D53 represses rice blast resistance by directly targeting phenylalanine ammonia lyases
Edited by: Yidan Ouyang, Huazhong Agricultural University, China
Graphical Abstract
Phytohormones play important roles in orchestrating plant immune responses to pathogen attacks. Strigolactones (SLs), a group of carotenoid-derived phytohormones, modulate diverse biological processes in plants, including shoot branching, plant height, root architecture, leaf senescence, seed germination of parasitic plants, and symbiosis of arbuscular mycorrhizal fungi (Burger and Chory, 2020). Recently, increasing evidence has indicated potential roles for SLs in regulating responses against biotic stresses, including defense responses against certain pathogenic fungi and bacteria in roots and leaves (Yi et al., 2023).
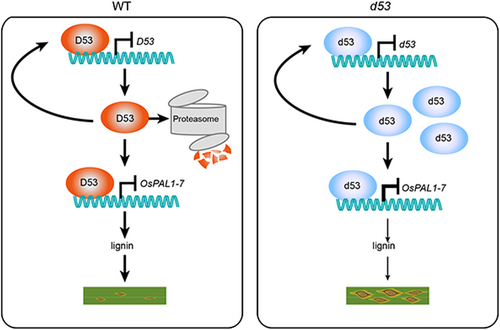
dwarf53 (d53) is an SL-insensitive rice (Oryza sativa) mutant resulting from a gain-of-function allele of D53 that encodes a mutated protein resistant to SL-induced ubiquitination and degradation (Jiang et al., 2013; Zhou et al., 2013). D53 and its homologous proteins are key transcriptional repressors in SL signaling through interaction with transcription factors and repression of their transcriptional activation activities. In Arabidopsis thaliana, the D53 homologs Suppressor of MAX2-Like 6/7/8 (SMXL6/7/8) and Suppressor of MAX2-1 (SMAX1) mediate SL and karrikin signaling, respectively. Both SMXL6/7/8 and SMAX1 can directly bind to their own promoters and function as autoregulated transcription factors (Wang et al., 2020; Xu et al., 2023). However, whether D53 and its homologs can function as transcription factors to regulate development and environmental responses in rice remain elusive.
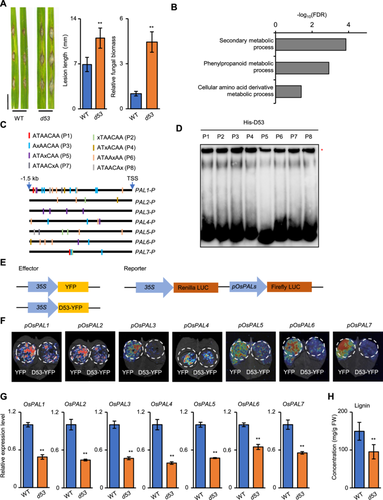
To explore the role of D53 in plant resistance to blast disease, the most destructive disease of rice, we inoculated d53 and the corresponding wild-type (WT) plants with the compatible Magnaporthe oryzae isolate Zhong10-8-14 by spraying fungal spores on rice leaves. At 7 d post-inoculation, the leaves of d53 plants exhibited more lesions than the wild-type (WT) (Figure S1). In a punch inoculation assay with detached rice leaves, the d53 leaves developed larger lesions and greater fungal biomass than WT (Figure 1A). Consistently, overexpression of D53 in Zhonghua 11 (ZH11) also conferred elevated susceptibility to blast (Figure S2). Together, these results indicate that the accumulation of D53 protein compromises rice resistance to M. oryzae.
Dwarf53 (D53) targets phenylalanine ammonia lyases (PALs) to negatively regulate rice blast resistance
(A) Punch inoculation of wild-type (WT) and d53 plants. Lesion length and fungal biomass were quantified at 7 d post-inoculation. Scale bar, 10 mm. Data are presented as mean ± SD (n = 15, **P < 0.01). (B) Representative biological processes of downregulated gene sets in d53 determined by analysis of significantly enriched Gene Ontology (GO) terms. (C) The ATAACAA and variant motifs present in the 1.5-kb promoter region of Oryza sativa PAL1 (OsPAL1) to OsPAL7. X represents variations in the base group, and P1–P8 represent different variants. TSS, transcriptional start site. (D) Binding of His-D53 to ATAACAA and variant motifs in electrophoretic mobility shift assays (EMSAs). Variant probes according to (C) were labeled with biotin. *Indicates shifted bands. (E–F) D53 represses the transcription of OsPAL1 to OsPAL7 promoters. Agrobacterium tumefaciens carrying plasmids as indicated in (E) were infiltrated into Nicotiana benthamiana leaves with different reporter and effector constructs. Light signal imaging shows luciferase (LUC) levels expressed from the OsPAL1 to OsPAL7 promoters in the presence or absence of D53 (F). Three biological replicates were carried out with similar results obtained. (G) The RNA expression levels of OsPAL1 to OsPAL7 in WT and d53 plants determined by quantitative reverse transcription-PCR (RT-qPCR). Rice ubiquitin5 was used as the reference. Data are presented as mean ± SD (Student's t-test, n = 3, **P < 0.01). (H) Quantification of lignin contents in WT and d53. Values are presented as means ± SD (Student's t-test, n = 3, **P < 0.01).
To elucidate the molecular basis underlying D53-mediated resistance in rice, we performed RNA sequencing (RNA-seq) analysis using 3-week-old seedlings of d53 and WT. A total of 469 differentially expressed genes were identified, including 160 upregulated and 309 downregulated genes in d53 compared with WT plants (Figure S3A; Table S1). Gene Ontology (GO) analysis revealed that the downregulated genes were enriched in the “secondary metabolic process”, “amino acid derivative metabolic process”, and their child term “phenylpropanoid metabolic process” (Figures 1B, S3B; Table S2). Pathway analysis revealed that the downregulated genes were enriched in Kyoto Encyclopedia of Genes and Genomes terms related to phenylpropanoid biosynthesis and biosynthesis of secondary metabolites (Figure S3C; Table S3). Phenylpropanoid metabolism plays a critical role in the plant immune response (You et al., 2020). We speculated whether D53 regulates blast disease resistance by modulating the phenylpropanoid metabolic pathway. To test this hypothesis, we detected expression levels of three representative genes encoding key enzymes involved in the phenylpropanoid metabolic process in rice seedling leaves, including OsPAL1 that encodes a phenylalanine ammonia-lyase (PAL), OsCAD2 that encodes a cinnamyl alcohol dehydrogenase, and Os4CL3 that encodes a 4-coumarate:coenzyme A ligase. The RNA expression levels of all three genes were repressed in d53 compared with those in WT (Figure S4).
In Arabidopsis, the ATAACAA motif is essential for the direct binding of SMXL6/7/8 and SMAX1 to their own promoters (Wang et al., 2020; Xu et al., 2023). Interestingly, we found that 96 downregulated genes harbor the ATAACAA motif in their 1.5-kb proximal promoter regions (Table S4). OsPAL1 contains an ATAACAA motif located at −1,443 to −1,437 bp upstream of the transcription start site (Figure S5A; Table S5). Our electrophoretic mobility shift assay (EMSA) showed that the addition of His-D53 fusion protein led to a shifted band, while the addition of unlabeled probe competitor reduced signal strength of the shifted band (Figure S5B), indicating that D53 directly binds to the ATAACAA motif on the OsPAL1 promoter.
Phenylalanine ammonia lyases function in the biosynthesis of lignin, salicylic acid (SA) and other phenylalanine-derived metabolites, and play critical roles in plant development and stress adaptation. Among the nine PAL genes in rice, OsPAL1 to OsPAL7 have been reported to be induced in response to M. oryzae infection (You et al., 2020). We found that the OsPAL1 and OsPAL7 promoters contain an ATAACAA motif, and the promoters of OsPAL1 to OsPAL7 contain seven variant motifs that harbor a single substitution compared with the ATAACAA motif (Figure 1C; Table S5). EMSA further showed that His-D53 directly bound to the intact ATAACAA motif and the seven variants in vitro, although the binding signal of D53 on the P5 (ATAxCAA) motif was relatively weak (Figure 1D). Moreover, a DNA affinity purification – quantitative polymerase chain reaction (DAP-qPCR) assay showed that fragments containing ATAACAA and variant motifs within the OsPAL1 to OsPAL7 promoters were notably enriched by His-D53 compared with the control (Figure S6). To determine whether D53 regulates the transcription of OsPAL1 to OsPAL7 in rice, we first performed a transcriptional repression assay in Nicotiana benthamiana leaves and found that the luciferase (LUC) activities of reporters pOsPAL1-LUC ~ pOsPAL7-LUC were significantly lower in leaves coexpressing D53-YFP (yellow fluorescent protein) than those coexpressing empty YFP control (Figure 1E, F). Similarly, the SL-resistant d53 protein also exhibited transcriptional repression activity (Figure S7). Consistently, transcript levels of OsPAL1 ~ OsPAL7 in the d53 plants were significantly lower than those in WT (Figure 1G). More importantly, the lignin content decreased by 37% in d53 compared with that in WT (Figure 1H), and the total SA level was reduced by 12% (Figure S8). Together, these results indicate that D53 represses the RNA expression of OsPAL1 ~ OsPAL7 by directly binding to their promoters, leading to impaired biosynthesis of lignin and SA, which are crucial to plant cell wall strengthening and immune response activation in the prevention of M. oryzae infection.
As a repressor in the SL signaling pathway, D53 has been proven to regulate tillering, root development, nutrient use efficiency and signaling homostasis by recruiting the transcriptional corepressors TOPLESS (TPL) and TPL-related (TPRs) proteins and repressing the activities of different transcription factors (Yuan et al., 2023). It is still unclear whether D53 confers immunity to. M. oryzae. In this study, we found that D53 can directly bind to and repress OsPAL promoters that contain the ATAACAA motif and/or seven variants, indicating that D53 functions as a transcriptional repressor in rice. Moreover, both D53 and degradation-resistant d53 proteins bind to their own promoter and repress transcription (Figure S9), functioning as autoregulated transcription factors similar to SMXL6,7,8 and SMAX1. Importantly, the d53 plants with decreased OsPAL expression levels and lignin contents were sensitive to M. oryzae, indicating that D53 represses defense responses to M. oryzae potentially by repressing the transcription of OsPALs (Figure S10). In addition, the four Arabidopsis PALs are also involved in defense response (Huang et al., 2010) and also contain the ATAACAA and variant motifs within 0.5-kb proximal promoters (Figure S11; Table S6), suggesting that SMXL6,7,8 may share a similar mechanism to regulate the immune response in Arabidopsis. A previous study indicated that SL signaling was downregulated in response to M. oryzae infection, and the SL biosynthesis mutant d10 and perception mutant d14 were less susceptible to M. oryzae (Lahari et al., 2024). Although d10, d14 and d53 plants show similar dwarf plant architecture due to SL deficiency or insensitivity, d53 exhibits compromised blast resistance, which is different from d10 and d14. We propose that a D53-mediated signaling pathway might function independently of SL biosynthesis and signal perception. Comprehensive identification of the unknown components involved in D53-mediated signal transduction could provide valuable insights, helping to elucidate the relationship between D53 and SL-mediated plant disease resistance in the near future.
ACKNOWLEDGEMENTS
This work was supported by NSFC (32072043, 32272116, 32122012), Fok Ying Tung Education Foundation (171023), Sichuan Science and Technology Program (2023ZYD0086, 2023NSFSC0155, 2023NSFSC1937, 2024NSFTD0022).
CONFLICTS OF INTEREST
These authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
J.W. and B.W. conceived and designed the experiments. H.Y., H.L., and Q.H. performed most of the experiments. H.S., D.W., Y.C., T.X., and M.W. performed some experiments on phenotypic and biochemical assays. M.H., J.Y., X.L., Y.T., X.Z., and L.Z. collected the data. J.W., B.W., Q.H., X.C., and J.L. analyzed the data, and wrote the manuscript. All authors read and approved this manuscript.