In vivo haploid induction in cauliflower, kale, and broccoli
Edited by: Kejian Wang, China National Rice Research Institute, China.
Graphical Abstract
Modifying the centromeric histone CENH3 or PHOSPHOLIPASE D genes in cauliflower (Brassica oleracea var. botrytis) created haploid induction lines, which can be widely used for in vivo haploid induction in cauliflower, kale, and broccoli, thus enabling rapid utilization of germplasm resources and improving breeding efficiency.
Brassica oleracea (family Brassicaceae) represents a group of economically valuable vegetables cultivated worldwide, including cabbage (var. capitata), cauliflower (var. botrytis), broccoli (var. italica), and kale (var. acephala). These crops are typically self-incompatible and require vernalization for flowering, thereby contributing to their long growth cycles. For hybrid breeding, 6–9 years of continuous selfing or backcrossing are required to develop pure parental lines. Alternatively, doubled haploid (DH) technology can be used to produce homozygous lines within two generations, thus increasing breeding efficiency and decreasing costs. However, traditional haploid induction (HI) methods for Brassica mainly rely on microspore or anther cultures, which require tissue culture and are highly limited by the plant genotype. Notably, rapid progress has been made in developing haploid inducer lines that produce haploid offspring directly through outcrossing. This in vivo haploid seed induction method does not require tissue culture, making it potentially useful for DH breeding.
Several HI genes have been identified, including genes associated with centromere functions (CENTROMERIC HISTONE H3 (CENH3) and KINETOCHORE NULL 2 (KNL2)) and the phospholipase pathway (MATRILINEAL (MTL), also reported as PHOSPHOLIPASE A1 (PLA1) and NOT LIKE DAD (NLD), and Zea mays PHOSPHOLIPASE D3 (ZmPLD3)), the membrane protein gene DUF679 DOMAIN MEMBRANE PROTEIN (DMP), and the peroxidase gene ZmPOD65 in maize (Zea mays) (Ravi and Chan, 2010; Liu et al., 2017; Zhong et al., 2019; Li et al., 2021; Jiang et al., 2022). DMP‑triggered HI has been reported for Brassica napus and B. oleracea (Li et al., 2022; Zhao et al., 2022; Zhong et al., 2022).
In this study, we assessed the utility of CENH3 and PLD as HI genes in the cauliflower inbred line “Korso”. We modified the Korso CENH3 gene (BolKCENH3) in three ways: (i) we knocked out the gene using a single guide RNA (sgRNA) targeting exon 8 of BolKCENH3 via clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated nuclease 9 (Cas9)-mediated gene editing (Figures 1A, B, S1); (ii) we transformed plants with the construct sgRNA-BMCENH3 designed to knock out BolKCENH3 as above and simultaneously expressing BMCENH3 (encoding the N terminus of BolKCENH3 fused to the C terminus of ZmCENH3; Figures 1A, S2) to rescue the lethality associated with null cenh3 alleles; and (iii) we expressed the construct sgRNA-BE3 targeting BolKCENH3 and expressing a cytosine base editor gene (BE3) to introduce the L133F mutation in the histone fold domain of BolKCENH3 (Wang et al., 2022) (Figure 1A). A BLAST analysis of the Korso genome (https://www.ncbi.nlm.nih.gov/bioproject/term=PRJNA546441) using ZmPLD3 as the query identified a gene (BolK_1g48490) annotated as encoding phospholipase D alpha 1 (BolKPLD1) that shares the highest similarity to ZmPLD3 (71.3% sequence identity). We knocked out BolKPLD1 to assess its utility for HI in B. oleracea, with a specific sgRNA targeting exon 2 (Figures 1B, S3). In addition, we reconstructed a phylogenetic tree of the phospholipase D (PLD) family across the monocots and dicots, including Brassicacea species (Figure S4; Table S1), to guide the selection of other PLD genes that may be used for HR.
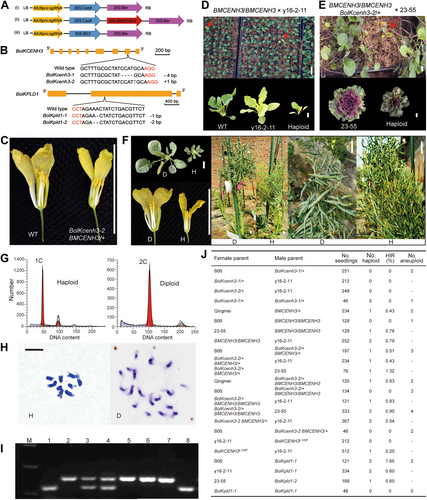
In vivo haploid induction in Brassica oleracea
(A) Diagrams of the constructs used in this study. (i) Clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated nuclease 9 (Cas9) knockout vector targeting BolKCENH3 or BolKPLD1 respectively; (ii) gRNA-BMCENH3 comprising a single guide RNA (sgRNA) targeting BolKCENH3 and expressing the modified coding sequence BMCENH3; (iii) base-editing vector used to modify BolKCENH3 to introduce the L133F mutation. (B) Diagrams of the BolKCENH3 and BolKPLD1 loci, showing the sgRNA target sites and the mutations in the identified plants. The protospacer-adjacent motif (PAM) sequence is highlighted in red. (C) Representative flowers of wild-type (WT) Korso and BolKcenh3-2 BMCENH3/+ plants. (D) Phenotypic screening of the F1 hybrid offspring derived from a cross to y16-2-11. The red arrows indicate chlorotic haploid seedlings. (E) Phenotypic screening of the F1 hybrid offspring derived from a cross to 23–55. The red arrow indicates a haploid plant with split leaves similar to those of 23–55. (F) Representative photographs of diploid and haploid plants from the tester line Qingmei (seedling, flower, whole plant, and seed pod). (G) Flow cytometry analysis of DNA content in the putative haploids. (H) Chromosome number in a meiotic sample of haploid (n = 9) and diploid (n = 18). (I) Genotyping polymerase chain reaction of the various cultivars and their putative haploid plants using molecular markers. Lanes: M, DNA marker; 1, Korso; 2, 23–55; 3 and 4, two F1 hybrids from a cross between BolKcenh3-2/+BMCENH3/BMCENH3 and 23–55; 5–7, three haploids from a cross between BolKcenh3-2/+BMCENH3/BMCENH3 and 23-55; 8, BolKcenh3-2/+BMCENH3/BMCENH3. (J) Summary of haploid induction rate (HIR) data, as determined by crossing. Scale bars, 10 μm (H), and 2 cm (C, D, E, F).
After Agrobacterium (Agrobacterium tumefaciens)-mediated transformation of Korso cotyledons (Wang et al., 2022), we identified heterozygous edited T0 plants with a 4-bp deletion or 1-bp insertion in BolKCENH3, resulting in translational frameshifts and premature stop codons (Figure 1B; BolKcenh3-1 and BolKcenh3-2). We obtained no homozygous BolKcenh3 mutants in the T1 or T2 generation. For the sgRNA-BMCENH3 construct, we generated BolKcenh3-2 mutants expressing BMCENH3. We obtained different genotype combinations for the BMCENH3 transgene and the BolKcenh3-2 mutant via segregation in T1 and T2 plants. Notably, we identified homozygous BolKcenh3-2 mutant plants carrying one copy of the BMCENH3 transgene (BolKcenh3-2+/BMCENH3), indicating that BMCENH3 can rescue the growth defects of the BolKcenh3 null mutant; these plants produced fewer pollen grains than wild-type plants (Figures 1C, S5). For the base-editing approach, a previous study generated a CENH3 variant with an L133F (CTT-TTT) substitution in the histone fold domain of BolKCENH3 (BolKCENH3L133F) for HI (Wang et al., 2022). We obtained two homozygous T0 BolKpld1 mutant plants (BolKpld1-1 and BolKpld1-2) with a 1-bp or 2-bp deletion at the target site, respectively, resulting in frameshifts and premature stop codons (Figure 1B). These mutants grew normally and produced viable pollen.
To assess whether the above plants are useful haploid inducers, we crossed them (as female or male parents) to the tester line “y16-2-11”, a completely yellow cauliflower line (Figure 1D, J). Plants heterozygous for the BolKcenh3 mutation (BolKcenh3-1/+ and BolKcenh3-2/+) produced no haploids in our limited number of F1 seedlings. Similarly, a plant homozygous for BolKCENH3L133F transgene did not induce maternal haploids when used as the male parent. We speculate that the haploid induction rate (HIR) is very low under these conditions. Fortunately, when used as the female parent, plants with the genotypes BMCENH3/BMCENH3, BolKcenh3-2/ + BMCENH3/+, BolKcenh3-2/+BMCENH3/BMCENH3, BolKcenh3-2 BMCENH3/+, BolKCENH3L133F, and BolKpld1-1(used as male parent) produced several F1 progenies with a completely chlorotic phenotype (Figure 1D, J). Flow cytometry analysis revealed that these chlorotic plants are all haploids.
We outcrossed most of the above mutant lines to other Brassica crops: the cauliflower hybrid “Qingmei,” the male-sterile broccoli inbred line “B06,” and the curly red kale inbred line “23–55”. We identified their haploid progenies based on plant phenotypes (Figure 1E, F), flow cytometry analysis (Figure 1G), and chromosome number (Figure 1H). Genotyping with molecular markers confirmed that the haploid plants carry only the nuclear genome of the tester parents (Figure 1I). Haploid plants were morphologically similar to their corresponding tester parents but were shorter in stature, had narrower leaves and smaller organs, and were sterile. The seed pods of haploid plants were small, withered, and seedless. An assessment of the HIR showed that overexpression of BMCENH3 and different combinations of BMCENH3 and BolKcenh3 (BolKcenh3-2/+BMCENH3/+, BolKcenh3-2/+BMCENH3/BMCENH3) could induce both maternal and paternal haploids (Figure 1J). The average HIR ranged from 0.43% to 1.32%. BMCENH3/+ induced maternal haploids in the cross QingmeiⅹBMCENH3/+ at HIR 0.43%. Although we did not conduct more outcrosses, based on our existing results, we speculate that BMCENH3/+ and BolKcenh3-2 BMCENH3/+ should be able to induce haploids in both male and female sides. In addition, although most of the transgenic lines above harboring a mutation in BolKCENH3 and expressing BMCEMH3 did not trigger haploid induction when outcrossed to B06, they produced haploid progeny when outcrossed to 23–55 and Qingmei, which may indicate that the tester germplasm influences HIR. Our results also suggest that different expression levels of multiple CENH3 alleles (BMCENH3 and/or BolKCENH3) might result in subtle differences in HI frequency. However, exploring this question will require quantification of gene expression and extensive outcrossing. We also determined that loss-of-function mutations in PLD1 (BolKpld1-1) trigger maternal haploid plants in cauliflower, broccoli, and kale, when used as the paternal parent, with an average HIR of 0.60%–1.65%. No haploids were detected in the 48 selfed progenies of BolKpld1-1.
In summary, we demonstrate that CENH3 and PLD are useful for HI in Brassica species. In terms of the CENH3 variants, HI may be triggered by the overexpression of a modified CENH3, different combinations of BMCENH3 and BolKcenh3 mutations, and point mutations in CENH3 (L133F), with HIR ranging from 0.20% to 1.32%. This is the first report that a loss-of-function in the ZmPLD3 homolog BolKPLD1 can trigger maternal HI in B. oleracea, with HIR ranging from 0.60% to 1.65%. A cauliflower HI line capable of inducing haploids in cauliflower, kale, and broccoli may be able to induce haploids in other species belonging to the same genus. The results of this study may thus be useful for increasing HIR and expanding the utility of HI lines among Brassica species and other dicotyledonous plants. Further on, HI lines as maternal parents can be combined with cytoplasmic male sterility (CMS) lines for one-step creation of CMS pure lines. And HI lines as paternal parents can be combined with the CMS restorer genes for rapid utilization of excellent gene resources in CMS hybrids.
ACKNOWLEDGEMENTS
This work was funded by the National Key Research and Development Program of China (2022YFF1003000), the Natural Science Foundation of China (31972401) and the Innovation Program of the Beijing Academy of Agricultural and Forestry Sciences (KJCX20230203).
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
G.W., N.G., and F.L. designed the study. G.W., M.Z., S.H., and H.Z. performed the experiments, the other authors analyzed the data. G.W. wrote the manuscript. N.G. and F.L. revised the manuscript. All authors read and approved the contents of this paper.