Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals
Summary
Polyphenols are secondary plant metabolites which have been shown to exert antioxidative and antiinflamma tory effects in cell culture, rodent and human studies. Based on the fact that conditions of oxidative stress and inflammation are highly relevant in farm animals, polyphenols are considered as promising feed additives in the nutrition of farm animals. However, in contrast to many studies existing with model animals and humans, potential antioxidative and antiinflammatory effects of polyphenols have been less investigated in farm animals so far. This review aims to give an overview about potential antioxidative and antiinflammatory effects in farm animals. The first part of the review highlights the occurrence and the consequences of oxidative stress and inflammation on animal health and performance. The second part of the review deals with bioavailability and metabolism of polyphenols in farm animals. The third and main part of the review presents an overview of the findings from studies which investigated the effects of polyphenols of various plant sources in pigs, poultry and cattle, with particular consideration of effects on the antioxidant system and inflammation.
Introduction
Since the ban of feed antibiotics by the EU in the year of 2006, there is intensive search for feed additives in farm animals which are able to maintain or even improve animal health and animal performance. Natural compounds produced by plants might be relevant candidates in this respect. Plants are producing a great variety of secondary metabolites. Many of these secondary plant metabolites have been shown to exert a broad range of beneficial effects on health in humans and rodent models (e.g. Cornwell et al., 2004; Hooper et al., 2008; Lavecchia et al., 2013; Martin and Bolling, 2015). Among the great number of secondary plant metabolites, the group of polyphenols might be most promising due to its well-established antioxidative and gene regulatory properties (Baur et al., 2006; Chuang and McIntosh, 2011; Aguirre et al., 2014). It has been well established that polyphenols are able to act antiinflammatory both in vitro and in vivo by inhibiting the activation of nuclear factor kappa B (NF-κB) and to induce antioxidative and cytoprotective effects by inducing nuclear factor erythroid 2-related factor-2 (Nrf2) (Rahman et al., 2006; Scapagnini et al., 2011; Tangney and Rasmussen, 2013). In humans and experimental animal models, these effects of plant polyphenols are well established. In contrast, in farm animals, the effects of plant polyphenols with respect to their antiinflammatory, antioxidative and cytoprotective effects have been less investigated so far. This study aims to give a comprehensive overview about effects of polyphenols in farm animals with major emphasis on the effect of polyphenols on inflammation and oxidative stress, both of which are linked with each other. In the first chapters, however, the role of oxidative stress and inflammation in farm animals is highlighted.
Oxidative stress
Oxidative stress is known as the imbalance between the formation of oxidants and their detoxification by the antioxidant system and is traditionally recognized as detrimental to the body because it can cause damage to the cell constituents including lipids, DNA, proteins and carbohydrates thus leading to tissue damage (Halliwell, 2007). Oxidants include both radical and non-radical molecules, containing either oxygen, nitrogen or chlorine, called reactive oxygen species (ROS), nitrogen or chlorine species respectively. The formation of oxidants can occur during normal metabolism, for example superoxide radicals are produced within the respiratory chain in the mitochondria, during inflammatory reactions, for example superoxide radicals are formed by NADPH oxidase from activated immune cells, and due to exogenous noxious agents, for example high concentrations of free transition metals like iron and copper which induce the formation of the highly reactive hydroxyl radicals from hydrogen peroxide (Gutteridge, 1994). The antioxidant system, which is able to prevent oxidative stress through reducing the formation and/or scavenging of oxidants, is comprised of three components: (i) antioxidant enzymes, such as superoxide dismutase (SOD), glutathione peroxidase (GPX) and catalase (CAT); (ii) low molecular mass antioxidants, such as tocopherols (vitamin E), ascorbic acid (vitamin C), carotenoids including β-carotene, uric acid, glutathione, and polyphenols; and (iii) proteins that are able to sequestrate free transition metals. The latter group of proteins includes storage and transport proteins for metal ions, such as ferritin, ceruloplasmin and metallothionein (Halliwell, 1996). As amino acids and trace elements (copper, zinc, iron, selenium) are constituents of endogenous antioxidants (glutathione, SOD, GPX, CAT) and different vitamins (thiamine, riboflavin, niacin, folic acid) involved as coenzymes in various antioxidant pathways, an inadequate supply of these nutrients impairs the antioxidant defence system thereby inducing oxidative stress (Evans and Halliwell, 2001).
Oxidative stress can be induced by dietary factors, such as polyunsaturated fatty acids or oxidized fatty acids representing an oxidative burden to the body through the formation of lipid peroxidation products and an increased consumption of endogenous antioxidants, in particular vitamin E. In broilers, for instance, it was observed that feeding a diet containing 6% linseed oil as a source of polyunsaturated fatty acids in combination with a low dietary vitamin E supply (15 mg vitamin E/kg diet) strongly depletes vitamin E and increases lipid peroxidation in tissues (Eder et al., 2005). Similarly, rats fed a diet with 10% fish oil in combination with a low vitamin E supply (10 mg vitamin E/kg diet) had increased levels of lipid peroxidation products and markedly reduced levels of vitamin E in blood and tissues (Ringseis and Eder, 2002). In pigs and different laboratory animals, feeding a diet rich in oxidized fatty acids also causes a strong reduction in plasma and tissue vitamin E concentrations and an increase in lipid peroxidation products in tissues (Liu and Huang, 1995; Eder et al., 2002; Keller et al., 2004). Further dietary sources for the generation of oxidants are undesirable substances in food such as pesticides, organic solvents or mycotoxins, because these compounds induce the hepatic xenobiotic system generating oxidants as by-products (Banerjee et al., 2001; Alpsoy and Yalvac, 2011).
Link of oxidative stress with inflammation
Oxidative stress is directly linked with inflammation due to the fact that the above-mentioned oxidants are activators of NF-κB, the key regulator of inflammation (Pantano et al., 2006). NF-κB is a protein complex which is present in almost all animal cell types and, in the inactive state, is bound to inhibitory proteins in the cytosol. Upon stimulation by oxidants and several other stimuli, such as cytokines, bacterial stimuli, viruses and ultraviolet radiation, the inhibitory proteins are released from NF-κB which facilitates the active NF-κB to translocate into the nucleus and to activate the transcription of a large set of genes involved in all aspects of inflammation (e.g. vasodilation, chemotaxis, cell adhesion, leucocyte extravasation, phagocytosis). Thus, typical proteins encoded by NF-κB target genes are proinflammatory cytokines, chemokines, inflammatory enzymes, adhesion molecules and various receptors (Hiscott et al., 1993; Barnes, 1997; Aggarwal, 2004). As several of the NF-κB-regulated proteins, such as cytokines and chemokines, stimulate the production of oxidants by activated neutrophils (respiratory burst) and in the mitochondria, thereby, promoting oxidative stress, a vicious cycle is triggered (Pillarisetti and Saxena, 2004). If the vicious cycle cannot be broken due to sustained and overwhelming production of oxidants, the inflammatory process becomes chronic and the body′s cells and tissues are damaged. This situation is the underlying cause of many chronic inflammatory disorders in humans, such as asthma, chronic obstructive pulmonary disease, inflammatory bowel disease, rheumatoid arthritis and many others, also called free radical diseases (Ciz et al., 2012). However, such free radical diseases also occur in farm animals and examples are pneumonia, enteritis and sepsis in pigs, mastitis and pneumonia in ruminants and recurrent airway obstruction, exercise-induced pulmonary haemorrhage or joint disease in horses (Lykkesfeldt and Svendsen, 2007).
Role of physiological levels of oxidants for stress adaptation
Besides the harmful effects of sustained and overwhelming production of oxidants, it has been recognized in recent years that low levels of oxidants have physiological functions for stress adaptation. This is explained by the role of many oxidants as signalling molecules of important intracellular pathways. For instance, several oxidants are activators of stress-sensitive transcription factors such as Nrf2 (Zhang and Hannink, 2003). Upon activation of Nrf2 the expression of a great number of genes involved in cytoprotection, such as the above-mentioned antioxidant enzymes, several enzymes involved in the detoxification of xenobiotics, and antiinflammatory genes, is induced (Kim et al., 2010). Thus, physiological levels of oxidants are even useful for the adaptation of the body to cellular stress due to improving defence and detoxification mechanisms. In line with this, it has been proven that moderate production of ROS in the mitochondria improves health and even extends the life span of different model organisms (C. elegans and mice), a phenomenon that has been named mitochondrial hormesis or abbreviated mitohormesis (Yun and Finkel, 2014). For instance, sirtuins, a family of histone deacetylases, were found to promote C. elegans longevity through generating a mitohormetic ROS signal, whereas this effect of sirtuins is blocked by antioxidants (Schmeisser et al., 2013). Moreover, studies in humans demonstrated that supplementation with high doses of vitamins C and E prevents the health-promoting effects, such as improvements of antioxidant defence and insulin sensitivity, of physical exercise, during which substantial amounts of ROS are formed in skeletal muscle (Ristow et al., 2009). This clearly indicates that ROS play an essential role for inducing health-promoting effects in the body. Also in agreement with this view is that the vast majority of placebo-controlled intervention trials in humans failed to demonstrate any health improvements in response to supplementation with high doses of antioxidants (vitamin C, E and β-carotene) (Fortmann et al., 2013; Grodstein et al., 2013). Moreover, according to meta-analyses of large human intervention trials, supplementation of antioxidants even increases overall mortality (Bjelakovic et al., 2014). The biological concept of mitohormesis also applies to farm animals as evidenced from a recent study with dairy cows showing that supplementation with vitamin E during the dry period increases clinical mastitis incidence post-partum through the induction of oxidative stress (Bouwstra et al., 2010a,b), likely as a result of suppression of the cows' own antioxidant and cytoprotective system. Thus, it can be summarized that the classic view of ROS as generally harmful products that destroy cellular structures is regarded as outdated, whereas physiological ROS levels should be considered as protective by stimulating defence mechanisms that prevent cellular damage.
General significance of inflammation and its regulation
Inflammation is the normal, protective and usually temporary response of the innate immune system to pathogens or injury. It is typified by redness, swelling, heat and pain. These responses occur as a result of increased blood flow, increased permeability across blood capillaries which increases the movement of leucocytes and large molecules (e.g. antibodies, cytokines) from the blood into the surrounding tissue. The aim of inflammation is to induce immunological processes to eliminate invading pathogens and toxins and to repair damaged tissue (Bertoni et al., 2009). Inflammation is triggered by the production of a broad spectrum of cytokines, chemokines, adhesion molecules, eicosanoids and complement proteins (Newton and Dixit, 2012). These molecules form complex regulatory networks to promote increased blood flow to the infected tissue, immune cell infiltration and activation, and systemic responses, including increased body temperature, increased heart rate and decreased appetite (Bradford et al., 2015). At the molecular level, the inflammatory process is mainly regulated by NF-κB, the above-mentioned key regulator of inflammation. The NF-κB is activated by ROS, which explains the mechanistic link between oxidative stress and inflammation, but also by viruses, bacterial toxins, proinflammatory cytokines and many other stressors. Upon activation, NF-κB target genes, such as proinflammatory cytokines, chemokines, inflammatory enzymes, adhesion molecules and various receptors are induced (Vendrame and Klimis-Zacas, 2015).
Consequences of inflammation
Hepatic acute-phase response
One important secondary response to inflammation is the acute-phase response (APR) which is mainly triggered by proinflammatory cytokines such as interleukin (IL)-6, tumour necrosis factor-α (TNF-α) or IL-1β. APR is characterized by an increased production of more than 200 acute-phase proteins (APP), mainly in the liver (Venteclef et al., 2011). APP play major roles in several aspects of systemic reaction to inflammation, such as opsonization of pathogens, scavenging of toxic substances and the overall regulation of different stages of inflammation (Ceciliani et al., 2012). While the concentrations of APP such as haptoglobin, ceruloplasmin, serum amyloid A and C-reactive protein in the blood are very low under healthy condition, their concentrations are greatly elevated during systemic inflammation (Bradford et al., 2015). Therefore, concentrations of APP have gained widespread acceptance as markers of inflammation (Ceciliani et al., 2012). While APP play a central role in restoring tissue homoeostasis during the early phase of inflammation, the production of several other proteins typically secreted by the liver such as albumin, apolipoproteins, transferrin or retinol-binding protein is reduced during inflammation. The downregulation of the hepatic synthesis of these proteins, which are denominated as negative APP, increases the pool of free amino acids which are utilized partially for gluconeogenesis and the production of APP (Ceciliani et al., 2012).
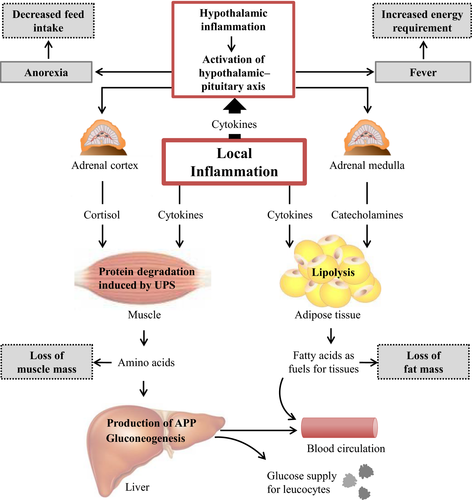
Hypothalamic inflammation
The production of cytokines is not only important for the regulation of immunological responses against pathogens or toxins. Cytokines have also profound effects on several metabolic pathways that are directly linked with performance of farm animals. Recent studies have shown that circulating cytokines and inflammatory mediators increase production and release of cytokines within the mediobasal hypothalamus, creating a paracrine inflammatory milieu which initiates alterations in the activity of neuronal populations that regulate appetite, body temperature and metabolic programs controlling body mass and energy homeostasis (Burfeind et al., 2015). Metabolic alterations that are regulated in the hypothalamus during a proinflammatory condition mainly aim to shift energy and amino acids away from stores towards metabolic responses that support the immune system. In this respect, accelerated muscle protein degradation is one key event. Increased proteolysis in skeletal muscle during inflammation is mediated by an activation of the ubiquitin proteasome system (UPS). Recent studies suggest that the activation of the UPS is mainly due to increased concentrations of glucocorticoids as a result of the activation of the hypothalamic–pituitary axis in the brain during proinflammatory conditions (Karrow, 2006; Burfeind et al., 2015). Besides glucocorticoids, cytokines such IL-1, IL-6 or TNF-α are able to directly activate the UPS in skeletal muscle (Bowen et al., 2015). Cytokines such as IL-1 moreover have been shown to inhibit the anabolic effects of insulin on skeletal muscle (Klasing and Johnstone, 1991). The aim of both muscle protein degradation and inhibition of muscle protein synthesis is to supply the liver with amino acids available for the synthesis of APP. During inflammation, the uptake of amino acids into the liver is increased, and it has been suggested that at least 60% of the amino acids required for synthesis of APP are deriving from proteolysis in skeletal muscle (Andus et al., 1991; Johnson, 1997). Amino acids deriving from muscle are moreover utilized for gluconeogenesis – a process which is stimulated by increased glucocorticoid secretion – to supply immune cells with fuels. Many immune cells, such as neutrophils and macrophages, rely heavily on glucose to meet their increased metabolic demands during inflammation (Bradford et al., 2015). An increased rate of lipolysis, induced by an increased secretion of catecholamines – a process that is also controlled in the hypothalamus –, is another metabolic alteration which aims to supply the body with metabolic fuels to meet the extraordinary energetic costs of increasing body temperature (fever) and expanding the immune responses (Thaler et al., 2010).
One key event occurring during inflammation, which is negatively linked with animal performance, is the induction of anorexia. Systemic inflammation, triggered for example by injection of lipopolysaccharides (LPS), causes an activation of neurons that produce the anorexigenic peptides pro-opiomelanocortin and cocaine- and amphetamine-regulated transcript, while the secretion of the orexigenic agouti-related peptide and neuropeptide Y is reduced, overall leading to a reduced appetite (Burfeind et al., 2015). Besides effects on the release of orexigenic and anorexigenic peptides in the brain, central cytokines moreover have profound effects on gastrointestinal secretions and motility. Changes in this respect resemble those of satiated animals, such as a decrease in gastric secretion or an inhibition of gastric motility (Johnson, 1997). The loss of appetite in sick animals is not a maladaptive response or the effect of debilitation, but rather an organized, evolved strategy that facilitates recovery. Systemic inflammation causes a shift of the anabolic–catabolic balance towards catabolism meaning that animals have a lower requirement of energy and nutrients for anabolic processes. Thus, anorexia in sick animals can be considered as a means to avoid intake of nutrients that are unusable in their current physiological state (Johnson, 1998). Indeed, it has been shown that survival of infected animals is positively related to anorexia and weight loss, at least in the short term (Murray and Murray, 1979). An overview of hormonal and metabolic changes triggered by inflammation is given in Fig. 1.
Consequences of inflammation on performance of farm animals
The effects of systemic inflammation on performance of important farm animal species, such as pigs, poultry and cattle, have been mostly investigated by injection of LPS or proinflammatory cytokines.
Effects of inflammation on performance of pigs
Several studies have shown that typical responses to LPS challenge in pigs are reduced feed intake, increase in body temperature (fever), strong increases in the concentrations of proinflammatory cytokines, APP and cortisol and reduction in the plasma concentration of insulin-like growth factor (IGF)-1. Reduced feed intake, increased energy requirement for the production of fever and hormonal changes shift the metabolism into a more catabolic state which results in reduced body weight gains and an impairment of the gain-to-feed ratio (Webel et al., 1997; Liu et al., 2003; Campos et al., 2014; Wyns et al., 2015). Increases of plasma non-esterified fatty acids (NEFA) and urea concentrations after LPS challenge are indicative of an increased lipolysis in adipose tissue and an increased protein breakdown in muscle (Johnson, 1997).
In pigs, besides several diseases associated with systemic inflammation, the weaning phase represents one important stage in which a local inflammation in the small intestine is occurring, which is called weaning-associated intestinal inflammation. This inflammation is characterized by an increased production of cytokines and adverse changes in intestinal morphology, including reduced villus height, increased villus width, increased crypt depth and reduced absorptive capacity and brush border enzyme activity (McCracken et al., 1995, 1999; Pié et al., 2004). It has been originally suggested that these effects are mainly due to a depressed feed intake (McCracken et al., 1995, 1999). Recent studies, however, have shown that gut function is adversely affected by an activation of the hypothalamic–pituitary–adrenal axis which is caused by weaning stress conditions, including psychosocial (maternal deprivation), immunological, infectious, metabolic and nutritional stress. Downstream events of hypothalamic–pituitary–adrenal axis activation include an increased secretion of corticotrophin-releasing factor and glucocorticoids, and an activation of intestinal mast cells, all of which might be involved in disruption of the intestinal barrier (Smith et al., 2010; Pohl et al., 2015). The compromised intestinal barrier allows the passage of luminal antigens into the lamina propria where an inflammatory response can be initiated. Villus atrophy in this stage might be caused by an activation of matrix metalloproteinases which are induced by proinflammatory cytokines (McCracken et al., 1999). A disturbance of the barrier integrity moreover favours the transepithelial passage of micro-organisms and thus promotes bacterial infections and diarrhoea. Noteworthy, adverse effects of weaning stress on gut function are not limited to the weaning phase. Rather, weaning stress triggers long-term defects in intestinal barrier function (Smith et al., 2010). Therefore, the weaning-associated intestinal inflammation process is negatively linked with health and growth performance of piglets, and controlling this process is a challenge in managing post-weaning health and optimum growth performance (Pié et al., 2004).
Recent studies have shown that lactation is another metabolic condition which is associated with the development of inflammation in the liver of pigs (Rosenbaum et al., 2012a,b). It is likely that the induction of inflammation in the liver of lactating sows – which was also associated with the development of stress of the endoplasmic reticulum (ER stress), a condition triggered by inflammation – might be due to oxidative stress caused by the high metabolic activity or the occurrence of infectious diseases such as mastitis or metritis which are commonly observed in sows after parturition (Gessner et al., 2015a,b). Physiological consequences of inflammatory conditions in lactating sows have been less investigated so far. However, with respect to animal welfare and performance, prevention of proinflammatory conditions in lactation sows should be given more consideration.
Effects of inflammation on performance of poultry
Like in pigs, LPS-induced systemic inflammation in broilers commonly leads to a strong increase in the production of various proinflammatory cytokines and to an increase in body temperature (De Boever et al., 2009, 2010; Zhang et al., 2010; Shen et al., 2010). With respect to animal performance, a decrease in feed intake, a reduction in body weight gain and an increase in the feed conversion ratio were typically observed in broilers subjected to LPS injection (Korver et al., 1998; Shen et al., 2010; Tan et al., 2014). LPS injection in broilers caused also a strong increase in plasma concentration of cortisol, shifting the metabolism in a more catabolic state (Shini et al., 2008). Broilers injected with LPS moreover had increased relative weights (organ weight/body weight) of liver, spleen and intestine (Korver et al., 1998).
Effects of inflammation on performance of dairy cattle
Several studies in the last years have shown that most of the high-yielding dairy cows experience a proinflammatory condition during the peripartal phase (Bertoni et al., 2008; Bradford et al., 2015). The proinflammatory condition is caused by various stimuli, such as LPS, cytokines and ROS released in the course of infectious diseases, such as mastitis or metritis (Bradford et al., 2015). Although this inflammation is mostly of subclinical nature, it is of impact for health and performance of cows during early lactation. Studies with dairy cows, which were subjected to systemic inflammation induced by injection of either LPS or proinflammatory cytokines, typically found a decrease in food intake (Yuan et al., 2013) and a strong reduction in milk yield (Kushibiki et al., 2003; Trevisi et al., 2009; Yuan et al., 2013). In addition to the impairment of animal′s performance, it was reported that the induction of inflammation was associated with an increase in the concentration of NEFA as a result of increased rate of lipolysis in adipose tissue and increased concentration of β-hydroxybutyrate as a result of a stimulation of ketogenesis in the liver and the development of a fatty liver (Trevisi et al., 2009; Bradford et al., 2015). Moreover, cows subjected to TNF-α-induced inflammation had an increased incidence of various diseases, such as ketosis, mastitis, respiratory diseases, metritis and milk fever (Yuan et al., 2013). Conversely, inhibition of the proinflammatory condition during the peripartal phase in dairy cows by early post-partum treatment with non-steroidal anti-inflammatory drugs led to a significant increase in milk yield over the whole lactation period and caused a reduction in somatic cell count in milk being indicative of an improved udder health (Carpenter et al., 2016). According to these findings, investigations aiming to prevent the proinflammatory condition during the peripartal phase, either by optimization of feeding regimes pre-partum (Khan et al., 2015; Zhou et al., 2015; Vailati-Riboni et al., 2016) or by administration of potential antiinflammatory feed supplements (Gessner et al., 2015a,b; Hashemzadeh-Cigari et al., 2015; Winkler et al., 2015), are of great relevance.
Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals
Structure and classes of polyphenols
Polyphenols are secondary plant metabolites synthesized by plants to provide protection against invasive pathogens, such as bacteria, viruses and fungi, and to prevent damage to DNA and photosynthetic apparatus due to ultraviolet radiation. The polyphenols comprise a large group of more than 8000 different compounds with the phenol ring being the common structural feature. According to the number of phenol rings and the structural elements that bind these rings to one another, the polyphenols can be divided into the flavonoid-type and the non-flavonoid-type polyphenols (Tangney and Rasmussen, 2013).
The flavonoid-type polyphenols comprise the largest group of polyphenols with more than 4000 compounds identified and share as a common structure two benzene rings connected by three carbon atoms forming an oxygenated heterocycle. Based on the type of the heterocycle, the following flavonoid subclasses can be distinguished: flavonols, flavones, flavan-3-ols, flavanones, anthocyanins and isoflavones (Beecher, 2003). Examples of frequently studied flavonoids are the flavonols quercetin and myricetin, the flavones orientin, vitexin and homoorientin, the flavan-3-ols catechin, epicatechin, epigallocatechin and their gallate esters, the flavanone naringenin, the anthocyanin cyanidin and the isoflavones genistein and daidzein. Isoflavones have an oestrogen-like structure and thus are able to bind to oestrogen receptors (Uifălean et al., 2015). Almost all dietary flavonoids exist in glycosidic form, mostly in O-glycosidic form, but C-glycosylated flavonoids, particularly flavones, are also abundant in plants (Xiao and Jiang, 2015).
Phenolic acids, which comprise one group of non-flavonoid-type polyphenols, are frequent in cereals and can be subdivided into cinnamic acids, such as ferulic, caffeic, coumaric and sinapic acid, and the less abundant hydroxyl benzoic acids, such as gallic and vanillic acid. Typically, the cinnamic acids are bound to arabinoxylans of the plant cell wall which impairs their bioavailability (Andreasen et al., 2001). A second group of non-flavonoid-type polyphenols are the lignans, which are also diphenolic compounds with a 1,4-diarylbutane structure formed by dimerization of two cinnamic acid residues, and several representatives, such as secoisolariciresinol, pinoresinol and syringaresinol, are considered as phytoestrogens due to their oestrogen agonist and antagonist properties (Kitts et al., 1999). The last group of non-flavonoid-type polyphenols are the stilbenoids, from which resveratrol is the most famous compound due to its lifespan prolonging effects (Valenzano et al., 2006) and which have two phenol rings connected by a two-carbon methylene bridge.
Occurrence of polyphenols
Polyphenolic compounds occur in almost all plants, but their distribution at the tissue level and the cellular or subcellular level is not uniform. For instance, the outer layers of plants contain higher polyphenol levels than the inner layers or insoluble polyphenolic compounds are associated with the cell wall, whereas soluble ones are found in the cell vacuoles (Wink, 1997). Moreover, the concentrations and the proportions of polyphenolic compounds in plants are greatly affected by ripeness at the time of harvest, edaphic factors such as soil type, sun exposure, air temperature and rainfall, processing and storage (Manach et al., 2004). With regard to the latter, it can be generally stated that the concentration of polyphenolic compounds in plant material decreases with increasing storage time and when high temperatures are present during storage and processing because of the high susceptibility of polyphenols to oxidation. Collectively, these factors explain that the concentrations and the type of polyphenols vary greatly in plants and the plant products (e.g. extracts) manufactured from.
Typical polyphenol-rich plant materials are fruits, vegetables, leaves, legume seeds and beans. Correspondingly, humans regularly consuming black and green tea, tropical fruits and berries, spinach, onion, soybeans, fruit juices and wine have a high intake of polyphenols (Tangney and Rasmussen, 2013). Due to economic considerations, relevant sources of polyphenols for farm animals are agroindustrial by-products from juice, wine and beer making, such as pomace, peels, seeds, stems and brewery waste, and from processing of grains, seeds and nuts, such as hulls (rice, buckwheat, almond) and husks (coconut).
Bioavailability of polyphenols in monogastric animals
The bioavailability of dietary compounds describes the degree to which they become available in intact form to the target tissues following ingestion into the gastrointestinal tract. Generally it can be assumed that the bioavailability of polyphenolic compounds from the small intestine is very low. Based on estimations from Faria et al. (2014), less than 5% to 10% of plant polyphenols are absorbed in the small intestine. This is explained mainly by the fact that almost all polyphenols in plants exist as esters, glycosides or polymers, and many polyphenols are associated with cell wall constituents, such as arabinoxylans, with proteins or with other organic compounds such as organic acids and lipids (Andreasen et al., 2001; Bunzel et al., 2004). On the other hand, the capacity for hydrolysis and liberation from the plant matrix, respectively, by either digestive or microbial enzymes in the small intestine is generally limited due to either lack of appropriate enzyme activity or the low microbial colonization. Enzymatic hydrolysis of esters and glycosides and the release from cell wall constituents is a prerequisite step for the absorption of polyphenols because only the aglycones and few glycosides (e.g. mono- and diglucosides), but not more complex, polymeric polyphenols can be absorbed in the small intestine (Murota et al., 2002; Cermak et al., 2003; Zubik and Meydani, 2003; Manach et al., 2004). A further reason for the low bioavailability of many polyphenols in target tissues following absorption is that polyphenolic compounds are recognized as xenobiotics by the body′s biotransformation system. Thus, polyphenols are extensively biotransformed by classic detoxification pathways (methylation, glucuronidation, sulphation) in enterocytes and liver to a large number of hydrophilic conjugated (methylated, glucuronidated and sulphated) metabolites (Kuhnle et al., 2000; Vaidyanathan and Walle, 2002), which can partially reach blood and tissues, but are also rapidly excreted via urine and bile (Hackman et al., 2008). As the result of low absorption, extensive biotransformation and excretion of metabolites, concentrations of polyphenols such as quercetin in plasma and tissues after supplementation remain relatively low. Even long-term supplementation of polyphenols does not lead to an accumulation in plasma or tissues (Bieger et al., 2008; Reinboth et al., 2010). Moreover, the diverse potential health benefits of polyphenols, such as antiinflammatory, antioxidative, anticarcinogenic, antiatherogenic and immunomodulatory effects, observed in studies with laboratory animals in response to isolated polyphenols and/or polyphenol mixtures cannot be simply ascribed to the polyphenols themselves, but rather to their metabolites originating from biotransformation. This is a significant limitation for the ability to translate results from cell culture studies, in which incubations were performed only with the parent compounds, to the in vivo situation. Moreover, it has to be pointed that there are large sex-related (Mugford and Kedderis, 1998; Renaud et al., 2011; Liu et al., 2013; Ruiz et al., 2013; Dellinger et al., 2014; Prokopec et al., 2015) and species-dependent (Matal et al., 2008; Yamazaki et al., 2011; Helke and Swindle, 2013; Saengtienchai et al., 2014) differences in xenobiotic metabolism which largely explains that the spectrum of polyphenol metabolites, their tissue distribution and concentrations in blood following ingestion of polyphenols or polyphenol mixtures can differ markedly between species and between males and females (Weinert et al., 2012; Margalef et al., 2016). This is a clear hindrance for the ability to transfer data from male-to-female animals and from one species to another one.
The large proportion of polyphenols, which passes through the small intestine without being absorbed (approximately 90% to 95% of ingested polyphenols; Faria et al., 2014), together with conjugated polyphenol metabolites excreted from the liver via the bile enter the colon and are biotransformed with the help of enzymatic activities of the colon microbiota to various polyphenol metabolites. For instance, the conjugated polyphenol metabolites excreted via the bile are deconjugated by microbial glucuronidases and sulphatases into their aglycones, which can be further degraded by microbial enzymes leading to various metabolites (Hein et al., 2008). In the case of degradation of flavonols, flavones, flavanones and flavan-3-ols, for instance, a large number of different metabolites, such as dihydroxyphenylacetic acids, hydroxyphenylacetic acids, hydroxyphenylpropionic acids, dihydroxybenzoic acid, trihydroxybenzoic acid, dihydroxyphenylethanol, dihydroxybenzaldehyde phenylvalerolactones, phloroglucinol, hydrocaffeic acid, phloretic acid, benzoic acid, phenyllactic acid and vanillic acid, are formed (Meselhy et al., 1997; Déprez et al., 2000; Rechner et al., 2004; Hein et al., 2008).
The polyphenol metabolites in the colon can have different fates: one part is absorbed into the circulation after being conjugated once again in the enterocyte and the liver. Once absorbed into the circulation, the bioavailability of polyphenol metabolites in target tissues depends on their binding affinity to albumin, which was shown to differ according to the chemical structure of polyphenolic compounds (Dangles et al., 2001) and thus influences delivery of polyphenol metabolites to tissues and the rate of clearance from the circulation. Another part of polyphenol metabolites can serve as either growth-promoting substrates or antimicrobial substances for bacteria of the colon microbiota. In this context it is worth mentioning that several studies have shown that polyphenol metabolites can modulate the composition of the colon microbiota in a desirable manner due to simultaneously promoting the growth of beneficial bacteria in a pre-biotic-like manner, while inhibiting that of pathogenic bacteria (Axling et al., 2012; China et al., 2012; Queipo-Ortuño et al., 2012; Etxeberria et al., 2013, 2015; Moreno-Indias et al., 2016). This modulation of the colon microbiota is considered a key mechanism underlying the overall health-promoting effects of polyphenols, because the resulting eubiotic microbiota contributes to gut health, intact gut barrier function, and a properly working intestinal immune system, all of which are important to protect the animal from translocation of pathogens or other infectious noxious into the circulation. The remaining part of non-absorbed and non-metabolized polyphenol metabolites is excreted via the faeces. A schematic illustration of absorption and metabolism of plant polyphenols in farm animals is shown in Fig. 2.
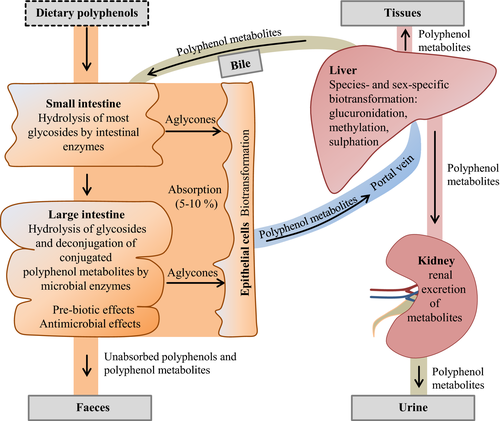
Summarizing the above, it can be stated that the bioavailability of plant polyphenols is critically influenced by their chemical structure (e.g. glycosylation, esterification and polymerization) and associations with other plant cell constituents (fibre, protein). Correspondingly, the most abundant polyphenols in plants or plant extracts are not necessarily those with the highest bioavailability. A second important message is that the two-way interaction between polyphenols and colon microbiota, meaning the biotransformation of polyphenols by the gut microbiota to polyphenol metabolites and the modulation of microbiota composition by polyphenol metabolites, is likely a key mechanism explaining many health-promoting effects of polyphenols.
Bioavailability of polyphenols in ruminants
In contrast to the great number of studies with monogastric animals, studies on the bioavailability of polyphenols in ruminants are very scarce. There are, however, few studies which indicate that at least a small part of polyphenols are absorbed from the diet in ruminants. Gohlke et al. (2013a) have recently shown that quercetin aglycon intraduodenally administered in lactating dairy cows is absorbed at a rate similar to that observed in pigs. In dairy cows, it has moreover been shown that the administration of feedstuffs rich in isoflavones (genistein, daidzein) such as soybean meal or red glover silage causes an increase in the concentrations of those isoflavones in blood and milk (Höjer et al., 2012; Cools et al., 2014). In sheep, it was observed that concentrations of different polyphenols, such as epicatechin, in plasma are increasing following the administration of four different polyphenol-rich plant extracts (rosemary, grape, citrus, marigold) via a rumen cannula (Gladine et al., 2007). Those studies indicate that at least a part of the isoflavones present in the diet is protected against degradation in the rumen and is available in the small intestine, the site of absorption of polyphenols. Besides, few reports exist demonstrating that feeding polyphenol-enriched feeding rations to heifers modulates several parameters of rumen fermentation (pH and concentration of fermentation products in rumen fluid) and rumen bacterial community (Balcells et al., 2012; De Nardi et al., 2016). With regard to the latter, it was reported in one study that feeding a flavonoid-rich plant extract increases the numbers of lactate-consuming and propionate-producing bacteria (Balcells et al., 2012) and in another study that a daily dose of a commercial polyphenol-essential oil mixture as part of a total mixed ration enhances the abundance of many taxa belonging to Bacteroidetes, Firmicutes and Tenericutes phyla (De Nardi et al., 2016). These reports indicate that, such as in monogastrics, the composition of the microbiota in the rumen of ruminants is modulated by plant polyphenols.
Effects of polyphenols on oxidative stress and inflammation in studies with farm animals
A large number of studies with either cell cultures (e.g. intestinal cells, immune cells, endothelial and smooth muscle cells, adipocytes) or experimental animal models of inflammation (intestinal inflammation, systemic inflammation associated with obesity, metabolic syndrome and atherosclerosis) convincingly demonstrated that isolated polyphenolic compounds or polyphenol-rich plant extracts (e.g. green tea, hop, cocoa, grape) suppress experimentally induced inflammation processes (Martín et al., 2004; Romier et al., 2009; Biasi et al., 2011; González et al., 2011; Song et al., 2011; Gessner et al., 2012; Gupta et al., 2014). Effects of polyphenols against inflammation are mediated by complex cellular mechanisms, most of which are linked with an inhibition of NF-κB, the master regulator of inflammation. Polyphenols are able to block the activation of NF-κB by inhibiting phosphorylation and proteasomal degradation of IκB, an effect which is at least in part due to the antioxidant properties of polyphenols (Vendrame and Klimis-Zacas, 2015). Polyphenols are able to directly scavenge ROS and moreover induce the activation of Nrf2 which in turn leads to an activation of various antioxidant enzymes (Na and Surh, 2008). Both, direct scavenging of ROS and activation of Nrf2 helps to prevent the development of oxidative stress which triggers proinflammatory pathways by activating NF-κB, mitogen-activated protein kinases and activating protein 1 (Chuang and McIntosh, 2011). Moreover, polyphenols are able to activate transcription factors such as peroxisome proliferator-activated receptor γ which is antagonizing inflammation by blocking NF-κB activation (Chuang and McIntosh, 2011). A simplified overview of some key mechanisms by which polyphenols prevent inflammation is shown in Fig. 3.
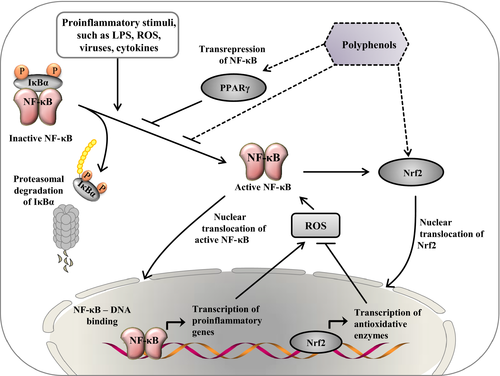
The activation of Nrf2 by polyphenols is one example of hormetic pathways which are typically activated by polyphenols and many other phytochemicals. In the context of polyphenols, the hormesis concept states that high doses of certain polyphenols are toxic, whereas subtoxic doses ingested by animals consuming plants induce mild cellular stress responses, like activation of Nrf2, leading to the induction of so-called vitagenes, such as genes encoding antioxidant enzymes, biotransformation enzymes and heat-shock proteins, thereby preserving cellular homeostasis during stressful conditions and conferring resistance to a more severe stress (Calabrese et al., 2012). Due to this, the stress-inducing phytochemicals not only protect the cell against higher doses of the same agent, but also against other agents or even less-specific stressors including oxidative, metabolic, inflammatory, and thermal stress (Son et al., 2008), all of which play an important role in farm animals. Interestingly, the activation of the cytoprotective Nrf2 pathway in response to such unspecific stressors like ROS and reactive nitrogen species stimulates autophagy (Ishii et al., 2002), which is a conserved lysosomal ‘self-digestion’ pathway for the degradation of long-lived cellular proteins and organelles generating amino acids, fatty acids and nucleotides which can be recycled for protein synthesis and ATP generation during stressful conditions. Through this autophagic ‘self-cleaning’, the cell′s ability to process damaged proteins, which can trigger apoptosis, and damaged mitochondria, a source of apoptotic proteins and toxic ROS, and to cope with the consequences of cellular stress is enhanced (Lee et al., 2012). As consequence of the activation of autophagy by hormetic phytochemicals, such as epigallocatechin gallate (Kim et al., 2013), but also by caloric restriction (Lempiäinen et al., 2013), oxidative stress, ER stress and inflammatory stress are reduced thus making autophagy an important biological process contributing to cellular and organismic health (Lee et al., 2012).
Effects of polyphenols on oxidative stress and inflammation in studies with pigs
There are several studies which considered the effects of polyphenols on pathophysiological processes of various diseases in pigs as an animal model for humans. For instance, it has been shown that the stilbenoid resveratrol or resveratrol-rich grape extract lowers fat deposition, improves the lipid profile and glucose metabolism and prevents the development of atherosclerotic lesions in the aorta of pigs (Burgess et al., 2011; Azorín-Ortuño et al., 2012). Other studies observed that resveratrol favourably influences risk factors for coronary heart disease and improves myocardial function in porcine models of metabolic syndrome and myocardial ischaemia (Robich et al., 2010; Burgess et al., 2011; Chu et al., 2011).
In contrast to studies with biomedical background, the number of studies dealing with effects of polyphenols on the antioxidant status and inflammation in pigs as farm animal species is relatively limited, with more studies focusing on potential antioxidant effects of polyphenols. One of these studies has shown that supplementation of the flavonol quercetin (at a level of 10 mg/kg body weight/day) is able to increase plasma and liver concentrations of α-tocopherol and reduce plasma lipid peroxidation products in growing pigs fed fish oil in combination with a low dietary vitamin E supply (7 mg/kg diet) (Luehring et al., 2011) indicating that quercetin exerts a tocopherol-sparing effect at a low vitamin E intake. In pigs treated with subcutaneous LPS injection, supplementation of the diet with ampelopsin (at a level of 400 mg/kg diet), which is also known as dihydromyricetin, the most common flavonoid in dry tender stems and leaves, ameliorated the antioxidative capacity in plasma and lowered concentrations of malondialdehyde (MDA) in the liver (Hou et al., 2014). This study suggested that the flavonoid ampelopsin improves the antioxidative status in pigs subjected to inflammation and oxidative stress. In a study with gilts which were treated with zearalenone, a mycotoxin known to induce oxidative stress, supplementation of isoflavones lowered MDA concentration in plasma (Wang et al., 2012). This finding suggests that isoflavones are able to counteract oxidative stress induced by mycotoxin treatment. In contrast to pigs which were either fed diets with insufficient dietary vitamin E levels or subjected to prooxidative treatments, plant polyphenols had less effect on the antioxidative status in pigs without prooxidative treatment. In the study of Augustin et al. (2008), supplementation of a green tea extract rich in catechins did not increase tocopherol concentrations in plasma and various tissues and did not increase the antioxidative capacity in pigs fed a diet with an adequate supply of vitamin E. In another study, supplementation of grape seed and grape marc extract with a total polyphenol content of 60 mg/g at a level of 10 g/kg diet did also not influence concentrations of tocopherols and thiobarbituric acid-reactive substances (TBARS) in plasma and liver and the antioxidative capacity of plasma in piglets (Gessner et al., 2013). In the study of Zhang et al. (2014), supplementation of a basal diet with a mixture of polyphenols extracted from apple, grapes, green tea and olive leaves (at a level of 1 g/kg diet) reduced concentration of MDA in plasma of weaned piglets but had no effect on the total antioxidative capacity and activities of several antioxidant enzymes in plasma. The overall conclusion of the few studies performed so far is that supplementation of polyphenols improves the antioxidative status and ameliorates oxidative stress in pigs subjected to prooxidative treatment. In pigs without prooxidative treatment, polyphenols have either no or only marginal effects on the antioxidant status.
Regarding potential effects of polyphenols on inflammation in pigs, very few studies are available considering the effects of polyphenol-rich plant extracts on the expression of proinflammatory genes in the intestine. In the study from Gessner et al. (2013), the diet containing grape seed and grape marc meal extract caused a downregulation of various proinflammatory genes in the duodenum of growing pigs. In that study, supplementation of grape seed and grape marc meal extract also caused an increase in the villus height-to-crypt depth ratio, suggesting that plant polyphenols could influence intestinal microarchitecture in a beneficial manner. In another study, two polyphenol-rich plant extracts were used, either grape seed and grape marc meal extract or hop extract, both at dietary levels of 10 g/kg diet. Both plant extracts were able to lower the expression of several proinflammatory genes in various parts of the intestine (duodenum, ileum, colon) (Fiesel et al., 2014). The apparent digestibility of nutrients was not changed by the treatment in that study. However, supplementation of both grape seed and grape marc meal extract and hop extract lowered the abundance of some potentially pathogenic bacteria (Streptococcus spp., Clostridium cluster XIVa) and concentrations of volatile fatty acids in faecal samples. These findings suggest that polyphenols exerted an antimicrobial effect on pathogenic bacteria in the intestine of pigs. In both of the above-mentioned studies, feeding polyphenol-rich plant extracts caused an improvement of the gain-to-feed ratio in pigs, an effect which could be due to an inhibition of proinflammatory processes in the intestine or to antimicrobial effects. A very recent study from Jang et al. (2016) reported that feeding different doses of cocoa powder (2.5, 10 and 20 g) containing 51, 205 and 410 mg flavanols, respectively, to pigs for 4 weeks decreased gene expression of TNF-α and Toll-like receptors -2, -4 and -9 in the ileal Peyer′s patches, mesenteric lymph nodes and the proximal colon. Interestingly, it has been shown in in vitro studies that the antiinflammatory potential of cocoa polyphenols is attributed mainly to the monomeric compounds, such as catechin and epicatechin, whereas the intermediate or large-sized oligomers, the procyanidins, which are also abundant in cocoa powder, have proinflammatory activities as evidenced by an increased secretion of TNF-α from macrophages (Mao et al., 2002; Park et al., 2000). In the context of the above-mentioned study from Jang et al. (2016), this possibly indicates that the anti-inflammatory effects of the monomeric compounds in cocoa powder dominated over the proinflammatory actions of procyanidins. Performance data were not shown in this study, but the authors stated that body weights were not influenced. Besides inhibiting proinflammatory gene expression in the intestine, this study revealed that feeding the cocoa powder increases the abundance of some beneficial bacterial strains, such as Lactobacillus and Bifidobacterium spp., in the proximal colon and faeces. These bacteria are known to inhibit the growth of pathogenic bacteria by the production of lactic acid by fermentation of sugars, reducing the intestinal pH and thus making the environment unfavourable for the growth of pathogens, competition for receptors on the intestinal epithelium and production of antimicrobial compounds (Alakomi et al., 2000; Servin, 2004). In line with findings in pigs, it was reported in a study with healthy humans that consumption of cocoa-derived flavanols has a pre-biotic effect stimulating the growth of beneficial bacterial strains while inhibiting that of potentially pathogenic ones (Tzounis et al., 2011). Thus, these findings indicate that flavanol-rich cocoa powder contributes to gut health through modulation of the colon microbiota composition and reducing expression of inflammatory genes in the intestine. In agreement with the modulation of intestinal microbial populations by polyphenol-rich diets in pigs (Fiesel et al., 2014; Jang et al., 2016), a very recent study from Magistrelli et al. (2016) found that feeding cocoa husk meal (75 g/kg diet), a polyphenol-rich waste from agroindustry representing the integuments of the cocoa beans, to male pigs for 3 weeks increased faecal abundance of Bacteroides–Prevotella and Faecalibacterium prausnitzii. Both bacterial populations are considered beneficial for the intestine due to the production of large amounts of butyrate, which stimulates growth and differentiation of enterocytes and exerts antiinflammatory effects. Unfortunately, these authors did not report on the effect of the cocoa husk meal-enriched diet on inflammatory parameters in the intestine. Performance data of the pigs such as feed intake, weight gain and gain-to-feed ratio were not influenced by feeding of the cocoa husk meal. One limitation of the study form Magistrelli et al. (2016) is that the inclusion of the large amount of cocoa husk meal into the cereal-based test diet not only increase the polyphenol content but also nearly doubled the percentage of acid detergent lignin. Thus, it is unclear to which extent the change of the intestinal microbial populations can be ascribed to cocoa polyphenols. Studies dealing with the effects of plant polyphenols on the expression of proinflammatory genes in tissues other than intestine have not yet been published. However, we have recently observed that supplementation of grape seed and grape marc meal extract or hop extract does not influence the expression of proinflammatory genes in the liver of pigs (Gessner et al., unpublished data).
In conclusion, the few studies published so far suggest that polyphenols are able to suppress inflammation in the small intestine of pigs. Considering similar effects of polyphenol-enriched diets in laboratory animals (Larrosa et al., 2009; Qiao et al., 2014; Etxeberria et al., 2015), it can be postulated that a key mechanism of this beneficial effect of polyphenol-rich diets in pigs is their contribution to gut health by exerting a pre-biotic activity favouring the growth of beneficial bacteria while inhibiting the growth of pathogenic bacteria. This beneficial modulation of the gut microbiota is known to enhance gut barrier function, decrease the translocation of bacterial components into the circulation and stimulate the host immune system (Mosele et al., 2015). In line with this, it was reported that the modulation of the colon microbiota by plant polyphenols correlated with reduced levels of systemic inflammatory markers, such as C-reactive protein, and decreased expression of inflammatory genes in tissues (Axling et al., 2012; Queipo-Ortuño et al., 2012; Moreno-Indias et al., 2016). With regard to the improvements of systemic antioxidative status and the reduction in ROS levels by polyphenol-rich diets observed in some studies with pigs (Luehring et al., 2011; Hou et al., 2014; Wang et al., 2012), we postulate that these effects are unlikely to be mediated by direct antioxidant effects of polyphenols, because of their poor bioavailability, but are secondary due to the improvements of gut health and consequently less translocation of proinflammatory and prooxidative stimuli. However, undoubtedly, further studies are warranted to get more evidence of potential beneficial effects of plant polyphenols in pigs.
Effects of polyphenols on oxidative stress and inflammation in studies with poultry
Similar to pigs, the number of studies dealing with effects of polyphenols in broilers and laying hens is limited and only a few of these studies focused on the antioxidant system or inflammation. In the study of Goni et al. (2007), supplementation of 5, 15 or 30 g grape pomace per kg diet had no effect on growth performance and digestibility of amino acids and also did not influence the antioxidant capacity in plasma and MDA concentration in breast and thigh muscle. However, the susceptibility of lipids in breast and thigh muscle during refrigerated storage was reduced by grape pomace supplementation in that study. In a follow-up study from the same group, doses of 15, 30 and 60 g of grape pomace concentrate per kg diet were tested in broilers (Brenes et al., 2008). In this experiment, doses of 30 and 60 g/kg diet caused a moderate increase in the antioxidant capacity in serum. In agreement with the study of Goni et al. (2007), supplementation of grape pomace concentrate caused a reduced formation of MDA in breast meat during refrigerated storage. In the study of Wang et al. (2008), supplementation with grape seed proanthocyanidin extract improved weight gains and lowered the mortality of broilers infected with Eimeria tenella, but had less effect on antioxidative parameters (MDA concentration, activity of SOD) in plasma. An improved performance and also reduced oxidative stress in broilers fed different polyphenols was reported in a study from Starčević et al. (2015). Theses authors found that the inclusion of different phenolic compounds into the broiler diet, either thymol (200 mg/kg diet), tannic acid (5 g/kg diet) or gallic acid (5 g/kg diet), improved feed utilization and/or final body weight (only tannic acid) and improved oxidative stability (decreased concentration of TBARS) in liver and breast muscle. In a study of Viveros et al. (2011), broiler diets were supplemented with either 60 g/kg diet of grape pomace concentrate or grape seed extract. Supplementation with grape pomace concentrate improved the feed efficiency in broilers, an effect that could be explained by beneficial effects on the intestinal microbial population (increased abundance of Enterococcus, decreased abundance of Clostridium) and an increase in the villus height-to-crypt depth ratio. Interestingly, these effects were not observed in broilers supplemented with grape seed extract, suggesting that polyphenols from different sources exert different biological effects. In the study of Eid et al. (2003), supplementation of green tea polyphenols improved the feed conversion ratio and lowered TBARS concentrations in muscle and liver of broilers treated with corticosterone. In contrast, green tea polyphenols did not lower TBARS concentrations in muscle and liver and even impaired the feed efficiency in broilers which were not treated with corticosterone. In the study of Sridhar et al. (2015), supplementation of resveratrol (at a level of 1% of diet) improved the antioxidative status of serum in broilers, both without and with further treatment with aflatoxin B1. In that study, dietary treatment with resveratrol, however, impaired body weight gains and feed conversion ratio. In a study with Hessian laying hens, the effect of different doses of dietary quercetin (0.2, 0.4 and 0.6 g quercetin/kg diet) on performance, egg quality, caecal microflora populations and antioxidant status was investigated (Liu et al., 2014). This study demonstrated an increased performance (increased laying rate, decreased feed-to-egg ratio) and improvements of caecal microflora status (decrease in the abundance of total aerobes and coliforms, increased abundance of Bifidobacteria) in laying hens fed quercetin. However, no consistent effect of quercetin on the antioxidant status was found; while the activity of SOD in the liver was increased, the concentration of MDA, Trolox equivalent antioxidative capacity and the activity of GPX were not influenced by quercetin supplementation. In a very recent study from Yuan et al. (2016), the effect of different doses of tea polyphenols (600 and 1000 mg/kg diet) on production performance, egg quality and hepatic antioxidant status was investigated in laying hens treated with different concentrations of dietary vanadium (5, 10 and 15 mg vanadium/kg diet) for 8 weeks. This study found that the high concentrations of vanadium caused reduced albumen quality, bleaching effect on eggshell colour and oxidative stress in the liver, while the tea polyphenols prevented the adverse effect of vanadium on egg quality and increased the activity of GPX in the liver being indicative of induction of the antioxidant defence system. To sum up, most of the studies with broilers and laying hens revealed increases of performance and moderate improvements of antioxidant status or oxidative stability of tissues by plant polyphenols. Nevertheless, two studies even reported an impairment of performance of broilers fed polyphenol-enriched diets.
With regard to potential effects of polyphenols on inflammation in broilers, only one study is available so far. That study investigated the effect of tea polyphenols (0.03–0.09 g/kg body weight) on the expression of various proinflammatory cytokines (IL-1B, IL-4, IL-6, IL-10, TNF, IFN-γ) in the intestine of broilers (Li et al., 2015). It was found that most of these genes were downregulated by tea polyphenols. Unfortunately, no performance data were reported in that study. Therefore, it remains unclear whether downregulation of proinflammatory genes by tea polyphenols was associated with an increase in animal performance. In the study of Huang et al. (2013), broilers were fed diets supplemented with either 50 or 100 mg of green tea polyphenols per kg body weight. This study did not consider pathways of inflammation. However, it was found that broilers supplemented with green tea polyphenols have a reduced deposition of body fat, probably as a result of a reduced rate of de novo-fatty acid synthesis and an increased rate of β-oxidation. In overall, the few studies performed so far indicate that polyphenols might exert beneficial effects in the intestine of broilers, like in pigs. It is likely that effects of polyphenols in this respect could be mediated by favourable effect on the microbial composition. However, future studies are warranted to get more evidence of potential beneficial effect of polyphenols in poultry.
Effects of polyphenols on oxidative stress and inflammation in studies with cattle
The focus of most of the studies dealing with effects of polyphenols in dairy cattle was on the production of methane. According to these studies, certain types of polyphenols, particularly tannins, are able to suppress ruminal methanogenesis, both under in vitro and in vivo conditions. The suppression of ruminal methanogenesis was found to be mainly due to a reduction in the number of protozoa and toxic effects of tannins on methanogens (Patra and Saxena, 2009; Cieslak et al., 2013). With regard to an effect of polyphenols on the antioxidant status of dairy cattle, only few studies with divergent results exist. In an earlier study from Colitti and Stefanon (2006), feeding grape polyphenols at a dose of 10 g/day to heifers during the transition period, it was observed that the leucocyte mRNA concentration of the Nrf2 target gene SOD increased after calving. This indicated that grape polyphenols may induce cytoprotective mechanisms after calving to cope with oxidative and inflammatory stress. In the study of Shabtay et al. (2008), supplementation of pomegranate peel as a source of flavonoids increased the concentration of α-tocopherol in plasma of Holstein–Friesian bull calves. In that study, however, no other indices of the antioxidant system were reported. In dairy cows, feeding of anthocyanin-rich corn silage did not influence the antioxidative capacity of plasma (Hosoda et al., 2012), despite a marked increase in the activity of SOD in plasma. Similarly, supplementation of a polyphenol-rich plant extract did not improve concentrations of MDA, α-tocopherol and total antioxidative status of plasma in dairy cows (Gobert et al., 2009). In the study of Gohlke et al. (2013b), 4-week intraduodenal supplementation of quercetin did not influence mRNA concentrations of various antioxidant enzymes in the liver of dairy cows. In a recent study of our group, supplementation of polyphenol-rich grape seed and grape marc meal extract at a dietary level of 1% also did not improve the plasma antioxidant status of dairy cows (as assessed by determination of α-tocopherol, β-carotene, TBARS and total antioxidative capacity in plasma) (Gessner et al., 2015a). In another study, feeding a polyphenol-rich plant product consisting of green tea and curcuma extract also did not exert antioxidant effects in dairy cows (Winkler et al., 2015). Collectively, it can be stated that, like in most studies with monogastric animals, the antioxidant status of dairy cattle is less influenced by supplementation of feed rations with polyphenols from plant sources.
Regarding potential antiinflammatory effects of polyphenols in cattle, there are only very few studies available so far. In a study from Oliveira et al. (2010), the effect of polyphenols from pomegranate extract on health, nutrient digestion and immune competence was investigated in Holstein calves. The calves received either 5 or 10 g pomegranate extract per day throughout the first 70 days of age. Feeding the pomegranate extract polyphenols impaired digestibility of crude protein and fat, increased the secretion of IFN-γ and IL-4 by peripheral blood mononuclear cells and improved total IgG responses to ovalbumin vaccination. These results led the authors to suggest that feeding pomegranate extract polyphenols suppresses nutrient digestibility, likely because of the high tannin content, but enhances mitogen-induced cytokine production and response to vaccination, which might be beneficial for immune competence and health of calves. In the study from Gessner et al. (2015a), feeding a polyphenol-rich grape seed and grape marc meal extract in dairy cows caused a consistent reduction (25–65%) of the mRNA concentrations of a large set of genes involved in inflammation and ER stress in the liver, even though the effects were not statistically significant. Moreover, feeding grape seed and grape marc meal extract to dairy cows in that study caused a significant downregulation of fibroblast growth factor (FGF)-21, a key marker of ER stress and a surrogate marker of liver fat accumulation. In this study, supplementation of the polyphenol-rich product also improved milk performance, which could be – at least in part – the result of a suppression of inflammation and ER stress in the liver. In the study from Winkler et al. (2015), the effect of feeding a polyphenol-rich plant product was also investigated on genes of inflammation and ER stress in the liver of dairy cows. Feeding the polyphenol-rich plant product caused a downregulation of a set of genes involved in inflammation and ER stress, which, however, failed to be significant for most genes. However, in the group of cows supplemented with the plant product, there was also a significant downregulation of FGF21, a reduced fat content in the liver and an increased milk performance. As dry matter intake was not different between cows supplemented with the plant product and control cows, it has been suggested that the plant product improved the utilization of energy for milk production. One plausible explanation for this might be a potential reduction in ER, inflammatory and metabolic stress in the liver by the polyphenols from the plant product.
Overall, the few studies in dairy cows published so far show that plant sources rich in polyphenols could have the potential to lower inflammation and ER stress in the liver during the transition period and even improve milk performance. An interesting observation of the few studies is the downregulation of hepatic FGF21, because of its role for liver fat accumulation, a frequent phenomenon in high-yielding dairy cows. Future studies have to clarify whether a modulation of the rumen microbiota by plant polyphenols (Patra and Saxena, 2009; Cieslak et al., 2013) might contribute to the improvements of the inflammatory status in dairy cows and the significant improvements of milk performance. However, given that rumen disorders, such as subacute rumen acidosis and abomasal displacement (Plaizier et al., 2008; Zebeli and Metzler-Zebeli, 2012; Guzelbektes et al., 2010), which frequently occur during parturition and contribute to the systemic inflammatory condition during early condition, are accompanied by a marked impairment of the rumen microbial community and an increased translocation of inflammatory stimuli, such as LPS and cytokines, this is not unlikely.
Conclusions and future perspectives
Increasing evidence from studies with pigs, poultry and cattle indicates that plant polyphenols are helpful to attenuate both local and systemic inflammatory conditions, which are of particular relevance during the weaning phase in monogastric species and during the periparturient period in dairy cattle, and through this improve animal′s performance. The main reason for improvements of animal′s performance, such as increases in gain-to-feed ratio and milk yield, in response to plant polyphenols is that the inflammatory condition reduces feed intake, increases energy requirement for the production of fever and induces several hormonal changes shifting the metabolism into a more catabolic state in farm animals. Given the generally poor absorption of polyphenols from the intestine and the mutual interaction between polyphenols and gut microbiota (biotransformation of polyphenols to metabolites by the microbiota and modulation of the microbiota by polyphenols), it can be postulated that a key mechanism underlying the antiinflammatory action of polyphenols is their contribution to a stable intestinal microbiota and gut health, both of which are associated with enhanced gut barrier function and decreased translocation of bacterial components and prooxidative stimuli into the circulation (Landete, 2012; Mosele et al., 2015). It is well known that polyphenols have pronounced antioxidative properties in vitro, such as scavenging of ROS, chelating of metal ions and induction of antioxidant enzymes (Fraga, 2007). Nevertheless, supplementation of plant polyphenols has less effect on the antioxidant capacity, at least in healthy animals. The reason for this might be that, due to their low rate of absorption and their fast degradation in the body by the xenobiotic system, systemic concentrations of polyphenols when compared with other antioxidants in plasma such as ascorbic acid, tocopherols, albumin, uric acid and glutathione are very low (Surai, 2014). Direct antioxidant effects of polyphenols are expected to occur in vivo only in the gut, because of the markedly higher concentration of polyphenols in the intestinal lumen compared with systemic concentrations and the direct exposition of polyphenols to the intestinal epithelium following ingestion of polyphenol-rich diets. Accordingly, the view that plant polyphenols are able to replace other, much better bioavailable antioxidants in the diet, such as tocopherols, and to improve the total antioxidant system of the body, should be considered as doubtful. The replacement of tocopherols in the diet with plant polyphenols is also questionable because polyphenols can act as antioxidants in vivo only in blood and cytosol due to their hydrophilic structure and thus cannot replace the unique antioxidant function of vitamin E, which due to its lipophilic structure is integrated in the biological membranes and there effectively neutralizes fatty acid radicals and other ROS (Surai, 2014). Unlike in healthy animals, improvements of systemic antioxidative status and the reduction in ROS levels by plant polyphenols were observed in some studies with challenged or stressed animals (Luehring et al., 2011; Hou et al., 2014; Wang et al., 2012). These effects could be explained by the systemic antiinflammatory action of polyphenols, which might be caused mainly by improvements of gut health and reduced translocation of proinflammatory and prooxidative stimuli into the circulation. However, regarding the relatively low number of studies performed with farm animals, there is no doubt that more studies are necessary to substantiate this hypothesis with experimental data.
A further issue, which has not been addressed in the present review due to a lack of studies with farm animals but which should receive attention in the future, is the in vitro antiviral activity of certain polyphenols, such as epigallocatechin-3-gallate (Ciesek et al., 2011; Calland et al., 2012), delphinidin (Calland et al., 2015) and many others (Date and Destache, 2015), against many viruses including hepatitis C, herpes simplex, influenza and even Ebola (Hsu, 2015). It has been demonstrated that the antiviral properties of many polyphenols are mediated by inhibiting an early and critical step of virus infection, namely the virus entry by acting directly on the viral particle and preventing its attachment to the cell surface (Calland et al., 2015). Since infectious diseases with involvement of viral pathogens, such as mastitis or metritis, are considered to contribute to the proinflammatory condition observed in dairy cows (Bradford et al., 2015), it is conceivable that the anti-inflammatory action of polyphenols in farm animals is partially due to inhibition of the entry of viral pathogens. This, however, is a matter of speculation and needs to be clarified in future studies.
With regard to several adverse effects of plant polyphenols, such as formation of complexes with trace elements, rendering them less available for absorption (Afsana et al. 2004; Gaffney et al. 2004), and inhibition of digestive enzymes (Cermak et al., 2004; Williamson, 2013; Buchholz and Melzig, 2015), future studies with farm animals are necessary to clarify which dose of polyphenols is appropriate to induce beneficial effects without significantly impairing nutrient digestibility.




