Sex-specific differences in chronic thromboembolic pulmonary hypertension. Results from the European CTEPH registry
Funding information
The CTEPH registry is supported by a research grant from Actelion Pharmaceuticals Ltd. Actelion did not participate in registry management or in data analyses.
Abstract
Background
Women are more susceptible than men to several forms of pulmonary hypertension, but have better survival. Sparse data are available on chronic thromboembolic pulmonary hypertension (CTEPH).
Methods
We investigated sex-specific differences in the clinical presentation of CTEPH, performance of pulmonary endarterectomy (PEA), and survival.
Results
Women constituted one-half of the study population of the European CTEPH registry (N = 679) and were characterized by a lower prevalence of some cardiovascular risk factors, including prior acute coronary syndrome, smoking habit, and chronic obstructive pulmonary disease, but more prevalent obesity, cancer, and thyroid diseases. The median age was 62 (interquartile ratio, 50-73) years in women and 63 (interquartile ratio, 53-70) in men. Women underwent PEA less often than men (54% vs 65%), especially at low-volume centers (48% vs 61%), and were exposed to fewer additional cardiac procedures, notably coronary artery bypass graft surgery (0.5% vs 9.5%). The prevalence of specific reasons for not being operated, including patient′s refusal and the proportion of proximal vs distal lesions, did not differ between sexes. A total of 57 (17.0%) deaths in women and 70 (20.7%) in men were recorded over long-term follow-up. Female sex was positively associated with long-term survival (adjusted hazard ratio, 0.66; 95% confidence interval, 0.46-0.94). Short-term mortality was identical in the two groups.
Conclusions
Women with CTEPH underwent PEA less frequently than men, especially at low-volume centers. Furthermore, they had a lower prevalence of cardiovascular risk factors and were less often exposed to additional cardiac surgery procedures. Women had better long-term survival.
Essentials
- No studies focused on sex differences in chronic thromboembolic pulmonary hypertension (CTEPH).
- We analyzed the use of pulmonary endarterectomy and survival within the European CTEPH registry.
- Women underwent PEA less often than men, particularly at low-volume centers.
- Women required fewer major cardiac surgery procedures and had better long-term survival.
1 INTRODUCTION
Chronic thromboembolic pulmonary hypertension (CTEPH), a distinct form of pulmonary hypertension, is often associated with and possibly caused by incomplete resolution of prior venous thromboembolism events.1-3 The presence of chronic thrombi in the pulmonary arteries and the progressive microvascular arteriopathy cause an increase in pulmonary vascular resistance, which leads to pulmonary hypertension and right heart failure.2-5 Surgical treatment by pulmonary endarterectomy (PEA) improves the hemodynamic status and cures most of the patients with CTEPH.6 However, if the obstructions are technically challenging or the risks of undergoing surgery are too high, patients may be subjected to balloon angioplasty or will receive medical treatment.2, 7 This latter patient category is characterized by worse prognosis in terms of functional limitations and survival than patients undergoing PEA.6-9
It has been reported that women are more susceptible than men to several subtypes of pulmonary hypertension.10, 11 General understanding of how and why incidence, presentation, and pathophysiology differ between sexes is still limited.12 A role for hormonal factors and alterations in their metabolism has been postulated, especially for idiopathic forms,10, 13-15 whereas other subtypes of pulmonary hypertension may be influenced by prevalent risk factors and comorbidities, including autoimmune diseases, human immunodeficiency virus infection, left heart disease, and chronic obstructive pulmonary disease.11, 16 Similarly, sex variations in the transformation of cardiopulmonary and vascular structures with aging may influence how pulmonary hypertension manifests.10, 17 Indeed, it appears that female sex is associated with longer survival after diagnosis of other subtypes of pulmonary hypertension,10, 18, 19 possibly because of a more effective adaptive remodelling of the right ventricle and burden of concomitant risk factors.13, 20 Sex differences in response to medical therapy for pulmonary hypertension have also been described.21
To the best of our knowledge, no comprehensive analysis of sex-specific factors in CTEPH has been performed thus far.17, 22 In our analysis of the European CTEPH registry, we studied the influence on survival of sex-specific variations in concomitant risk factors, clinical presentation, hemodynamic parameters, and performance of PEA.
2 METHODS
2.1 Study setting and population
We accessed the prospective European CTEPH registry, which included adult patients who did not receive pulmonary hypertension-targeted treatment before CTEPH diagnosis and were followed at 27 selected centers in Europe and Canada for a minimum of 3 years between 2007 and 2012. Data cutoff was February 26, 2012. Further details of the registry have been previously presented; the diagnosis of CTEPH was made based on clinical guidelines valid at the time of study design.6, 23 In brief, CTEPH had to be confirmed as the cause of pulmonary hypertension by abnormalities in a ventilation/perfusion scan (at least one mismatched segmental perfusion defect) and in a computed tomography scan or via pulmonary angiography, and could be established only after a 3-month course of efficient anticoagulation.
Data were obtained from routine diagnostic workup including medical history, clinical presentation, results of diagnostic tests, diagnostic delay, and treatment.6, 23, 24 Predefined criteria for considering a patient inoperable included the presence of distal pulmonary artery obstructions, signs of microvascular disease, elevated pulmonary vascular resistance (>1500 dyn × s × cm−5), age (subjectively decided by the treating physicians), the burden of comorbidity, and patient refusal.6, 23
2.2 Aims and primary outcome
The aim of the present analysis was to assess potential sex-specific differences in the characteristics of clinical presentation, results of functional tests, proportion of PEA performed, and course of patients diagnosed with CTEPH. The primary outcome was all-cause death. Additional analyses included an evaluation of diagnostic delay, probability of PEA being performed, postoperative complications, and death from cardiovascular causes, as defined in previous reports.6, 23-25
2.3 Statistical methods
Adequate measures of central tendency and counts (percentages) were used to describe the characteristics of the study population. Absolute risks and annualized rates (95% confidence interval [95% CI]), absolute risk differences (ARD; 95% CI) and relative measures of risk (odds ratio [OR; 95% CI] or hazard ratio [HR; 95% CI]) served to compare groups. Separate analyses were performed for patients categorized by sex, performance of PEA, and inclusion at a high-volume center.6 A generalized additive model with linear, quadratic, or cubic functions, which were chosen based on relationships depicted by polynomial locally weighed regression, was used to fit the curve depicting the probability that women or men underwent PEA across age. In the model, the linear predictor depended on the function and the values were based on the intercept and age estimates with all the other covariates set to zero. Univariate and parsimonious fully adjusted multivariable Cox regression models estimating HR were run under the proportional hazard assumption. The covariates included in the multivariable models were selected on the basis of their clinical meaningfulness, utilization in prior reports,6 and the number of events observed per variable. Because of the pattern and amount (~20% for some variables) of missing values, we decided not to perform multiple imputation.26 Variables with a high frequency of missing values were primarily excluded from the multivariable model; in addition, we fit a model including all variables deemed clinically relevant. In light of the explorative and hypothesis-generating nature of the present work, we did not apply techniques to correct for multiple testing. R (v. 3.4.3; RStudio v. 1.0.153; packages survival and ggplot2), jamovi (v.0.9; R package jmv), and SPSS v. 23.0 (IBM) served for data analysis.
3 RESULTS
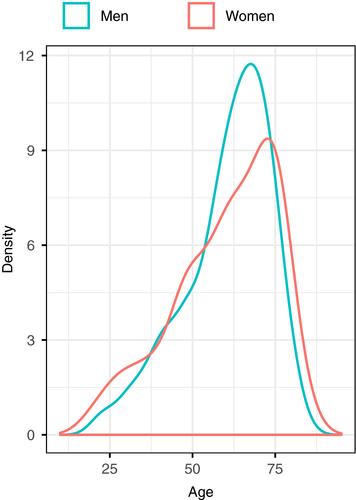
A total of 339 (49.9%) of the 679 patients included in the European CTEPH Registry were women. Median age was 62 years (interquartile range [IQR], 50-73) in women and 63 in men (IQR 53-70; P = .796, Mann-Whitney test); Table 1. Age distribution in women and men is depicted in Figure 1.
| Variablesa | Total (N = 679) | Not operated/PEA- (n = 275) | Operated/PEA+ (n = 404) | |||
|---|---|---|---|---|---|---|
| Women (n = 339) | Men (n = 340) | Women (n = 156) | Men (n = 119) | Women (n = 183) | Men (n = 221) | |
| Age (years), median (IQR) | 62 (50-73) | 63 (53-70) | 57 (45-69) | 62 (51-69) | 68 (58-75) | 66 (58-73) |
| Smokers, n (% of total) | 75 (22.1) | 148 (43.5) | 35 (22.4) | 54 (45.4) | 40 (21.9) | 94 (42.5) |
| Obesity | 71 (20.9) | 49 (14.4) | 30 (19.2) | 23 (19.3) | 41 (22.4) | 26 (11.8) |
| Diabetes type 2 | 18 (5.3) | 17 (5.0) | 10 (6.4) | 7 (5.9) | 8 (4.4) | 10 (4.5) |
| Coronary disease/MI | 26 (7.7) | 55 (16.2) | 15 (9.6) | 25 (21.0) | 11 (6.0) | 30 (13.6) |
| History of VTE | 249 (73.5) | 259 (76.2) | 99 (63.5) | 87 (73.1) | 150 (82.0) | 172 (77.8) |
| Cancer | 49 (14.5) | 39 (11.5) | 27 (17.3) | 14 (11.8) | 22 (12.0) | 25 (11.3) |
| Thyroid disorders | 42 (12.4) | 15 (4.4) | 22 (14.1) | 7 (5.9) | 20 (10.9) | 8 (3.6) |
| Prior splenectomy | 5 (1.5) | 18 (5.3) | 3 (1.9) | 11 (9.2) | 2 (1.1) | 7 (3.2) |
| COPD | 23 (6.8) | 41 (12.1) | 11 (7.1) | 22 (18.5) | 12 (6.6) | 19 (8.6) |
| Neurological diseases | 14 (4.1) | 7 (2.1) | 8 (5.1) | 2 (1.7) | 6 (3.3) | 5 (2.3) |
| Major trauma | 2 (0.6) | 19 (5.6) | 1 (0.6) | 10 (8.4) | 1 (0.5) | 9 (4.1) |
| Prior fractures | 14 (4.1) | 23 (6.8) | 6 (3.8) | 10 (8.4) | 8 (4.4) | 13 (5.9) |
| Congestive heart failure | 13 (3.8) | 18 (5.3) | 8 (5.1) | 9 (7.6) | 5 (2.7) | 9 (4.1) |
| Women-related factors | ||||||
| Use of contraceptive before diagnosis | 27 (8.0) | - | 3 (1.9) | - | 24 (13.1) | - |
| HRT | 7 (2.1) | - | 4 (2.6) | - | 3 (1.6) | - |
| One pregnancy | 19 (5.6) | - | 11 (7.1) | - | 8 (4.4) | - |
| Two pregnancies | 48 (14.2) | - | 21 (13.5) | - | 27 (14.8) | - |
| Three pregnancies | 26 (7.7) | - | 11 (7.1) | - | 15 (8.2) | - |
| Four or more pregnancies | 22 (6.5) | - | 9 (5.8) | - | 13 (7.1) | - |
| One miscarriage | 7 (2.1) | - | 4 (2.6) | - | 3 (1.6) | - |
| Two or more miscarriages | 6 (1.8) | - | 2 (1.3) | - | 4 (2.2) | - |
| Symptoms at the time of clinical presentation, n (% of total) | ||||||
| Syncope | 43 (12.7) | 50 (14.79 | 19 (12.2) | 12 (10.1) | 24 (13.1) | 38 (17.2) |
| Chest pain | 58 (17.1) | 46 (13.5) | 26 (16.7) | 13 (10.9) | 32 (17.5) | 33 (14.9) |
| Fatigue | 123 (36.3) | 91 (26.8) | 58 (37.2) | 32 (26.9) | 65 (35.5) | 59 (26.7) |
| Dizziness | 20 (5.9) | 18 (5.3) | 11 (7.1) | 3 (2.5) | 9 (4.9) | 15 (6.8) |
| Hemoptysis | 13 (3.8) | 17 (5.0) | 3 (1.9) | 7 (5.9) | 10 (5.5) | 10 (4.5) |
| NYHA class III | 244 (72.0) | 223 (65.6) | 114 (73.1) | 76 (63.9) | 130 (71.0) | 147 (66.5) |
| NYHA class IV | 44 (13.0) | 42 (12.4) | 19 (12.2) | 17 (14.3) | 25 (13.7) | 25 (11.3) |
Note
- The prevalence of comorbidities in women and men across age is depicted in Figure S1.
- Abbreviations: COPD, chronic obstructive pulmonary disease; HRT, hormone replacement therapy; IQR, interquartile range; MI, myocardial infarction; NYHA class, New York Heart Association class; PEA, pulmonary endarterectomy; PVR, pulmonary vascular resistance; VTE, venous thromboembolism.
- aMissing values (n): history of venous thromboembolism (1), obesity (167), diabetes (168), coronary disease (167), smoking (163), major trauma (167), cancer (162), thyroid disorders (166), splenectomy (171), congestive heart failure (172), use of hormonal treatment before chronic thromboembolic pulmonary hypertension diagnosis (175).
We identified sex differences in the following baseline comorbidities: smoking habit (ARD, −21.4% in women; 95% CI: −28.1 to −14.4), obesity (ARD, 6.5%; 95% CI, 0.8-12.3), prior acute coronary syndrome (ARD, −8.5%; 95% CI, −14.3 to −3.7), chronic obstructive pulmonary disease (−5.3%; 95% CI, −9.8 to −0.9), prior trauma (ARD, −5.0%; 95% CI, −8.0 to −2.5), cancer (ARD, 5.9%; 95% CI, 0.6-11.2), thyroid disease (ARD, 8.0%; 95% CI, 3.9-12.3), and prior splenectomy (ARD, −3.8%; 95% CI, −6.9 to −1.1). A summary of patients′ baseline characteristics is provided in Table 1. The age- and sex-dependent probabilities of presenting with specific risk factors or comorbidities are summarized in the Figure S1.
At the time of CTEPH diagnosis, women had lower right atrial pressure, but no differences in mean pulmonary artery pressure, pulmonary vascular resistance, and cardiac index were observed with the current sample size (Figure S2). Similarly, no clinically meaningful difference was observed between women and men for the results of imaging tests (eg, dilation of bronchial arteries, mosaic perfusion pattern, proximal vs distal localization of lesions, ventilation/perfusion patterns); Table S1.
3.1 Pulmonary endarterectomy and surgical complications
A total of 291 (85.8%) women and 311 (91.5%) men were evaluated for PEA (ARD, −5.3% in women; 95% CI, −10.1 to −0.6). In those receiving evaluation (n = 602), 176 (60.5%) of 291 women and 209 (67.2%) of 311 men were initially scheduled for PEA for an ARD of −6.7% (95% CI, −14.3 to +1.0). PEA was performed in 183 (54.0%) of 339 women and 221 (65.0%) of 340 men (ARD, −11.0%; 95% CI, −18.2 to −3.6); Table 1. The time elapsing from CTEPH diagnosis to surgery was 74 days (IQR, 28-146) in women vs 90 days (IQR, 34-146) in men (P = .473, Mann-Whitney test).
Among 275 patients who were not operated, women had a longer median diagnostic delay than men (15.8 [IQR, 9.0-37.1] vs 10.8 [IQR, 7.0-26.1] months; P = .035, Mann-Whitney test). The diagnostic delay was shorter in women (12.8 [IQR, 6.2-27.3]) vs men (17.3 [IQR, 8.9-38.8]) who underwent PEA (P = .009, Mann-Whitney test). With the current sample size, several of the sex-specific differences captured in the Registry concerning specific reasons for not being operated did not reach statistical significance at standard probability threshold of 5%; Table 2. Microvascular disease (+6% in women [95% CI +0.4 to +11.5]) accounted for the largest difference between women and men.
| Women not operated / PEA- | Men not operated / PEA- | All women | All men | |||||
|---|---|---|---|---|---|---|---|---|
| Total (n = 156) | Low-volume centers (n = 91) | High-volume centers (n = 65) | Total (n = 119) | Low-volume centers (n = 61) | High-volume centers (n = 58) | Total (n = 339) | Total (n = 340) | |
| Clot not accessible (distal lesions) | 14 (9.0) | 12 (13.2) | 2 (3.1) | 14 (11.8) | 12 (19.7) | 2 (3.4) | 14 (4.1) | 14 (4.1) |
| Imbalance between severity of pulmonary hypertension and morphological lesion (microvascular disease) | 66 (42.3) | 27 (29.7) | 39 (60.0) | 46 (38.7) | 15 (24.6) | 31 (53.4) | 66 (19.5) | 46 (13.5) |
| Pulmonary vascular resistance > 1500 dyn*s*cm−5 | 4 (2.6) | 3 (3.3) | 1 (1.5) | 0 | 0 | 0 | 4 (1.2) | 0 |
| Comorbidity | 15 (9.6) | 11 (12.1) | 4 (6.2) | 17 (14.3) | 9 (14.8) | 8 (13.8) | 15 (4.4) | 17 (5.0) |
| Age | 4 (2.6) | 2 (2.2) | 2 (3.1) | 1 (0.8) | 1 (1.6) | 0 | 4 (1.2) | 1 (0.3) |
| Patient′s refusal (being otherwise operable) | 19 (12.2) | 11 (12.1) | 8 (12.3) | 19 (16.0) | 10 (16.4) | 9 (15.5) | 19 (5.6) | 19 (5.6) |
| Other or unknown | 34 (21.8) | 25 (27.5) | 9 (13.8) | 22 (18.5) | 14 (23.0) | 8 (13.8) | 34 (10.0) | 22 (6.5) |
Note
- Overall, 4 (of 27) high-volume centers included a total of 347 (of 679) patients, corresponding to 51.1% of the study population. Numbers in the table represent numbers of patients; percentages are in brackets.
- Abbreviation: PEA, pulmonary endarterectomy.
In women, the probability of receiving PEA decreased linearly as a function of age, namely from 85% in patients aged ~30 to 30%-40% older than 75 years, whereas in men this probability remained higher than in women between 55 and 75 years of age (Figure 2).
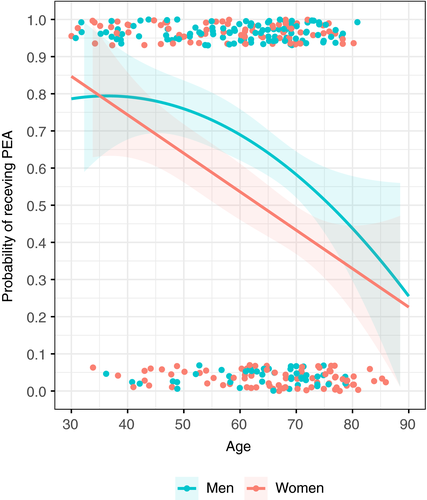
We fitted univariate and multivariable logistic regression models evaluating the association of sex with PEA after adjustment for age, sex, inclusion at high-volume centers, and diagnostic delay. Sex remained inversely associated with performance of PEA (adjusted OR, 0.58; 95% CI, 0.41-0.81) independently from these factors (Table 3). Four high-volume centers included a total of 347 patients, corresponding to 51.1% of the study population. Because inclusion at high-volume centers corresponded to a higher proportion of patients screened for operability (93.9% vs 83.1% at low-volume centers), a higher proportion of patients deemed operable (55.1% vs 44.9%), and, finally, a higher proportion of patients subjected to PEA (56.3% vs 43.7%), we also included in a model the potential interaction between sex and type of center with similar direction of the estimates (adjusted OR, 0.48; 95% CI, 0.30-0.78); Table 3.
| Model | Adjustment | Odds ratio (female to male sex) for PEA being performed (95% CI) | Missing values |
|---|---|---|---|
| Model 1 | No adjustment (univariate model) | 0.63 (0.46-0.86) | 0 |
| Model 2 | Adjustment for age | 0.60 (0.44-0.83) | 0 |
| Model 3 | Adjustment for age, NYHA class, inclusion at a high-volume center | 0.61 (0.44-0.84) | 1 |
| Model 4a | Adjustment for age, diagnostic delay, NYHA class, inclusion at a high-volume centera | 0.58 (0.41-0.81) | 42 |
| Model 5b | Adjustment for age, diagnostic delay, NYHA class, site volume, interaction between sex and inclusion at a high-volume centerb | 0.48 (0.30-0.78) | 42 |
- Abbreviations: CI, confidence interval; NYHA, New York Heart Association; PEA, pulmonary endarterectomy.
- aAdjusted odds ratio (95% CI): age 0.96 (0.95-0.97); inclusion at a high-volume center 1.51 (1.08-2.12); higher tertile of diagnostic delay 1.17 (0.81-1.67), NYHA III-IV 1.04 (0.67-1.63).
- bAdjusted odds ratio (95% CI): age 0.96 (0.95-0.97); inclusion at a high-volume center 1.25 (0.77-2.04); higher tertile of diagnostic delay 1.18 (0.83-1.70), NYHA III-IV 1.03 (0.66-1.61), interaction between sex and inclusion at a high-volume center 1.43 (0.73-2.81).
The proportion of operated women at high-volume centers was much higher than at low-volume centers (ARD of PEA exposure between high- and low-volume centers +12.4% [95% CI, 1.7-22.6]); in men, this difference was +7.2% (95% CI, −3.0 to +17.2). Performance of PEA at low-volume centers was 48% in women and 61.1% in men (ARD of PEA exposure between women and men −13.2% [95% CI, −23.4 to −2.4]). Specific reasons for not undergoing PEA at low- vs high-volume centers are listed in Table 2.
During PEA, women were exposed to additional cardiac surgery procedures less often than men (ARD, −9.1% in women [95% CI, −15.9 to −2.0]), particularly to coronary artery bypass graft surgery (0.5% vs 9.5%, ARD, −9.0% [95% CI, −13.6 to −4.9]; Table S2). Surgical complications were 48.1% in women vs 51.6% in men. Intraoperative mortality was 3.8% and 5.4%, respectively.
3.2 Long-term mortality
A total of 127 deaths were recorded over long-term follow-up: 57 (17.0%) of 339 women and 70 (20.7%) of 340 men died for an unadjusted HR of 0.75 (95% CI, 0.49-1.16). Causes of deaths are depicted in Table S3. Cardiovascular causes, accounting for right heart failure, perioperative complications, cardiac arrhythmia, myocardial infarction, and pulmonary embolism, occurred in 24 (15.7%) women and 22 (18.8%) men not undergoing PEA, and in 9 (4.9%) women and 19 (8.6%) men undergoing PEA (overall ARD, −2.3%; 95% CI, −7.1 to 2.5).
One-year mortality rate (death from all causes) was 5.5% in women and 6.8% in men who underwent PEA (OR, 0.79; 95% CI, 0.35-1.81), and 13.1% in women vs 12.0% in men (OR, 1.10; 95% CI, 0.53-2.30) who did not undergo PEA; Table 4.
| Not operated / PEA- (n = 275) | Operated / PEA+ (n = 404) | Total (N = 679) | ||||
|---|---|---|---|---|---|---|
| Women | Men | Women | Men | Women | Men | |
| Year 1 | ||||||
| n/N | 20/153 | 14/117 | 10/183 | 15/221 | 30/336 | 29/338 |
| % (95% CI) | 13.1 (8.6-19.3) | 12.0 (7.3-19.1) | 5.5 (3.0-9.8) | 6.8 (4.2-10.9) | 8.9 (6.3-12.5) | 8.6 (6.0-12.1) |
| Odds ratio (95% CI) | 1.10 (0.53-2.30) | Reference | 0.79 (0.35-1.81) | Reference | 1.05 (0.61-1.78) | Reference |
| Total period | ||||||
| n/N | 42/153 | 40/117 | 15/183 | 30/221 | 57/336 | 70/338 |
| % patient-years (95% CI) | 10.6 (7.4-13.8) | 14.1 (9.7-18.4) | 2.7 (1.3-4.1) | 4.8 (3.1-6.5) | 6.0 (4.5-7.6) | 7.7 (5.9-9.5) |
| Hazard ratio (95% CI) | 0.75 (0.49-1.16) | Reference | 0.59 (0.31-1.09) | Reference | 0.79 (0.56-1.13) | Reference |
- Abbreviations: CI, confidence interval; PEA, pulmonary endarterectomy.
Women had better long-term survival than men with annualized death rates of 10.6% (95% CI, 7.4-13.8) vs 14.1% (95% CI, 9.7-18.5) among nonoperated patients, and 2.7% (95% CI, 1.3-4.1) vs 4.8% (95% CI, 3.1-6.5) after PEA, corresponding to HR of 0.75 (95% CI, 0.49-1.16) and 0.59 (95% CI, 0.31-1.09), respectively. This difference appeared more evident beyond year 2 after CTEPH diagnosis, irrespective of whether patients underwent PEA or not (Figure 3).
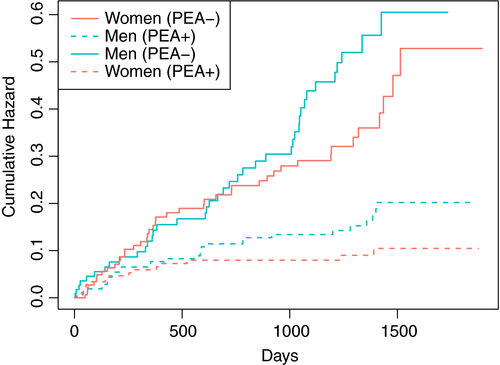
The results of parsimonious Cox regression models adjusted for clinically relevant covariates and parameters of severity suggested similar magnitudes of the effect of female sex in operated (adjusted HR [aHR], 0.67 [95% CI, 0.35-1.27] and nonoperated patients (aHR, 0.70 [95% CI, 0.45-1.09]). In the whole cohort, aHR for female sex was 0.66 (95% CI, 0.46-0.94; Table 5). An exploratory analysis of potential interactions was performed (data not shown): none of the independent variables appeared to interact with sex for the primary outcome. In the sensitivity analysis including variables selected based on their clinical relevance irrespective of the rate of missing values, we obtained similar risk estimates (aHR, 0.65 for women [95% CI, 0.43-0.99]); Table 5.
| Adjusted hazard ratio (95% CI) | ||||
|---|---|---|---|---|
| Whole cohortb | Whole cohort (sensitivity analysis)c | Not operated / PEA- | Operated / PEA+ | |
| Women (vs men)a | 0.66 (0.46-0.94) | 0.65 (0.43-0.99) | 0.70 (0.45-1.09) | 0.67 (0.35-1.27) |
| Age at diagnosis (per unit increase) | 1.01 (1.00-1.03) | 1.004 (0.99-1.02) | 1.01 (0.99-1.03) | 1.02 (0.99-1.04) |
| Pulmonary endarterectomy | 0.32 (0.22-0.47) | 0.23 (0.15-0.37) | - | - |
| Mean pulmonary artery pressure (per unit increase) | 1.02 (1.004-1.04) | 1.02 (1.001-1.04) | 1.03 (1.01-1.05) | 0.99 (0.97-1.02) |
| NYHA class III (vs I-II) | 1.89 (0.99-3.59) | 2.12 (1.04-4.36) | 2.27 (0.96-5.35) | 1.35 (0.50-3.34) |
| NYHA class IV (vs I-II) | 5.08 (2.51-10.28) | 7.15 (3.24-15.8) | 5.35 (2.06-13.87) | 4.89 (1.69-14.13) |
| History of venous thromboembolism | 0.71 (0.49-1.03) | 0.61 (0.40-0.93) | 0.85 (0.54-1.36) | 0.54 (0.28-1.02) |
| Additional cardiac procedures | - | - | - | 1.83 (0.91-3.69) |
| Persistent pulmonary hypertension | - | - | - | 2.36 (1.16-4.79) |
| All other complications | - | - | - | 1.64 (0.88-3.05) |
| Active cancer | - | 1.94 (1.21-3.13) | - | - |
| Coronary heart disease | - | 1.46 (0.91-2.36) | - | - |
| Obesity | - | 0.95 (0.58-1.55) | - | - |
| Smoking | - | 0.89 (0.58-1.37) | - | - |
- Abbreviations: CI, confidence interval; NYHA, New York Heart Association; PEA, pulmonary endarterectomy.
- aAn explorative analysis of potential interactions was performed: none of the other independent variables, including PEA, appeared to interact with sex for the outcome of all-cause death.
- bN = 7 patients had missing values for some of the variables included in the model (n = 127 events recorded in 672 patients).
- cN = 179 patients had missing values for some of the variables included in the model (n = 103 events recorded in 500 patients).
4 DISCUSSION
The results of our study confirm the general hypothesis that the prevalence of comorbidities and cardiovascular risk factors, the clinical presentation, and the course of disease differ between women and men diagnosed with CTEPH. Although pulmonary hemodynamic and radiological appearance were similar between sexes, the therapeutic approach varied considerably because PEA was less frequently performed in women. This trend was much more evident at low-volume centers and predominantly involved women aged 55-75 years. As observed in the general population, women were characterized by a better long-term survival, irrespective of the performance or not of PEA, but a similar short-term mortality. This may have been due to the lower prevalence of cardiovascular risk factors observed in women.
Women were evaluated for and exposed to PEA less often than men (Figure 2), although no large differences were observed in echocardiographic and functional parameters. Indeed, high-volume centers exhibited higher proportions of women undergoing PEA (60.4%) than low-volume centers (48%). We observed sex-specific differences for specific contraindications to surgery (Table 2), particularly concerning the presence of microvascular disease, high pulmonary vascular resistance, and older age (but not distal lesions, or comorbidities), which were more prevalent in nonoperated women. Although referral bias may have played a role, we cannot exclude that, in general, the pattern of CTEPH may be different between sexes, leading to greater technical complexity.
Among nonoperated patients, women had a longer diagnostic delay than men, which may represent a factor increasing the likelihood of secondary microvascular disease. Data from other registries and cohort studies also showed that the proportion of women is higher among nonoperable and nonoperated patients than among those undergoing PEA.27-32 We were not able to confirm the results of a recent report suggesting that the patient′s refusal (12.2% among nonoperated women vs 16.0% among nonoperated men in our cohort, corresponding to 5.6% and 5.6% of all women and men, respectively) may explain the observed sex-specific discrepancy in performed PEA procedures.33 Whether subjective or disease-related factors play a role in determining operability should be the goal of future ad hoc investigations.
In the European registry, the ratio of women vs men was 1:1, which is similar to what reported by the authors of other registry-based studies.6, 34, 35 In contrast, a recent population-based study conducted in the United Kingdom suggested that women might be more prone to develop CTEPH than men.36 This latter observation is indirectly supported by the higher prevalence of women observed in Japanese,22 Mexican,37 and Korean cohorts,38 and in some European cohorts of CTEPH patients.18, 27-29, 39 It must be noted, however, that these figures are conditional probabilities that depend on eligibility criteria of each cohort and national differences in the baseline characteristics and demographics. As indirect as this evidence is, one might hypothesize that, also based on our results, the number of women diagnosed with CTEPH may exceed that of men during the reproductive age, when women are exposed to hormonal use and experience pregnancy (Figure 1), as has been observed for venous thromboembolism.40-42
Another element that might explain the differential use of PEA between sexes is that men more often required major cardiac surgery procedures for other indications, although this correlation does not necessarily imply causation. This was the case in 43 men and 19 women, corresponding to 19.5% and 10.4% of operated patients: of note, 9.5% and 0.5% underwent coronary artery bypass surgery, respectively. Consistently, women had a lower prevalence of major cardiovascular risk factors, such as smoking, prior acute coronary syndrome or coronary heart disease, and chronic obstructive pulmonary disease. In cohorts of patients with venous thromboembolism, men were characterized by a heavier burden of cardiovascular risk factors than women.43 A predisposition to coronary artery disease is known to be partially determined by genetic factors related to the Y chromosome44: its prevalence is higher in men than in women in the general population 45-47 as well as among patients undergoing major cardiothoracic surgery 48, 49.
The lower burden of comorbidities may have also contributed to a better long-term survival in women (aHR, 0.66; 95% CI, 0.46-0.94); Figure 3. No clear interaction was demonstrated with PEA, suggesting that the benefit of surgery was similar in women and men. Interestingly, no difference in the cumulative rate of death over the first year after CTEPH diagnosis was observed with the current sample size, despite more prevalent cancer at baseline in women. To the best of our knowledge, only another study of 73 inoperable CTEPH patients, the results of which have been presented at the latest American Thoracic Society conference, addressed this issue and seemed to confirm a positive association between female sex and survival 50. The main explanation to this difference may be found in deaths resulting from cardiopulmonary causes, which are influenced by genetic, hormonal, and behavioral factors. In this perspective, the results are consistent with the longer life expectancy in women globally 51.
We acknowledge several limitations of our study. First, the statement that “absence of evidence is not evidence of absence” applies to our results. Although we found that sex is an independent prognostic factor in patients with CTEPH, the current sample size did not allow us to confirm this observation in patients stratified by PEA being performed or not. The current study population was neither powered to study marginal associations nor to investigate whether an interaction between risk factors and sex occurs. Consistently, the relatively small sample size of the study population and subgroups limits the interpretation of the reasons at the basis of the different proportion of “operable” women and men. Second, some variables were characterized by a high proportion of missing data. Being a post hoc analysis, the exploratory nature of the present study must be acknowledged as must that multiple comparisons may have led to erroneous inferences. In addition, the data have been obtained over the past decade: during this time, the treatment of CTEPH has changed considerably with approval of drugs for nonoperable patients and introduction of balloon pulmonary angioplasty. It is unclear whether these results would still be applicable today. Finally, caution must be paid when interpreting the different causes of deaths reported in the manuscript since, as for many other variables collected, central adjudication had not been performed.
Women diagnosed with CTEPH underwent PEA, the main curative therapy for CTEPH, less frequently than men. This was especially the case for patients managed at low-volume centers. Despite this, they exhibited better long-term survival than men, similarly to what has been described for other forms of pulmonary hypertension. Men had a higher prevalence of cardiovascular risk factors than women and more often received concomitant cardiac surgery procedures, such as coronary artery bypass surgery. Our data should be interpreted with caution because of the exploratory nature of this study; it therefore remains to be investigated whether sex-specific differences in the use of PEA may represent an example of disparity in the access to the cure, be dependent on the experience of the treating physicians and surgeons, depend on subjective (eg, the perception of the disease) and disease-related factors, or characterize different preoperative assessment strategies.
ADDENDUM
S. Barco contributed to the concept and design of the study, statistical analysis, interpretation of the results, writing of the manuscript, and gave final approval. F.A. Klok contributed to the concept and design of the study, interpretation of the results, and gave final approval. S.V. Konstantinides, M. Delcroix, and I.M. Lang contributed to the design of the study, interpretation of the results, critical revision of the manuscript for important intellectual content, and gave final approval. All other authors provided important intellectual content, including interpretation of the results, critical revision of the manuscript, and gave final approval.
ACKNOWLEDGEMENTS
The authors are grateful to the steering committee and investigators of the European CTEPH Registry for being allowed to use the data for the present analysis. The work of Stefano Barco, Frederikus Klok, and Stavros Konstantinides is supported by the German Federal Ministry of Education and Research (BMBF 01EO1003 and 01EO1503). The work of Frederikus Klok was supported by the Dutch Heart Foundation (2017T064).
CONFLICT OF INTEREST
Stefano Barco has received congress and travel payments from Daiichi-Sankyo and Bayer HealthCare, and personal fees from BTG outside the submitted work. Frederikus Klok reports research grants from Bayer, Bristol-Myers Squibb, Boehringer-Ingelheim, MSD, Daiichi-Sankyo, Actelion, the Dutch thrombosis association, and the Dutch Heart Foundation. Stavros Konstantinides has received consultancy and lecture honoraria from Bayer HealthCare, Boehringer Ingelheim, Daiichi-Sankyo, and Pfizer-Bristol-Myers Squibb; payment for travel accommodation/meeting expenses from Bayer HealthCare; and institutional grants from Boehringer Ingelheim, Bayer HealthCare, and Daiichi-Sankyo. Nick Kim reports research support from Actelion, Bayer, J&J, and Merck, and received lecture honoraria from Bayer. Joanna Pepke-Zaba or her institution have received research, educational grants from Actelion, Merck, and Bayer; she served at advisory boards of Actelion, J&J, Merck, Bayer, and GSK. David Jenkins reports grants and personal fees from Bayer, personal fees from Actelion, outside the submitted work. Hiromi Matsubara reports personal fees from Actelion Pharmaceuticals Japan, Ltd., personal fees from AOP Orphan Pharmaceuticals AG, personal fees from Bayer Yakuhin, Ltd., personal fees from Nippon Shinyaku, Co., Ltd., personal fees from Pfizer Japan, Inc., and personal fees from Kaneka Medix Corporation, outside the submitted work. Eckhard Mayer reports personal fees from Actelion, personal fees from Bayer, personal fees from MSD, and personal fees from Pfizer, outside the submitted work. Irene Lang reports personal fees from MSD, grants and personal fees from AOPORphan Pharma, and grants and personal fees from Actelion, outside the submitted work. Marion Delcroix has been an investigator, speaker, consultant, or steering committee member for Actelion, Bayer, Eli Lilly, GlaxoSmithKline, MSD, and Pfizer; and has received research grants from Actelion. All the other authors have no conflicts of interest to declare.




