TG, peak TG, and endogenous thrombin potential (ETP), which is the area under the curve.38 Increased ex vivo TG is a marker of thrombogenic potential and has predictive value for recurrent VTE.39 TG assays remain limited to specialized laboratories, and standardized protocols and data normalization should lead to better reproducibility and ability to compare data from different laboratories.40 A more standardized automated TG method has become available: the ST Genesia (Diagnostica Stago, Asnières sur Seine Cedex, France).41 TG is not currently suitable for anticoagulant monitoring, as it is not standardized and no therapeutic ranges have been established.
4 WARFARIN AND OTHER VITAMIN K ANTAGONISTS
Warfarin or an alternative VKA is the standard anticoagulation treatment for thrombotic APS.12-14 Standard-intensity VKA (target INR 2.5, range 2.0-3.0) is used following a first VTE. The optimal anticoagulation intensity for APS-related arterial thrombosis is not defined or agreed, as existing data are not conclusive.13, 14, 42, 43 Recommended options include standard-intensity VKA, with or without LDA or high-intensity VKA, target INR 3.5 (range 3.0-4.0), considering the individual's risk of bleeding and recurrent thrombosis.13, 14, 43 Should recurrent thrombosis occur while on standard-intensity VKA, other therapeutic options may include an increased target INR range, treatment dose LMWH, or the addition of antiplatelet therapy.12-14
Warfarin should be used with caution in patients with aPL-related thrombocytopenia, in view of its long half-life of 26 to 48 hours;44 split-dose LMWH, half-life approximately 4 hours after subcutaneous injection,45 should be considered instead. The prevalence of thrombocytopenia (platelets < 100 x 109/L) was 26.9% in 1000 APS patients in the Euro-Phospholipid project.46 Thrombocytopenia appears to be associated with higher thrombotic risk in APS patients with platelets < 150 x 109/L, with a reported hazard ratio (HR) of 2.95, 95% confidence interval (CI) 1.11-7.88.47 The risk of teratogenicity with VKAs is extremely low, provided that women are switched from VKA to LMWH before 6 weeks of gestation.48
4.1 Anticoagulant monitoring of VKAs in APS
The PT-INR is traditionally used to monitor VKA anticoagulation intensity. LA-induced PT prolongation, typically unassociated with bleeding, is unusual, likely due to the high phospholipid concentration in PT reagents (Table 1). Use of an instrument-specific ISI reduced the differences observed between reagents in earlier studies, for samples with INRs within the therapeutic range, with similar results in APS and non-APS patients.49 A multicenter study demonstrated that differences in PT-INR when measured with the majority of commercial thromboplastins are not enough to cause concern, if thromboplastins insensitive to LA and instrument-specific ISI calibration are used19. Recombinant thromboplastins produce consistently higher INRs,19 which could potentially alter clinical management.18 New thromboplastins should be checked for sensitivity to LA.17 LA can prolong the PT when associated with the rare LA-hypoprothrombinemia syndrome with acquired factor II deficiency, caused by anti-prothrombin antibodies.50 Despite its limitations for monitoring VKA-treated APS patients, the INR remains in general use.
| Monitoring test |
Target therapeutic ranges |
Comments |
|---|---|---|
|
PT-INR Clotting assay INR = (PT/MNPT)ISI |
2.0-3.0 3.0-4.0a |
-Standard method to monitor VKA anticoagulation intensity in APS patients -PT-INR raised in subset of APS patients (effect of LA on thromboplastin) -Most thromboplastins: low sensitivity to LA with instrument-specific ISI -Check new thromboplastins for sensitivity to LA before use to monitor VKA -Local INR calibration to minimize between-laboratory INR variation -Check baseline PT prior to anticoagulation wherever possible -If baseline PT prolonged, check for acquired factor II deficiency (rare LA-hypoprothrombinaemia syndrome) |
|
POC INR Clotting assay INR = (PT/MNPT)ISI |
2.0-3.0 3.0-4.0a (INR) |
-Several POC coagulometers available -Reliable and accurate INRs compared to lab INRs in general population -Variable POC INR results in APS patients -Divergence between POC and lab INRs increases as INR values rise -Interpret POC INR results with caution in APS patients17 -Pragmatic approach: check for concordance between POC and venous lab INRs initially and every 6-12 months; use POC if INR difference is < 0.5 -Ensure regular IQC: if liquid IQC samples are available, they should be run at least when testing with a new batch of test strips or if an unexpectedly high or low result occurs, and at least once every 6 months (2C)27 -Ensure regular EQA which should take place at least every 6 months (2C)27 |
|
Chromogenic FX (CFX) Chromogenic assay |
-CFX range of ~ 20-40 % ≅ INR range of 2.0-3.0 -Therapeutic ranges not established |
-CFX provides an LA-independent measure of anticoagulation intensity -Inverse linear relationship between CFX and INR; therefore, may provide a guide to anticoagulation intensity18, 19 -Poor utility of CFX at INRs > 3.0 (when functional FX ≤ 12.0 IU/dL)18 -Therapeutic ranges are not established, and the assay is not well standardized, nor widely available or practicable for routine use -Notwithstanding its limitations, CFX could assist in monitoring LA-positive APS who have a prolonged PTs prior to commencement of VKA or who experience recurrent VTE while on apparently therapeutic VKA anticoagulation |
|
Coagulation factor II and X assays Clotting assays |
Therapeutic ranges not established |
-Coagulation FII and X levels proportional to INRs between 2.0-3.5 in general population patients32 -Non-proportional relationship at INR > 3.532 -Unclear whether useful for VKA monitoring in APS -FII coagulation assay not applicable for monitoring VKA anticoagulation in patients with acquired factor II deficiency (rare LA-hypoprothrombinaemia syndrome) |
|
Thrombin generation (TG) Fluorogenic substrate: continuous measurement of TG |
Therapeutic ranges not established |
-ETP and peak TG show significant inverse correlations with INR in non-APS and APS patients, including in those with INR ≥ 3.518 -Concordance with in vivo coagulation activation markers being normal in most non-APS and APS patients with VTE who have an INR ≥ 2.071, 73 -A subgroup of APS patients with increased peak TG, despite therapeutic INR and CFX, suggests that TG might identify an ongoing prothrombotic state18 -The use of TG remains limited to specialized laboratories due to lack of standardization and no established therapeutic ranges |
- Abbreviations: APS, antiphospholipid syndrome; CFX, chromogenic factor X; EQA, external quality assessment; ETP, endogenous thrombin potential; INR, international normalized ratio; ISI, international sensitivity index; IQC, internal quality control; LA, lupus anticoagulant; MNPT, mean normal prothrombin time (geometric mean of the prothrombin times of the healthy adult population); POC, point-of-care; PT, prothrombin time; TG, thrombin generation; VKA, vitamin K antagonist.
- a See text regarding use of high-intensity VKA.
POC INR testing, however, shows variable results in APS patients. A study of 29 APS versus 31 non-APS patients using the ProTime InRhythmTM System (finger stick or venous non-citrated blood) showed a difference of < 0.4 INR units compared to venous citrated blood laboratory INR (both measured with human recombinant thromboplastin) in 97% of patients, suggesting that this device is accurate in APS- and non-APS patients.51 However, in a comparative study, 5/59 (8%) patients with APS and both elevated aβ2GPI and LA had non-measurable ProTime® INR results and generally higher HemochronTM Signature INR results than the plasma-based INR, indicating elevated POC INRs in a subset of APS patients. The divergence between POC and laboratory INRs increased as INR values rose.52 Taylor et al found that the mean INR difference between the CoaguChek XS and laboratory INR was 0.68, with a difference significantly higher in APS patients than in controls with a target INR of 2 to 3.53 Isert et al did not observe a higher disagreement between INR results from CoaguChek versus laboratory INR, using two thromboplastins, in APS patients.54 However, INR variations above 0.5 were more frequent in APS patients compared to controls (55.6% vs. 67.8%, P = .05). No in-depth analysis has been performed to identify the reason for variability in INR by POC devices compared to plasma-based laboratory INR in APS patients, or the reason for variability between POC devices. More investigation to unravel potential differences in PT-INR measured in APS patients by POC devices is needed.
Pending further data to establish the place of POC INR testing in LA-positive patients, results should be interpreted with caution.17 A pragmatic approach is to restrict POC testing to APS patients in whom concordance of POC and laboratory INRs (INR difference < 0.5 units) is demonstrated at therapeutic intensity initially and every 6-12 months; ensure regular internal quality control (IQC), and ongoing review of external quality assessment (EQA) results to check that concordance is maintained.27, 55 It is prudent to apply this to all APS patients rather than only those who are LA-positive, as LA testing could yield false negative results, but might influence INR monitoring.
CFX provides an LA-independent assessment of VKA anticoagulant intensity. This is because the CFX assay requires minimal phospholipid, and the initial dilution is large, which would make the assay less sensitive to LA.31 A literature review of CFX use that selected 9/55 articles for their relevance, concluded that in a subgroup of APS patients, INRs may be falsely elevated and thus may not accurately inform the dose of warfarin.56 Notwithstanding its limitations, CFX could assist in monitoring LA-positive APS patients, particularly in those who have a prolonged PT prior to commencement of VKA or who experience recurrent VTE while on apparently therapeutic VKA anticoagulation.
A study of concurrent INR, CFX, and PT-based clotting FII levels in 36 patients (26 aPL positive), showed limited discordance between CFX and FII. The authors concluded that FII and CFX testing are well correlated in patients in whom anticoagulation with warfarin requires alternative monitoring to INR and thus that either test can be used in this population.57 In contrast to the study of Rosborough et al that showed, in 14 of 21 LA-positive patients, a significantly lower FII/CFX ratio than in LA-negative patients,58 Baumann Kreuziger et al did not find a difference in FII/CFX ratios between patients with or without LA.57 FII (clotting or chromogenic) assays should be avoided for anticoagulant monitoring in APS patients with LA-hypoprothrombinemia syndrome.50
ETP and peak TG parameters showed significant inverse correlations with INR in APS patients on warfarin, including those with ≥ 3.5 INR, suggesting that TG might inform anticoagulant intensity in patients on high-intensity VKA.18 Additionally, increased peak TG in a subgroup of APS patients, despite therapeutic INR and CFX, suggests that TG might be able to identify an ongoing prothrombotic state in VKA-treated patients.18 The normalized TG-derived peak height/lag time ratio identifies LA in plasma with high sensitivity, irrespective of the patient’s treatment with oral anticoagulants, if TG is performed on mixtures of patient plasma and pooled normal plasma.59
5 DIRECT ORAL ANTICOAGULANTS (DOACS)
Current first-line anticoagulation used for a first episode of VTE in the general population is a DOAC.60 Post hoc analysis of the RE-COVER, RE-COVER II, and RE-MEDY randomized controlled trials (RCTs) suggested that the efficacy and safety of dabigatran etexilate were not significantly affected by the presence of aPL,61 with no analogous data available for the other DOACs.
The European Medicines Agency (EMA), following a risk assessment triggered by the TRAPS (Rivaroxaban in Thrombotic APS) RCT,62 stated that DOACs are not recommended for thrombotic APS patients, especially those who are triple aPL-positive.63 The ISTH12 and 16th International Congress on Antiphospholipid Antibodies Task Force Report on APS Treatment Trends13 concur in recommending that in single- or double aPL-positive APS patients following a first VTE, DOACs initiated as standard care may be continued, with consideration of the perceived risks and uncertainties and discussion with the patient, for shared decision-making. The doses of DOACs used in APS patients reported in the literature have been shown to be as effective as warfarin at a target INR range of 2.0 to 3.0 in general-population patients following a first VTE. These DOAC doses may not be sufficient to prevent further thrombosis in APS patients who experience recurrent thrombosis while on therapeutic-intensity VKA or those with a history of arterial thrombosis.64 Notably, in animal models, stronger inhibition of Xa activity is required to protect against arterial versus venous thrombosis,65 although clinical studies are lacking. DOACs should be avoided in patients with APS-related arterial or small vessel thrombosis (proven or suspected),12, 13 except in the context of a clinical trial. The RISAPS (Rivaroxaban for Stroke Patients with Antiphospholipid Syndrome) phase 2/3 RCT aims to assess the efficacy of high-intensity rivaroxaban 15mg twice daily versus high-intensity warfarin in APS patients with stroke or other brain ischaemic injury (ClinicalTrials.gov Identifier: NCT03684564). All cases of DOAC use in APS patients should be reported to the ISTH-supported international registry (ClinicalTrials.gov Identifier: NCT04262492).12, 13
The teratogenic risk of DOACs in humans is uncertain, estimated to potentially affect 2.2% (7/137) pregnancies.66 ISTH guidance recommends that DOACs are switched to an alternative anticoagulant pre-conceptually, with the main options VKAs (to be switched to LMWH as soon as possible when pregnant and before 6 weeks gestation), or LMWH.67
5.1 Anticoagulant monitoring of DOACs in APS
As in non-APS patients, DOAC anticoagulant effect is not routinely monitored, but there is increasing demand on the laboratory to have the capacity to adequately assess DOAC anticoagulant effect (pharmacodynamics) or levels (pharmacokinetics) in emergent or routine situations (Table 2).68 DOAC levels should also be measured if testing for LA (as they may cause false positives and negatives), after the DOAC has been stopped for at least 48 hours and longer if there is renal impairment.69 Alternatively, LA can be measured after preanalytical neutralizing or adsorbing DOAC.70 As with VKAs, DOACs should be used with caution in thrombotic APS patients with thrombocytopenia, particularly as based on their half-lives,37 DOAC anticoagulant effects persist for > 48 hours; LMWH should be considered instead.
| Dabigatran | Rivaroxaban | Apixaban | Edoxaban | |
|---|---|---|---|---|
| Standard dose | 150mg bd | 20mg od | 5mg bd | 60mg od |
| Monitoring test |
-Dilute thrombin time -Ecarin clotting time -Ecarin chromogenic assay -Chromogenic anti-IIa |
-Specific chromogenic anti-Xa |
Specific chromogenic anti-Xa |
Specific chromogenic anti-Xa |
|
Peak concentration ng/mL |
175a (117-275) |
270b (189-419) |
132c (59-302) |
234d (149-317) |
|
Trough concentration ng/mL |
60a (39-95) |
26b (6-87) |
63c (22-177) |
19d (10-39) |
| Examples of medications contraindicated: strong inhibitors of both CYP3A4 & P-gp pathways |
-systemic azole antifungalse -strong CYP3A4 inducersf -HIV protease inhibitorsg |
-systemic azole antifungalse -strong CYP3A4 inducersf -HIV protease inhibitorsg |
-systemic azole antifungalse -strong CYP3A4 inducersf -HIV protease inhibitorsg* |
-systemic azole antifungalse -strong CYP3A4 inducersf -HIV protease inhibitorsg |
| Examples of medications or supplements to use with caution* |
-antiplatelet agents -NSAIDs -omega 3 fish oils -ginkgo biloba |
-antiplatelet agents -NSAIDs -omega 3 fish oils -ginkgo biloba |
-antiplatelet agents -NSAIDs -omega 3 fish oils -ginkgo biloba |
-antiplatelet agents -NSAIDs -omega 3 fish oils –ginkgo biloba |
|
Comments -Renal function, liver function, and full blood counts should be checked at baseline, at least annually, and more frequently as clinically indicated -DOAC levels should be measured in patients who weigh > 120kg as recommended in ISTH guidance,114 although a recent report suggests that standard rivaroxaban dosing is suitable at extremes of body weight, <50kg to 150kg115 -Other populations when DOAC monitoring could be clinically useful include: in the elderly, those at extremes of body weight, those requiring drugs that impact certain metabolic pathways, those with renal impairment (CrCl [C&G] is < 30 mL/min); when an interacting medication is prescribed; when there is doubt around oral absorption, to assess adherence68, 116 -Moreover, DOAC-treated patients that require acute intervention or in emergent situations, such as bleeding, overdose, acute stroke, trauma, surgery, may require assessment of their coagulation status to assure appropriate management68, 116 -For most of these scenarios, it is the clearance of the DOAC that is of interest, so trough concentrations are prioritized, except in the case of a bleeding emergency or to confirm oral absorption116 http://www.kingsthrombosiscentre.org.uk/index.php/anticoagulation/anticoagulation-monitoring -DOAC levels should be measured if testing for LA (as DOACs may cause false positives and negatives), after the DOAC has been stopped for at least 48 hours and longer if there is renal impairment69 -Alternatively, LA can be measured after preanalytical neutralizing or adsorbing DOAC70 -LC-MS/MS is considered the gold standard method for the measurement of DOACs. However, it is not widely available, and is time consuming, expensive, and requires a high level of expertise. Therefore, tests that are more accessible have been developed, based on existing methods Drug-specific calibrator should be used for anti-Xa and IIa assays and results expressed in mass concentration: ng/mL -Antithrombin supplement anti-Xa methods should not be used for DOAC assessment -Ecarin clotting time can be affected by hypoprothrombinaemia due to anti-prothrombin antibodies -Ecarin chromogenic assay has prothrombin added and is less sensitive to factor deficiencies -DOACs should be used with caution in patients with APS-associated thrombocytopenia -Anti-Xa inhibitors and dabigatran are contraindicated in Europe at creatinine clearance (C&G) < 15 mL/min and < 30 mL/min, respectively, with dose reductions for renal impairment, following the SmPC recommendations. |
||||
- Abbreviations: bd, twice daily; C&G, Cockcroft and Gault; CrCl, creatinine clearance; HIV, human immunodeficiency virus; IQR, interquartile range; LC-MS/MS, liquid chromatography with tandem mass spectrometry; NSAIDS, non-steroidal anti-inflammatory agents; SmPC, summary of product characteristics; SNRI, selective noradrenaline receptor inhibitor; SSRI, selective serotonin receptor inhibitor; VTE, venous thromboembolism.
- a Mean (25th–75th centile).
- b Mean (5th–95th centile).
- c Median (5th–95th centile).
- d Median (IQR).
- e eg, ketoconazole, itraconazole, voriconazole and posaconazole.
- f eg, rifampicin, phenytoin, carbamazepine, phenobarbital or St. John's Wort.
- g eg, ritonavir; *reduce dose by 50%.
- h citalopram.
- i eg, duloxetine and venlafaxine; *These agents, which should be used with caution in patients on any anticoagulant, inhibit platelet function and potentially increase bleeding risk.
Drug-calibrated chromogenic anti-Xa assays are suitable to provide quantitation of direct anti-Xa inhibitors33, 37, 68 in APS patients. Drug-calibrated dTT and anti-FIIa chromogenic methods are suitable ways to provide quantitation of dabigatran.33, 37, 68 The ECT, also generally suitable to monitor dabigatran, should not be used in patients with LA-hypoprothrombinemia syndrome.50 The ECA has prothrombin added and therefore could be used in this context.
TG has been found to be sensitive to all kinds of anticoagulants and may best represent inter-individual response more so than exploring merely plasma drug concentrations.68 In non-APS patients with VTE, rivaroxaban was reported to provide effective anticoagulation, as assessed by inhibition of TG and in vivo markers of coagulation activation.71 Notably, reduced TG parameters have been reported to persist at 12 hours after DOAC intake, despite low residual DOAC plasma levels < 50 ng/mL.72 In the RAPS (Rivaroxaban in Antiphospholipid Syndrome) RCT in APS patients with VTE requiring standard-intensity anticoagulation, the primary outcome, percentage change in ETP for rivaroxaban, did not reach the non-inferiority threshold. However, peak thrombin was significantly lower on rivaroxaban and the authors concluded that the overall TG curve, in which the higher ETP reflects the altered reaction kinetics with rivaroxaban, was not indicative of increased thrombotic risk.73
A more standardized method for TG measurement has become available recently: the ST Genesia (Diagnostica Stago, Asnières sur Seine Cedex, France), an automated analyzer for TG. This may be a “game changer” for the assessment of DOAC anticoagulant effects in the future.68 TG parameters measured with ST Genesia correlate with drug levels of anti-Xa DOACs. Peak thrombin and velocity index are of special interest for the determination of residual anticoagulant effect at low drug levels. For dabigatran-treated patients, only lag time shows a correlation with the dabigatran plasma levels.74 As with VKAs, TG is not currently suitable to monitor anticoagulant intensity in DOAC-treated APS patients.
6 LOW MOLECULAR WEIGHT HEPARIN
LMWH is used as initial bridging anticoagulation in patients treated with VKA24 and as initial and bridging anticoagulation when recurrent thrombosis occurs on standard-intensity VKA, ongoing options including high-intensity VKA or treatment dose LMWH.12-14 Should thrombosis recur while on high-intensity VKA, or standard-treatment dose LMWH, ie, anticoagulant-refractory thrombotic APS, high-intensity and subsequently escalated high-intensity LMWH, approximately one-quarter and one-third above standard intensity dose LMWH, respectively, may be considered.42, 75, 76 LMWH can provide bridging anticoagulation peri-operatively in VKA-treated patients, because of its relatively short half-life.45 The risk of heparin-induced thrombocytopenia (HIT) with LMWH is considered low (<0.1%) in medical and obstetric patients, after minor surgery or minor trauma, and intermediate (0.1%-1.0%) following major surgery or major trauma.77 Prolonged LMWH use, between 3 and 24 months, decreases mean bone mineral density (BMD) by 2.8% to 4.8% (depending on the site) versus 1.2% to 2.5% with VKA.78 The International Congress on Antiphospholipid Antibodies Task Force Report on APS Treatment Trends has recommended that vitamin D deficiency is corrected in APS patients, based on general population guidelines.13 As indicated above, LMWH should be considered rather than VKA or DOACs in thrombotic APS patients with thrombocytopenia.
LMWH or UFH do not cross the placenta. The optimal dose regimen for LMWH during pregnancy in thrombotic APS is not established. A history of thrombotic APS is associated with increased risk for future thrombotic events;79 thus, therapeutic dose heparin, together with LDA, during pregnancy appears prudent, as per the recommendation in the European League Against Rheumatism (EULAR) guidelines.14 The United Kingdom Royal College of Obstetricians and Gynaecologists (RCOG) guidelines, in alignment with other national guidelines, do not recommend routine anti-Xa monitoring during pregnancy in patients on LMWH except in certain circumstances: < 50 kg or > 90 kg or with other complicating factors, for example with renal impairment or recurrent VTE.85 The optimal dosage regimen of LMWH for treatment of VTE in pregnancy (once daily versus split-dosing, ie, two divided doses) and the value and role of anti-Xa monitoring merits further investigation.85 Limited data suggest that patients with a history of APS-related cerebrovascular events are at excess risk of recurrence during pregnancy,80, 81 thus high-intensity adjusted dose LMWH may be warranted.
6.1 Anticoagulant monitoring of LMWH in APS
The aPTT is not usable for LMWH monitoring (Table 3). The aPTT will be variably prolonged at peak levels, and often only shows a mild prolongation over that due to the LA, depending on the sensitivity of the individual reagents used for LMWH and LA.82 The anti-Xa chromogenic assay, unaffected by LA, is the most appropriate assay for monitoring LMWH in APS patients, as in non-APS patients. Evidence on LMWH dosing and monitoring in APS is lacking and may be extrapolated from use in general population patients. Dose reduction of LMWH, following the manufacturer's summary of product characteristics, should be undertaken in accordance with the SmPC in APS patients with creatinine clearance (CrCl; Cockcroft-Gault [C&G]) <30mL/min, with anti-Xa monitoring considered to check for accumulation. The most appropriate LMWH dosing in patients with obesity remains undefined.83
| Drug |
Monitoring test |
Dose |
Target therapeutic ranges |
Comments |
|---|---|---|---|---|
| LMWH |
Anti-Xa Chromogenic assay |
-Standard treatment dose (once daily) -Standard treatment dose (twice daily dosing, i.e., split-dose) -High/escalated-intensity LMWH ~ 25% or 33% above standard dose, respectively (twice daily dosing) |
-No routine anti-Xa monitoring -Peak anti-Xa 0.5-1.0 IU/mL -Peak anti-Xa 1.0-1.2 IU/mL |
-LA-independent measure of anticoagulant activity -Standard therapeutic dose LMWH with weight-based dosing, without anti-Xa monitoring, applies in most cases -Peak anti-Xa should be monitored 4 hours after dosing as levels peak at 3-5 hours -The therapeutic range for standard therapeutic dose LMWH administered twice daily is generally regarded to be 0.5 to 1.0 IU/mL80, 117 -A high-intensity anti-Xa therapeutic range, based on twice daily dosing, of 1.0-1.2 IU/mL, is reasonable -Empirical dose reduction of LMWH if CrCl (C&G) <30mL/min and/or anti-Xa (trough [maintain < 0.3units/mL] and peak) monitoring, following SmPC -Ensure adequate LMWH doses in obese patients, consider anti-Xa monitoring -Consider twice daily dosing if increased bleeding risk, eg, CrCl (C&G) <30mL/min; thrombocytopenia; and during pregnancy -Dose reduction for treatment and secondary thromboprophylaxis for thrombocytopenia (see text) -The optimal dosage regimen and monitoring during pregnancy is undefined (see text)85 -Monitor platelets for HIT in patients receiving LMWH after major surgery or major trauma77 -HIT monitoring not suggested during LMWH use in medical patients and after minor surgery or minor trauma77 or in obstetric patients77, 85 |
| UFH |
aPTT Clotting assay |
-IV bolus of UFH 5000 units / 75 units/kg (10,000 units for severe PE) -then 15-25 units/kg/hour, by continuous IVI, dose adjusted |
aPTT therapeutic range corresponding to UFH levels of 0.3 to 0.7 IU/mL anti-Xa activity33, 45 |
-For continuous UFH by IVI, samples for anticoagulant monitoring should be collected 4-6 hours after initiation and thereafter at least daily and after dose adjustment33 -For UFH monitoring by aPTT, a relatively LA insensitive APTT reagent should be used33, 69 -aPTT therapeutic range should be locally adapted to the responsiveness of the reagent and coagulometer used45 -If the baseline aPTT is prolonged, the aPTT should not be used for UFH monitoring as this may overestimate heparin effect -The aPTT may be unreliable in the acute phase situation -Perform anti-Xa level to distinguish real from apparent “heparin resistance,” ie, daily dose > 35,000 units; interpret results in the context of concomitant APTT -In all patients on UFH, monitor platelet counts for HIT, including in obstetric patients and consider HIT if platelets fall |
| UFH |
Anti-Xa Chromogenic assay |
0.3-0.7 IU/mL |
-The anti-Xa assay instead of the aPTT should be used in all cases in which a prolonged baseline aPTT is suspected; this would include LA positive patients -Particularly in view of the potential for unreliable aPTTs due to to LA-induced effects and patient safety issues as a result, anti-Xa rather than the aPTT is more appropriate to monitor UFH in APS patients -The anti-Xa assay has important advantages, including that it is unaffected by raised CRP or FVIII related to an acute phase reaction -In CAPS patients in the acute phase situation, use anti-Xa to monitor UFH; interpret results in the context of concomitant aPTT -An UFH reference standard traceable to the current International Standard for UFH should be employed for estimation of UFH in IU/mL if an anti-Xa assay is used for monitoring33 -Limitations of anti-Xa assays include that they are more complex to perform than the aPTT and not universally available -Further limitations include considerable inter-laboratory variation34 and inter-assay variability.35, 36 |
|
| UFH | TT with protamine titration | -Superseded by anti-Xa assays | ||
| UFH | ACT | In non-APS patients undergoing CPB, generally a dose of UFH of ~ 300-400 IU/kg is administered prior to CPB with additional boluses given as required |
In non-APS patients: -ACT in a non-anticoagulated patient is ~ 107s ± 13s -During CPB, UFH is titrated to maintain an ACT of 400-600s |
-The ACT is a phospholipid-dependent test widely used to monitor UFH during CPB -The ACT may be prolonged by LA -Suggested modification for use in APS: target clotting time twice the baseline ACT94 -Other suggested modifications: |
- Abbreviations: ACT, activated clotting time; aPTT, activated partial thromboplastin time; CAPS, catastrophic antiphospholipid syndrome; CrCl (C&G), creatinine clearance (Cockcroft and Gault); HIT, heparin-induced thrombocytopenia; IVI, intravenous infusion; LA, lupus anticoagulant; LMWH, low molecular weight heparin; SmPC, summary of product characteristics; TT, thrombin time; UFH, unfractionated heparin.
LMWH dosing should be adjusted for APS-associated thrombocytopenia. Therapeutic dosing in thrombocytopenic APS patients may be extrapolated from use in other thrombotic situations, such as in cancer patients. ISTH guidance recommends full-dose anticoagulation following acute VTE in cancer patients with a high risk of thrombus propagation and a platelet count > 50 x 109/L and advise platelet transfusion support in those with thrombocytopenia to maintain platelet counts of 40-50 x 109/L.84 In APS patients, immunomodulatory treatment may increase platelet counts sufficiently for therapeutic anticoagulation. Platelet counts should be monitored while on LMWH, during the first 14 days in patients at intermediate risk for HIT,77 and beyond, to guide consideration of dose reduction, if required, for secondary thromboprophylaxis.
The optimal dosage regimen of LMWH for treatment of VTE in pregnancy (once daily versus split-dosing, ie, two divided doses) and the value and role of anti-Xa monitoring merits further investigation.85
7 UNFRACTIONATED HEPARIN (UFH)
UFH has a shorter half-life than LMWH (60 minutes after an intravenous (IV) bolus of 100 units/kg)45 and may be preferred for anticoagulation in situations in which bleeding risk is high, and rapid reversal of heparin effect by protamine sulphate45 may be required, such as in patients with CAPS associated with severe thrombocytopenia (platelets < 20 x 109/L),86 or those who may need urgent critical site surgery. In the CAPS Registry, the majority of patients, 403 of 471 (85.6%), were treated with anticoagulation, which was usually IV UFH followed by oral anticoagulation. UFH has been recommended for use in CAPS, based on a meta-analysis of two studies including 325 CAPS registry patients, which showed significantly lower mortality in anticoagulated patients (odds ratio [OR] 0.18; 95% CI, 0.09, 0.38; with very low certainty of evidence).16 UFH is also standard treatment during cardiopulmonary bypass (CPB) surgery.87
UFH may be associated with a higher risk of bleeding compared to LMWH. A meta-analysis of 13 RCTs of LMWH versus UFH for VTE treatment showed no difference in risk for major bleeding (relative risk [RR] 0.63 [95% CI, 0.37-1.05]).88 However, an observational cohort study on 3066 hospitalizations of adults receiving therapeutic doses of UFH and LMWH over a year showed in multivariate analysis that UFH was a significant predictor of bleeding (OR 2.35, 95% CI 1.11-4.98; P = .005).89 The risk of HIT with UFH is considered intermediate (0.1-1%) in medical and obstetric patients, and high (>1.0) in surgical and trauma patients receiving postoperative UFH,77 which is a disadvantage compared to LMWH. A prospective matched cohort study in 50 patients reported that UFH therapy for more than 1 month during pregnancy was associated with a significant reduction in bone density, although fractures were uncommon, with no significant correlation between lumbar bone density and the dose or duration of heparin.90
7.1 Anticoagulant monitoring of UFH in APS
Monitoring of therapeutic doses of intravenous UFH is recommended and can be achieved using an anti-Xa assay or an aPTT with a therapeutic range corresponding to 0.3 to 0.7 IU/mL anti-Xa activity (Table 3).33, 45 The aPTT is typically prolonged in APS, particularly if reagents sensitive to LA are used. Thus, for UFH monitoring by aPTT, a relatively LA-insensitive aPTT reagent should be used.33, 69 If the baseline aPTT is prolonged, the aPTT should not be used for UFH monitoring as this may overestimate heparin effect, resulting in subtherapeutic anticoagulation and a potentially increased risk of recurrent thromboembolism. BSH guidelines state that the anti-Xa assay could be the test of choice to monitor UFH.33 There are great advantages to use of the chromogenic anti-Xa assay instead of the aPTT in all cases in which a prolonged baseline aPTT is suspected; this would include LA positive patients. Particularly as aPTTs may be unreliable due to LA-induced effects and the potential for patient safety issues as a result, anti-Xa rather than the aPTT is more appropriate to monitor UFH in APS patients.
Unusually high doses of UFH may be required to achieve a therapeutic aPTT, ie, “heparin resistance,”45 in the acute situation such as in CAPS, with potential mechanisms including raised levels of FVIII and fibrinogen, and other heparin-binding acute phase reactants, which lead to aPTT shortening91 Decreased antithrombin levels are also associated with “heparin resistance,”92 reported to be reversed by supplementation with antithrombin concentrate in patients undergoing cardiac surgery.93 Anti-Xa levels, interpreted in the context of concomitant aPTTs, are advised.86 Platelet counts should be monitored during UFH treatment and HIT considered should the platelet count fall.77
The activated clotting time (ACT), a phospholipid-dependent test widely used to monitor UFH during cardio-pulmonary bypass (CPB), may be prolonged by LA. There is no consensus in the literature regarding optimal anticoagulation for APS patients in this challenging situation. Reported approaches in CPB include targeting twice the baseline ACT, measuring heparin concentrations by an automated protamine titration device, using preoperative in vitro heparin-ACT titration curves, and also measuring heparin anti-Xa levels;94 and ACT coupled with thromboelastography.95
8 OTHER PARENTERAL ANTICOAGULANT OPTIONS IN THROMBOTIC APS PATIENTS
8.1 Fondaparinux
The specific anti-Xa activity of fondaparinux is seven times higher than that of LMWH (~700 units/mg and 100 units/mg, respectively), and its half-life after subcutaneous injection is longer than that of LMWH (17 and about 4 hours, respectively).45 The successful use of fondaparinux was reported in two patients with APS in combination with mycophenolate mofetil,96 and in three APS patients with recurrent thrombosis.97
The risk of fondaparinux-related HIT is low (<0.1%).77 In vitro studies show no significant inhibitory effect of fondaparinux on osteoblast proliferation or activity,98 although clinical data in this context are lacking. Fondaparinux has been reported to cross the placenta, resulting in low but measurable anti-Xa activity in umbilical-cord blood.85, 99 A review of maternal and pregnancy outcomes of 65 pregnancies in women using fondaparinux (11 on therapeutic dose) reported that it was well tolerated and the rate of pregnancy complications was similar to that observed in the general population.100 Fondaparinux is assigned to category B by the U.S. Food and Drug Administration (FDA), ie, that it should only be given during pregnancy when need has been clearly established (https://www.accessdata.fda.gov/drugsatfdadocs/label/2008/020883s014lbl.pdf).
8.2 Anticoagulant monitoring of fondaparinux in APS
Fondaparinux dosing is weight based. It is nearly completely eliminated by renal clearance and therefore contraindicated in patients with severe renal impairment (CrCl [C&G] <30mL/min; Table 4). Routine anticoagulation monitoring is not recommended; however, it may be useful in some circumstances, such as after re-thrombosis while on fondaparinux to assess patient adherence to inform consideration of an escalated dose. Fondaparinux-specific anti-Xa assays are available, although a therapeutic anti-Xa range has not been established.
| Drug | Monitoring test | Dose |
Target therapeutic ranges |
Comments |
|---|---|---|---|---|
| Fondaparinux | Anti-Xa |
Weight based as per SmPC (once-daily) |
Not established |
-Routine anti-Xa monitoring not recommended -Monitoring may be useful after re-thrombosis to assess patient adherence and inform consideration of high-intensity dose76 -Almost completely renally cleared, therefore contraindicated in severe renal impairment (CrCl [C&G] <30mL/min) -The mean expected peak steady-state plasma concentration on once-daily therapeutic dose fondaparinux can be expected to be 1.20 to 1.26 mg/L 3 hours post dose45 -Consider twice daily dosing (on an empirical basis) if used in anticoagulant-refractory APS patient who is also on antiplatelet treatment, or if use high-intensity fondaparinux dose, to limit bleeding risk76 -Suggested option for HIT77 |
| Argatroban |
-Dilute thrombin time -Ecarin clotting time -Ecarin chromogenic assay |
Continuous IV infusion, initial dose 1 to 2 mg/kg/min; dose is adjusted to maintain therapeutic levels |
Not established |
-Not renally excreted, therefore useful in APS patients with HIT and severe renal impairment, including CAPS -Routinely monitored by aPTT; however, the aPTT could give unreliable results due to LA-induced effects, as well as those of raised CRP or factor VIII levels -SmPC advises aPTT ratio between 1.5 and 3.0 but not exceeding 100 seconds -Metabolized by the liver via CP450 3A4/5; reduce dose to 0.5mg/kg/min if hepatic dysfunction, critically ill, after cardiac surgery, heart failure -Drug-calibrated chromogenic anti-IIa assays are suitable to monitor argabtroban -When bridging with argatroban to warfarin in LA-positive patients, check CFX and stop argatroban when CFX < 45 (equivalent to INR ~>2.0) -Suggested option for HIT77 |
| Bivalirudin | -As for argatroban |
IV bolus of 0.1 mg/kg followed by an infusion of 0.25 mg/kg/hr |
Not established |
-~ 20% is renally excreted; consider dose reduction in patients with moderate to severe renal impairment45 -consider dose reduction in patients with hepatic dysfunction77 -aPTT ratio is the usual monitoring test, target range 1.5-2.5 but could give unreliable results due to LA-induced effects -Drug-calibrated chromogenic anti-IIa assays are suitable to monitor bivalirudin -When bridging with argatroban to warfarin in LA-positive patients, check CFX and stop bivalirudin when CFX < 45 (equivalent to INR ~ > 2.0) -Suggested option for HIT77 |
| Danaparoid | -Anti-Xa |
Therapeutic dose as per SmPC, administered by continuous IVI |
0.5-0.8 U/mL |
-Contraindicated in severe renal and hepatic insufficiency, unless there is no alternative for HIT -In general, anti-Xa monitoring not necessary -Monitoring of anti-Xa activity is recommended in patients with renal insuffiency and/or if weight > 90kg -Suggested option for HIT77 -Serological cross-reactivity with heparin-platelet factor 4 antibodies ~ 5%, incidence of clinical cross-reactivity developing ~ 3%; SmPC advises monitoring platelets for HIT during treatment: https://www.medicines.org.uk/emc/product/5369/smpc -Danaparoid is currently unavailable in the United States; however, it is available in several other countries including Canada, Australia, New Zealand, European countries and Japan |
- Abbreviations: APS, antiphospholipid syndrome; aPTT, activated partial thromboplastin time; CAPS, catastrophic antiphospholipid syndrome; CFX, chromogenic factor X; CrCl (C&G), creatinine clearance (Cockcroft and Gault); HIT, heparin-induced thrombocytopenia; IVI, intravenous infusion; SmPC, summary of product characteristics.
8.3 Direct thrombin inhibitors: argatroban and bivalirudin
Argatroban, half-life 45 minutes after intravenous (IV) injection,101 is not renally excreted and is therefore an option in patients with HIT and severe renal impairment,77 and with CAPS.86 It is metabolized in the liver via the cytochrome P450 3A4/5 enzyme system and should therefore be used with caution in patients with hepatic dysfunction.101 Argatroban has been used successfully in case reports during pregnancy;102, 103 however, there are no controlled data in human pregnancy. Bivalirudin has a half-life of 25 minutes after IV injection.104 Approximately 20% is renally excreted and dose reduction should be considered in patients with moderate to severe renal impairment(45) or hepatic dysfunction.77 Bivalirudin is suggested as an option in patients with acute HIT or subacute HIT (ie, normal platelets, positive functional test for HIT) who require cardiovascular surgery that cannot be delayed.77 Both argatroban and bivalirudin are assigned to category B by the FDA.
8.4 Anticoagulant monitoring of argatroban and bivalirudin in APS
Argatroban is routinely monitored by the aPTT.106 However, the aPTT could give unreliable results due to LA-induced effects, as well as during the acute phase (Table 4).28, 29 In such situations, the dTT, which has shown comparable results using different analyzers, should be considered for the measurement of blood concentrations of the direct thrombin inhibitors argatroban107, 108 and bivalirudin.108 A therapeutic range for either remains to be established. ECT or ecarin chromogenic assays have been considered as alternatives, although the ECT has been demonstrated to have a slightly sigmoid response curve to concentration with argatroban109 and is falsely elevated by prothrombin deficiency,106 present in APS patients with LA-hypoprothrombinemia.50 The ECA, although unaffected by the presence of factor deficiencies (due to the addition of prothrombin) or LA, has been shown to have a low sensitivity to argatroban.108 In critically ill patients, it has been suggested that TT and rotational thromboelastography (ROTEM) parameters may provide better correlation to argatroban and lepirudin (unavailable since 2012) plasma concentrations than APTT.110 Drug-calibrated chromogenic anti-II assays are suitable to monitor argatroban and bivalrudin,33 and in APS patients, would not be affected by any LA effects.
8.5 Danaparoid sodium and anticoagulation monitoring in APS
Danaparoid sodium is a heparinoid that catalyzes the antithrombin inhibition of factor Xa (Table 4). It has a long half-life of approximately 25 hours, which is a disadvantage for patients requiring urgent surgery or invasive procedures.45 It has low cross-reactivity in vitro with sera containing HIT (heparin-platelet factor 4) antibodies and is suggested as an option for HIT.77 In APS, a case report describes thrombosis of a cardiopulmonary bypass circuit in an APS patient with acute HIT, despite therapeutic danaparoid.111 Danaparoid was reported to be effective for improving the live birth rate and safe for patients with obstetric APS.112 Monitoring is with a danaparoid calibrated anti-Xa assay.33 Danaparoid has also been assigned to category B by the FDA.
9 CONCLUSIONS
Anticoagulation monitoring in APS patients can be challenging because of LA-induced effects on phospholipid-dependent coagulation tests; therefore, strategies are required to achieve effective anticoagulant monitoring in thrombotic APS patients. For VKA monitoring, the PT-INR, using an LA-insensitive thromboplastin, instrument-specific ISI, and local INR calibration enables representative monitoring in the majority of patients. Until further evidence is available, the results of POC INR testing in APS patients should be interpreted with caution. Although a therapeutic range is not established for CFX use in APS patients on VKAs, this assay could assist in monitoring APS patients who have a prolonged PT prior to commencement of VKA or experience recurrent VTE while at apparently therapeutic VKA anticoagulation intensity.
In APS patients, the aPTT could give unreliable results due to LA-induced effects, as well as those of raised CRP or factor VIII levels. Given that the aPTT may not be representative of anticoagulant intensity in APS patients due to LA-induced and other effects, and the potential for patient safety issues as a result, the anti-Xa assay rather than the aPTT is more appropriate to monitor UFH in APS patients. The aPTT may also be unreliable to monitor argatroban and bivalirudin in APS patients and alternative methods are required.
The usual considerations for dosing and methods of anticoagulant monitoring, when required, can be applied to APS patients on therapeutic doses of DOACs and LMWH. However, optimal dosing and target therapeutic ranges are not established for LMWH use in thrombotic APS, including in patients who are anticoagulant-refractory or pregnant. Therapeutic dosing of fondaparinux and danaparoid are as for non-APS patients and routine anticoagulant monitoring is not generally required. TG assays remain limited to specialized laboratories and have not been standardized and validated for the assessment of anticoagulation intensity. Anticoagulant dosing and monitoring in APS patients would be optimized by systematic clinical and laboratory investigation to establish the utility of and to validate current and other potential approaches.
ACKNOWLEDGMENTS
We are most grateful to Dr Jignesh Patel for the critical reading of the manuscript and helpful suggestions.
CONFLICTS OF INTEREST
H Cohen reports, outside the submitted work, institutional research support and support to attend scientific meetings from Bayer Healthcare, and travel support from UCB Biopharma, with honoraria for lectures from Bayer Healthcare and consultancy fees from UCB Biopharma paid to University College London Hospitals Charity. M Efthymiou and KMJ Devreese report no conflicts of interest.
AUTHOR CONTRIBUTIONS
H Cohen wrote the first draft of the manuscript. H Cohen, M Efthymiou, and KMJ Devreese undertook critical revision of the manuscript.