Maize ZmSRO1e promotes mesocotyl elongation and deep sowing tolerance by inhibiting the activity of ZmbZIP61
Edited by: Feng Tian, China Agricultural University, China
ABSTRACT
Deep sowing is a traditional method for drought resistance in maize production, and mesocotyl elongation is strongly associated with the ability of maize to germinate from deep soil. However, little is known about the functional genes and mechanisms regulating maize mesocotyl elongation. In the present study, we identified a plant-specific SIMILAR TO RCD-ONE (SRO) protein family member, ZmSRO1e, involved in maize mesocotyl elongation. The expression of ZmSRO1e is strongly inhibited upon transfer from dark to white light. The loss-of-function zmsro1e mutant exhibited a dramatically shorter mesocotyl than the wild-type in both constant light and darkness, while overexpression of ZmSRO1e significantly promoted mesocotyl elongation, indicating that ZmSRO1e positively regulates mesocotyl elongation. We showed that ZmSRO1e physically interacted with ZmbZIP61, an ortholog of Arabidopsis ELONGATED HYPOCOTYL 5 (HY5) and showed a function similar to that of HY5 in regulating photomorphogenesis. We found that ZmSRO1e repressed the transcriptional activity of ZmbZIP61 toward target genes involved in the regulation of cell expansion, such as ZmEXPB4 and ZmEXPB6, by interfering with the binding of ZmbZIP61 to the promoters of target genes. Our results provide a new understanding of the mechanism by which SRO regulates photomorphogenesis and highlight its potential application in deep sowing-resistant breeding.
INTRODUCTION
Maize (Zea mays) is one of the most important grain crops worldwide, and drought has become an important limiting factor for maize production, especially in arid and semiarid regions. Deep sowing is the traditional method for drought resistance in maize production because it can ensure adequate seed zone moisture to promote germination (Zhang et al., 2012; Niu et al., 2019). However, most maize varieties have poor ability to germinate from deep soil; therefore, seedlingless ridges often occur. Thus, the development of deep sowing-tolerant maize cultivars is becoming increasingly important in arid and semiarid regions. The mesocotyl connects the coleoptilar node to the basal part of the seminal roots of maize seedlings. During seed germination, the mesocotyl rapidly elongates and pushes the shoots of the seedlings out of the soil. Thus, mesocotyl elongation is strongly associated with deep sowing tolerance (Niu et al., 2020). However, little is known about the functional genes and mechanisms regulating maize mesocotyl elongation. It is well known that light and phytohormones regulate the elongation of maize mesocotyl (Vanderhoef and Briggs, 1978; Kutschera and Wang, 2016). Some maize mutants involved in light signal transduction pathways show pronounced or suppressed mesocotyl elongation. For example, elongated mesocotyl1, a phytochrome-deficient maize mutant, exhibits mesocotyl elongation under red or far-red light conditions (Sawers et al., 2002). Mutants of the maize phytochrome genes phyB1 significantly inhibit mesocotyl elongation under red light (Sheehan et al., 2007). The zmpif3, zmpif4, and zmpif5 knockout mutants severely suppressed mesocotyl elongation in the dark (Wu et al., 2019). Recently, ZmMYB59 was found to be downregulated by deep sowing during maize seed germination, and the overexpression of ZmMYB59 in tobacco and rice significantly decreased hypocotyl/mesocotyl elongation, possibly by inhibiting the synthesis of gibberellin and cytokinin and promoting the synthesis of abscisic acid (Zhai et al., 2020). Despite these insights, the mechanisms through which these genes regulate mesocotyl elongation in maize remain unclear.
Light is a key environmental signal throughout a plant's life cycle. In nature, seeds undergo skotomorphogenesis after germination in the soil, forming etiolated and long hypocotyls (dicots), coleoptiles, or mesocotyls (monocots) (Dong et al., 2014; Kutschera and Wang, 2016). Upon emergence from the soil and sensing sufficient light, seedlings are characterized by the deceleration of hypocotyl/coleoptile/mesocotyl growth (Jiao et al., 2007). In recent decades, functional genes and mechanisms regulating hypocotyl growth have been widely exploited in the model plant Arabidopsis thaliana. HY5 functions as a major positive regulator of photomorphogenesis, and its mutant seedlings exhibit long hypocotyls under red, far-red, blue, and UV-B light (Gangappa and Botto, 2016). HY5 is a basic domain/leucine zipper (bZIP) transcription factor having a C-terminal domain for DNA binding and a leucine zipper for dimerization (Yoon et al., 2007). HY5 specifically binds to the G-box and ACE present in the promoters, affecting the expression of downstream genes in response to light (Gangappa and Botto, 2016). It has since been shown that HY5 directly binds to the promoters of nearly 3,000 genes and modifies their expression (Lee et al., 2007; Zhang et al., 2011). Although it has been characterized as a transcriptional activator, repressor, or both, HY5 does not have its own activation or repression domain and requires cofactors to regulate gene transcription (Burko et al., 2020). Numerous genetic and biochemical studies have identified several cofactors that interact with HY5, including PICKLE, Histone Deacetylase15 (HDA15), CALMODULIN7 (CAM7), HYH, BBX20/21/22, COLD-REGULATED (COR) proteins COR27 and COR28, ACTIN-RELATE PROTEIN6 (ARP6), and SWR1 complex core subunit C6 (SWC6) (Holm et al., 2002; Jing et al., 2013; Abbas et al., 2014; Bursch et al., 2020; Li et al., 2020; Mao et al., 2021). These proteins physically interact with HY5 and influence its transcriptional activity.
SIMILAR TO RCD-ONE (SRO) proteins participate in abiotic stress responses and modulate reactive oxygen species (ROS) homeostasis (Liu et al., 2014; Qin et al., 2021; Gao et al., 2022). In addition, they play important roles in plant growth and development. In A. thaliana, mutants of the SRO gene, RADICAL-INDUCED CELL DEATH1 (RCD1), display pleiotropic developmental phenotypes, such as reduced stature, malformed leaves, early flowering, and premature senescence (Teotia and Lamb, 2009; Vainonen et al., 2012). In wheat, TaSRO1 improves plant growth and abiotic stress resistance by modulating redox homeostasis and maintaining genomic stability (Liu et al., 2014). Recently, TaSRO1 was reported to regulate seed dormancy and pre-harvest sprouting resistance through interacting with TaVP1 (Liu et al., 2024). In maize, ZmSRO1e competes with ZmR1 for binding to ZmPL1 to repress anthocyanin biosynthesis (Qin et al., 2021), while ZmSRO1d modulates the trade-off between drought resistance and yield by affecting ZmRBOHC-mediated stomatal ROS production (Gao et al., 2022). Recently, some SROs have been reported to regulate the function of interacting proteins through adenosine diphosphate (ADP)-ribose modifications. For example, mono(ADP-ribosyl)ation (MARylation) of the Arabidopsis zinc finger proteins SZF1 and SZF2 by SRO2 can stabilize SZF proteins (Kong et al., 2021), and MARylation of ZmRBOHC by ZmSRO1d can promote the activity of ZmRBOHC (Gao et al., 2022). However, the function and molecular mechanisms of SRO in the regulation of plant growth and development remain unclear.
Accumulating evidence revealed that SROs play a role in light signaling. It was reported that the rcd1 mutant exhibits higher tolerance to UV-B irradiation with respect to shorter hypocotyl (Jiang et al., 2009). RCD1, working together with STO, enhanced the expression of COP1 and HY5-regulated genes. Furthermore, RCD1 could interact with many transcription factors (PIF3, PIF5, PIF7, STO, COL9, and COL10) belonging to families known to play a role in light responses (Jaspers et al., 2009). However, the mechanism for SRO-regulated hypocotyl growth remains unknown.
In the present study, we characterized the role of ZmSRO1e in mesocotyl elongation. Genetic and physiological analyses have shown that ZmSRO1e promotes mesocotyl elongation through physical interaction with ZmbZIP61 (an ortholog of Arabidopsis HY5), thus repressing the transcriptional activity of ZmbZIP61 toward target genes involved in cell expansion, such as ZmEXPB4 and ZmEXPB6. Our results provide a new understanding of the mechanism by which SRO regulates photomorphogenesis and highlights its potential application in deep sowing-resistant breeding.
RESULTS
ZmSRO1e promotes mesocotyl elongation
To determine the role of maize SRO in light signaling, we investigated the transcript abundance of ZmSROs in the maize inbred line B73 seedlings using quantitative real-time polymerase chain reaction (qRT-PCR). The expression levels of ZmSROs were markedly lower in the shoots of light-grown seedlings than in those of dark-grown seedlings (Figure 1A). Furthermore, the expression of ZmSROs gradually decreased in the shoots of dark-grown seedlings upon transfer to white light at the given time points (0, 1, 6, and 12 h) (Figure 1B), indicating that the expression of ZmSROs was inhibited by light. Among the six ZmSROs, the expression of ZmSRO1e is the most strongly inhibited upon transfer to white light, suggesting that ZmSRO1e plays an important role in maize light signaling (Figure 1B).
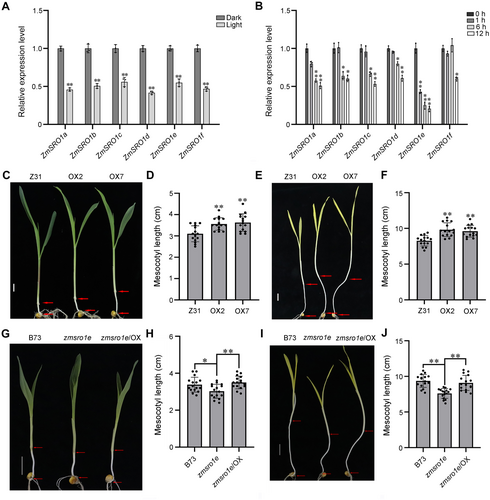
ZmSRO1e promotes mesocotyl elongation
(A) Relative expression levels of maize SROs in seedlings at the three-leaf stage grown in darkness and continuous light. (B) Relative expression levels of maize SROs in seedlings at the three-leaf stage grown in darkness and then transferred to white light for the given periods (0, 1, 6, and 12 h). (C, E) Phenotypes of 10-d-old ZmSRO1e overexpressor (OX) and wild-type (Z31) seedlings grown in long day (LD) (16 h light/8 h dark) (C) or constant darkness conditions (E). Scale bars: 2 cm. (D, F) Quantification of mesocotyl lengths of the genotypes in LD (D) and constant darkness conditions (F). Values are mean ± SE (n = 15). (G, I) Phenotypes of 9-d-old wild-type (B73), zmsro1e, and zmsro1e/OX complementation seedlings grown in LD (16 h light/8 h dark) (G) or constant darkness conditions (I). Scale bars: 2 cm. (H, J) Quantification of mesocotyl lengths of the genotypes in LD (H) and constant darkness conditions (J). Values are mean ± SE (n = 15). Asterisks indicate significant differences compared with the wild-type (**P < 0.01) determined by Student's t-test.
To investigate the function of ZmSRO1e in the regulation of maize growth and development, we generated transgenic plants harboring ZmSRO1e driven by the maize ubiquitin promoter in maize (overexpressor (OX) lines) and the cauliflower mosaic virus (CaMV) 35S promoter in Arabidopsis (overexpression (OE) lines) (Qin et al., 2021). Two independent OX transgenic lines exhibited longer mesocotyls than the wild-type (Z31) under both white light and dark conditions (Figures 1C–F, S1). We also investigated whether the constitutive expression of ZmSRO1e affects hypocotyl elongation in Arabidopsis. As expected, OE lines developed significantly longer hypocotyls than wild-type plants under light conditions (Figure S2A, B). Scanning electron microscopy revealed differences in the cell profiles of the hypocotyls of the wild-type and OE lines (Figure S2C). The length of hypocotyl epidermal cells was significantly increased in the OE lines compared to that in wild-type Col-0, suggesting that ZmSRO1e promotes cell expansion (Figure S2D). To further verify the role of ZmSRO1e in regulating mesocotyl elongation, we examined the mesocotyl phenotypes of a loss-of-function mutant of ZmSRO1e (zmsro1e) (Qin et al., 2021). The zmsro1e mutant seedlings exhibited a dramatically shorter mesocotyl compared to B73 under both white light and dark conditions, and the overexpression of ZmSRO1e rescued the shorter mesocotyl phenotype of zmsro1e mutant seedlings (Figure 1G–J). These results indicated that ZmSRO1e positively regulates mesocotyl elongation.
ZmSRO1e plays a role in deep sowing tolerance
The mesocotyl can directly push plumules above the soil surface during germination and is a significant trait affecting plant establishment under deep-sown conditions in maize. Therefore, we investigated whether ZmSRO1e is beneficial for deep sowing tolerance in maize. Maize seeds were planted at depths of 5, 10, and 15 cm; the emergence rate of seedlings was monitored, and the mesocotyl lengths of different lines were measured. The results showed that the zmsro1e mutant seedlings exhibited a dramatically shorter mesocotyl and lower emergence rate than the wild-type maize seedlings (Figure 2). In contrast, the overexpression of ZmSRO1e promoted mesocotyl elongation and improved the seedling emergence rate under deep sowing conditions, which were suitable for rainfed environments (Figure 2).
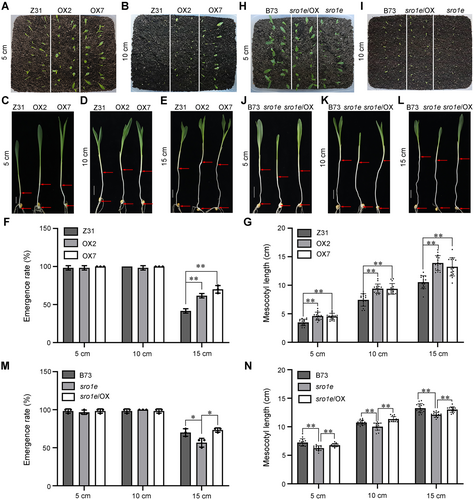
ZmSRO1e plays a role in deep sowing tolerance
(A–E) Phenotypes of ZmSRO1e-OX (overexpressor) seedlings buried at a 5 cm (A, C), 10 cm (B, D), and 15 cm (E) depth filled with soil. Scale bars: 2 cm. (F, G) The mesocotyl length (F) and emergence rate (G) of ZmSRO1e-OX seedlings buried at different depths filled with soil. (H–L) Phenotypes of zmsro1e mutant and zmsro1e/ZmSRO1e-OX seedlings buried at 5 cm (H, J), 10 cm (I, K), and 15 cm (L) depths filled with soil. Scale bars: 2 cm. (M, N) The mesocotyl length (M) and emergence rate (N) of zmsro1e mutant and zmsro1e/ZmSRO1e-OX seedlings buried at different depths filled with soil. Values are presented as mean ± SE. Asterisks indicate significant differences compared with the wild-type (*P < 0.05; **P < 0.01) determined by Student's t-test.
ZmSRO1e influence on the expression of genes regulating mesocotyl elongation
The fact that ZmSRO1e modulates mesocotyl elongation prompted us to investigate how ZmSRO1e regulates the downstream genes involved in cell expansion. We selected several cell expansion-related genes, including ALPHA EXPANSIN2 (ZmEXPA2), BETA EXPANSIN4/6 (ZmEXPB4/6), INDOLE-3-ACETIC ACID INDUCIBLE15 (ZmIAA15) and measured their expression levels. The expression level of these genes was lower in the shoots of light-grown maize seedlings than in those of dark-grown seedlings (Figure S3). Consistent with the morphological phenotype, the expression levels of ZmEXPA2, ZmEXPB4/6, and ZmIAA15 were dramatically elevated in the ZmSRO1e overexpression lines (Figure 3A, B). The abundance of transcripts generated from these four genes was lower in zmsro1e mutant seedlings than in the wild-type seedlings (Figure 3C, D), suggesting ZmSRO1e positively regulates the expression of cell expansion-related genes. We also detected the expression of some cell expansion-related genes in the Arabidopsis seedlings overexpressing ZmSRO1e. The results indicated that ZmSRO1e enhanced the expression of some cell expansion-related genes such as INDOLE-3-ACETIC ACID INDUCIBLE19 (AtIAA19), EXPANSIN2 (AtEXP2), DWARF4 (AtDWF4), and EXTENSIN3 (AtEXT3) (Figure S4).
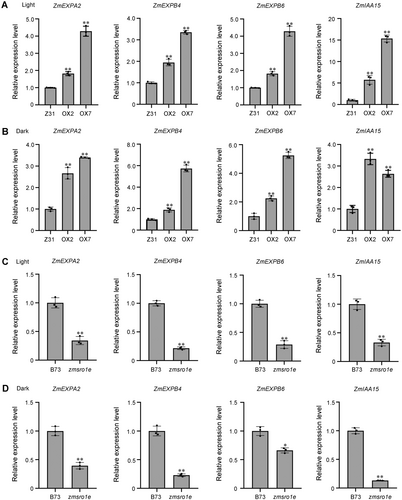
ZmSRO1e regulates the expression of cell expansion-related genes in maize seedlings
(A, B) Transcriptional profiling of genes involved in cell expansion in 10-d-old ZmSRO1e overexpression seedlings under long day (LD) (A) and constant darkness conditions (B). (C, D) Transcriptional profiling of genes involved in cell expansion in 10-d-old zmsro1e mutant seedlings under LD (C) and constant darkness conditions (D). Values are mean ± SE with at least three biological replicates. Asterisks indicate significant differences compared with the wild-type (*P < 0.05; **P < 0.01) determined by Student's t-test.
ZmSRO1e physically interacts with ZmbZIP61
To identify novel light signaling components with which ZmSRO1e can interact, we performed a yeast two-hybrid (Y2H) screen on a maize complementary DNA (cDNA) library using ZmSRO1e as bait. Among the 54 candidate proteins (Table S1), a bZIP transcription factor ZmbZIP61 was confirmed to interact with ZmSRO1e in yeast cells (Figure 4A, B). To verify the interaction between ZmSRO1e and ZmbZIP61, we performed a protein–protein co-immunoprecipitation (Co-IP) assay. We transiently co-expressed recombinant ZmSRO1e-MYC and ZmbZIP61-GFP (green fluorescent protein) in Nicotiana benthamiana leaves. ZmSRO1e-MYC successfully pulled down ZmbZIP61-GFP but not the negative control GFP (Figure 4C), suggesting that ZmSRO1e interacts with ZmbZIP61 in vivo. Split-luciferase (LUC) complementation (SLC) assays were used to verify the interaction between ZmSRO1e and ZmbZIP61. A strong luminescence signal in N. benthamiana leaves co-transformed with ZmSRO1e-nLUC and ZmbZIP61-cLUC (or ZmSRO1e-cLUC and ZmbZIP61-nLUC) was detected in the SLC experiment (Figure 4D, E). To determine which ZmbZIP61 domain participates in the interaction between ZmbZIP61 and ZmSRO1e, we divided the full-length coding sequence of ZmbZIP61 into N-terminal (N78) and C-terminal bZIP (ΔN78) domains and tested their interactions with ZmSRO1e using SLC assays (Figure 4F). We reconstituted LUC when ZmSRO1e-nLUC was co-expressed with ZmbZIP61-ΔN78-cLUC but not with ZmbZIP61-N78-cLUC, indicating that the C-terminal bZIP domain of ZmbZIP61 was sufficient for interaction with ZmSRO1e (Figure 4G). Similarly, we found that the C-terminal RST domain of ZmSRO1e was required for its interaction with ZmbZIP61 (Figure 4H). Together, these results show that ZmSRO1e interacts with ZmbZIP61 in plants.
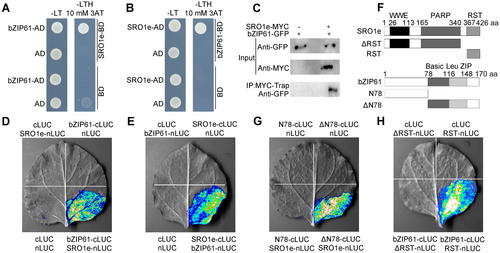
ZmSRO1e physically interacts with ZmbZIP61
(A, B) Yeast two-hybrid assays showed physical interaction between ZmSRO1e and ZmbZIP61 in yeast. (C) Co-immunoprecipitation assay shows direct interaction between ZmSRO1e and ZmbZIP61 in Nicotiana benthamiana leaves. Total protein was extracted from N. benthamiana leaves transiently expressing 35S::ZmSRO1e-MYC alone or with 35S::ZmbZIP61-GFP. The immunoprecipitates were detected using anti-green fluorescent protein (anti-GFP) and anti-MYC antibodies. (D, E) Split-luciferase (LUC) complementation assays show that ZmSRO1e interacts with ZmbZIP61. The ZmSRO1e-nLUC/ZmbZIP61-cLUC (D), ZmSRO1e-cLUC/ZmbZIP61-nLUC (E) constructs were transiently expressed into N. benthamiana leaves and LUC signal was captured using a charge-coupled device camera. (F) Schematic diagram of different truncated fragments of ZmSRO1e and ZmbZIP61. ΔRST, N-terminal fragment of ZmSRO1e without RST domain; RST, C-terminal RST domain of ZmSRO1e; N78, 1–78 amino acids in the N-terminal of ZmbZIP61; ΔN78, Basic leucine zipper in the C-terminal domain of ZmbZIP61. (G) ZmbZIP61 binds to ZmSRO1e via its C-terminal bZIP domain. (H) The N-terminal RST domain of ZmSRO1e is necessary for interaction with ZmbZIP61.
Phylogenetic and sequence analyses indicated that ZmbZIP61 is a homolog of Arabidopsis HY5 (Figure S5A, B). Y2H, SLC, and Co-IP assays showed that ZmSRO1e interacted with HY5 (Figure S6). Since HY5 is extensively involved in light signaling, we also determined whether the expression of ZmbZIP61 in maize seedlings was affected by light. We found that the expression of ZmbZIP61 was rapidly upregulated by light treatment and that the transcript level gradually decreased with increasing lighting time (Figure 5A, B). To further examine whether ZmbZIP61 performs functions similar to HY5, we heterologously overexpressed ZmbZIP61 in the Arabidopsis ecotype Ler-0 (ZmbZIP61-OE) and hy5-1 mutant (Ler-0 background). Since the Arabidopsis hy5-1 mutant showed a constitutive skotomorphogenesis-like phenotype with an elongated hypocotyl and reduced anthocyanin and chlorophyll content, the transgenic lines of ZmbZIP61-OE and ZmbZIP61-OE/hy5-1 were checked for hypocotyl length, anthocyanin content, and chlorophyll content. The light-grown ZmbZIP61-OE seedlings exhibited a similar hypocotyl as wild-type Ler-0; however, heterologous overexpression of ZmbZIP61 rescued the longer hypocotyl phenotype of hy5-1 mutant seedlings (Figure 5C, D). The expression of some cell expansion-related genes such as AtIAA19 and AtEXP2 were dramatically elevated in hy5-1 mutant seedlings, while heterologous overexpression of ZmbZIP61 inhibited their expression in hy5-1 mutant seedlings (Figure S7). The anthocyanin and chlorophyll content of ZmbZIP61-OE and hy5-1/ZmbZIP61-OE lines were higher than those of the hy5-1 mutant (Figure 5E, F). These results suggested that ZmbZIP61 and HY5 have conserved functions in regulating photomorphogenesis.
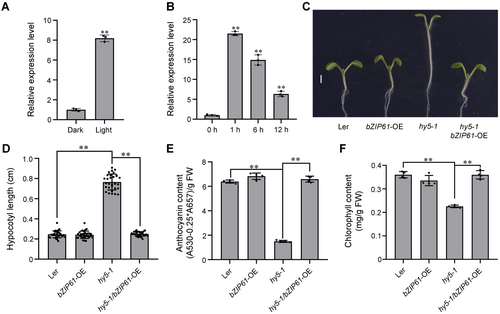
ZmbZIP61 is a functional ortholog of Arabidopsis HY5
(A) Relative expression levels of ZmbZIP61 in seedlings at the three-leaf stage grown in darkness and continuous light. (B) Relative expression levels of ZmbZIP61 in seedlings at the three-leaf stage grown in darkness and then transferred to white light for the indicated periods (0, 1, 6, and 12 h). (C) Heterologous overexpression of ZmbZIP61 rescued the longer hypocotyl phenotype of hy5-1 mutant seedlings. Scale bars: 0.5 cm. (D) The hypocotyl length of hy5-1 mutant, ZmbZIP61-OE (overexpression), and hy5-1/ZmbZIP61-OE lines. Values are mean ± SE (n = 25). (E) The anthocyanin content of hy5-1 mutant, ZmbZIP61-OE, and hy5-1/ZmbZIP61-OE lines. (F) The chlorophyll content of hy5-1 mutant, ZmbZIP61-OE, and hy5-1/ZmbZIP61-OE lines.
ZmSRO1e influences transcriptional activity of ZmbZIP61
Given that ZmbZIP61 is a bZIP transcription factor and ZmSRO1e interacts with ZmbZIP61, we tested whether ZmbZIP61 directly binds to the promoters of cell expansion-related genes regulated by ZmSRO1e. Sequence analysis revealed the presence of some conserved putative ZmbZIP61-binding sites (ACE elements and G-boxes) in the promoters of ZmEXPB4 and ZmEXPB6. We then recombined the promoters of ZmEXPB4 and ZmEXPB6 (EXPB4p and EXPB6p, respectively) into yeast one-hybrid reporter constructs to drive LacZ reporter gene expression. Yeast one-hybrid assays showed that ZmbZIP61 is bound to the promoters of ZmEXPB4 and ZmEXPB6. Mutation of the G-box motif (CACGTG) of ZmEXPB4 and ZmEXPB6 promoters to CTTTTG (EXPB4m and EXPB6m) completely abolished ZmbZIP61 binding in yeast cells (Figure 6A). Next, we performed chromatin immunoprecipitation (ChIP)-qPCR assay to show the in vivo binding of ZmbZIP61 to the promoter region of ZmEXPB4 and ZmEXPB6, and the results indicated that ZmbZIP61 mainly binds to the P2 region of ZmEXPB4 and ZmEXPB6 promoters, which contain G-box motifs (Figure 6B–D). Subsequently, we performed an electrophoretic mobility shift assay (EMSA), which confirmed that maltose-binding protein (MBP)-ZmbZIP61 could specifically bind to the oligonucleotide probes of ZmEXPB4 and ZmEXPB6 promoters having the G-box motif (Figure 6E, F). Moreover, transient expression assays in N. benthamiana leaves showed that ZmbZIP61 inhibited the transcription of EXPB4pro:LUC and EXPB6pro:LUC (Figure 6G–K). Thus, our results prove that ZmbZIP61 directly binds to the promoters of ZmEXPB4/ZmEXPB6 and inhibits their expression.
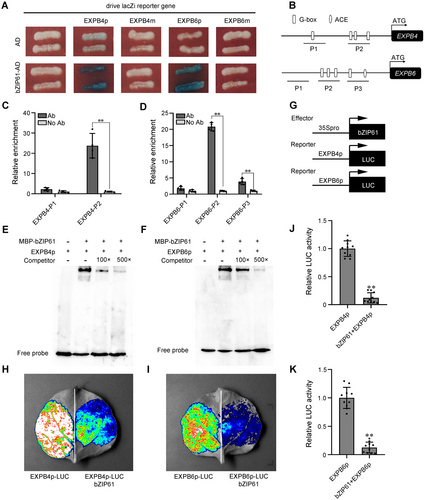
ZmbZIP61 directly binds to the promoter of ZmEXPB4/ZmEXPB6 and inhibits their expression
(A) Yeast one-hybrid assays confirmed the binding capability of ZmbZIP61 onto the promoters of ZmEXPB4 and ZmEXPB6. LacZ reporter gene was driven by promoter (p) and mutated promoter (m, with mutation in the G-box) of ZmEXPB4 and ZmEXPB6. (B) Diagrams of different regions of ZmEXPB4 and ZmEXPB6 promoters. (C, D) Chromatin immunoprecipitation – quantitative polymerase chain reaction assay indicated that ZmbZIP61 mainly binds to the P2 region of ZmEXPB4 (C) and ZmEXPB6 (D) promoters. (E, F) Electrophoretic mobility shift assay showed that maltose-binding protein (MBP)-ZmbZIP61 protein could bind to the ZmEXPB4 (E) and ZmEXPB6 promoters (F). (G) Diagrams of various constructs used in the transient transfection assay in Nicotiana benthamiana leaves. (H) ZmbZIP61 represses the expression of ZmEXPB4 in N. benthamiana leaves. Construct pairs were combined at a 1:2 ratio for EXPB4p-LUC: 35S-ZmbZIP61. (I) ZmbZIP61 represses the expression of ZmEXPB6 in N. benthamiana leaves. Construct pairs were combined at a 1:2 ratio for EXPB6p-LUC: 35S-ZmbZIP61. (J, K) Relative luciferase (LUC) activities were quantified by ImageJ software from the intensity of the mean color signals in (H) and (I). Values are as means ± SE (n = 10). **Shows significant differences (P < 0.01), as determined by Student's t-test.
As ZmSRO1e interacts with ZmbZIP61, and ZmbZIP61 directly binds to the promoters of ZmEXPB4/ZmEXPB6 and inhibits their expression, we investigated whether and how ZmSRO1e interferes with the inhibition of ZmbZIP61 on the expression of ZmEXPB4/ZmEXPB6. First, we performed an EMSA to analyze the binding of ZmbZIP61 to the G-box of ZmEXPB4 and ZmEXPB6 in the presence of ZmSRO1e. The addition of MBP-ZmSRO1e significantly decreased the binding of MBP-ZmbZIP61 to ZmEXPB4 and ZmEXPB6 promoters (Figure 7A, B). Subsequently, we used the ZmEXPB4 and ZmEXPB6 promoters as reporters in a transient transcription assay in N. benthamiana leaves to study how ZmSRO1e affects the activity of ZmbZIP61 in plant cells. Compared to ZmbZIP61 alone, the co-expression of ZmSRO1e with ZmbZIP61 dramatically enhanced the luminescence signal, confirming that ZmSRO1e can alleviate the inhibition of ZmbZIP61 to ZmEXPB4 and ZmEXPB6 (Figure 7C–F).
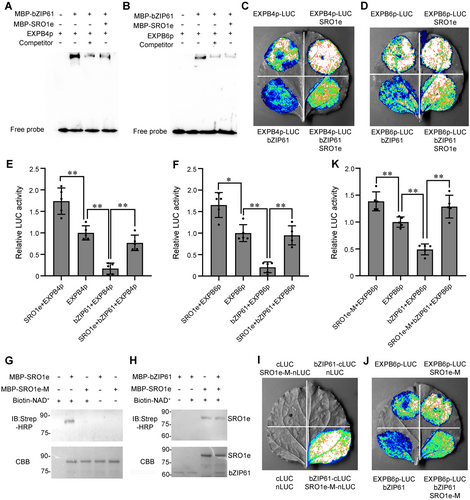
ZmSRO1e interferes with the binding of ZmbZIP61 to the promoters of ZmEXPB4 and ZmEXPB6
(A, B) Electrophoretic mobility shift assay showed that maltose-binding protein (MBP)-ZmSRO1e decreased the binding of MBP-ZmbZIP61 to the promoters of ZmEXPB4 (A) and ZmEXPB6 (B). (C, D) Transient activation assay proved the inhibition of ZmSRO1e on ZmbZIP61 to regulate the expression of the luciferase (LUC) reporter gene driven by the ZmEXPB4 (C) and ZmEXPB6 (D) promoters in Nicotiana benthamiana leaves. Construct pairs were combined at a 1:2:4 ratio for EXPB4p-LUC: 35S-ZmbZIP61: 35S-ZmSRO1e and EXPB6p-LUC: 35S-ZmbZIP61: 35S-ZmSRO1e. (E, F) Relative LUC activities were quantified by ImageJ software from the intensity of the mean color signals in (C) and (D). (G) ZmSRO1e but not ZmSRO1e-M showed auto-adenosine diphosphate (ADP)-ribosylation activity. (H) ZmSRO1e could not modify ZmbZIP61 by ADP-ribosylation. (I) ZmSRO1e-M interacted with ZmbZIP61. (J) Transient activation assay proved the inhibition of ZmSRO1e-M on ZmbZIP61 to regulate the expression of the LUC reporter gene driven by the ZmEXPB6 promoter in N. benthamiana leaves. Construct pairs were combined at a 1:2:4 ratio for EXPB6p-LUC: 35S-ZmbZIP61: 35S-ZmSRO1e-M. (K) Relative LUC activities were quantified by ImageJ software from the intensity of the mean color signals in (J). Values are presented as mean ± SE (n = 5). Asterisks indicate significant differences (**P < 0.01), as determined by Student's t-test.
As some SROs have been reported to have ADP-ribosyltransferase (ART) activity and regulate the function of their interacting proteins through ADP-ribose modification (Kong et al., 2021; Gao et al., 2022), we also tested whether ZmSRO1e has ART activity and affects ZmbZIP61 activity through ADP-ribose modification. An in vitro ADP-ribosylation assay indicated that ZmSRO1e showed auto-ADP-ribosylation activity (Figure 7G), whereas ZmSRO1e-M (with the Tyr-His-Trp triad in the catalytic core mutated to Leu-His-Asn, similar to Arabidopsis RCD1 with no ART activity) showed no auto-ADP-ribosylation activity, indicating that ZmSRO1e is a functional ART (Figure 7G). However, no ADP-ribosylation was detected on ZmbZIP61 when incubated with ZmSRO1e, indicating that ZmSRO1e could not modify ZmbZIP61 via ADP-ribosylation (Figure 7H). Furthermore, we found that ZmSRO1e-M could also interact with ZmbZIP61 and alleviate the inhibition of ZmbZIP61 to ZmEXPB6, indicating that ZmSRO1e influences the transcriptional activity of ZmbZIP61 not through its ART activity (Figure 7I–K).
DISCUSSION
ZmSRO1e functions in regulating mesocotyl elongation
SRO proteins are plant-specific proteins involved in both development and the response to abiotic stress. Multiple SROs act as key components in modulating ROS content and cellular redox homeostasis to regulate abiotic stress. However, the mechanisms through which SROs regulate plant development remain unclear. Previous studies have shown that rcd1 mutant seedlings exhibit a reduced hypocotyl phenotype under red light, blue light, and UV-B radiation (Jiang et al., 2009), and Y2H assays have shown that RCD1 interacts with some regulators of light signaling, such as BBX24 and PIF (phytochrome-interacting factor) transcription factors (Jaspers et al., 2009); however, the mechanism by which RCD1 regulates hypocotyl elongation is still unknown. In the present study, we demonstrated the function and mechanism of ZmSRO1e in regulating mesocotyl elongation. First, the expression of ZmSRO1e was repressed by light (Figure 1A, B). Overexpression of ZmSRO1e resulted in elongated mesocotyl/hypocotyl, whereas the loss-of-function zmsro1e mutant exhibited shortened mesocotyl, and overexpression of ZmSRO1e rescued the shortened mesocotyl phenotype of zmsro1e mutant (Figure 1C–J). Consistently, ZmSRO1e promotes the expression of genes that regulate cell expansion, such as ZmEXPA2, ZmEXPB4/6, and ZmIAA15 (Figure 3). Moreover, ZmSRO1e interacted with ZmbZIP61, an ortholog of Arabidopsis HY5, and influenced the binding of ZmbZIP61 to the promoters of cell expansion-related genes (Figures 4–7). These results indicated that ZmSRO1e promotes mesocotyl elongation.
Deep sowing-tolerant seeds can germinate from deep soil to avoid the unfavorable effects of topsoil drought (Lee et al., 2017). Further, deep sowing also evades damage from harmful chemical residues in shallow soil and improves lodging tolerance (Zhao et al., 2023). However, existing maize varieties exhibited poor emergence and weak seedling establishment when deeply sowed (Wang et al., 2022). In order to develop deep sowing-tolerant maize varieties, it is important to uncover the molecular mechanism of mesocotyl elongation. Since the mesocotyl pushes the shoot of the seedling out of the soil during seed germination, its growth is highly related to deep sowing tolerance. Quantitative trait loci (QTL) analysis results confirmed that maize seedling emergence under deep sowing conditions was mainly caused by mesocotyl elongation (Zhang et al., 2012). We found ZmSRO1e promoted mesocotyl elongation and improved seedling emergence rate under deep sowing conditions (Figure 2), indicating that SRO might have important application potential in maize breeding for deep sowing tolerance.
ZmSRO1e promotes mesocotyl elongation by interacting with ZmbZIP61
HY5 is a central regulator of light signaling, with the Arabidopsis hy5-1 mutant showing a constitutive skotomorphogenesis-like phenotype with an elongated hypocotyl and reduced anthocyanin and chlorophyll content. We found that the overexpression of ZmbZIP61 in hy5-1 mutant background complemented the phenotype of hy5-1 mutant, suggesting that ZmbZIP61 and HY5 have conserved functions in regulating photomorphogenesis and that ZmbZIP61 is a functional ortholog of HY5. HY5 is a transcription factor that directly or indirectly regulates one-third of gene expressions throughout the Arabidopsis genome (Lee et al., 2007; Zhang et al., 2011). In this study, ZmbZIP61 was shown to repress the transcription of light-responsive genes such as ZmEXPB4 and ZmEXPB6 by directly binding to the G-box present in their promoters. Since HY5 lacks any apparent transactivation or repression domains, cofactors are required to regulate gene transcription (Burko et al., 2020; Bursch et al., 2020). Several HY5-interacting proteins have functional roles in modulating the transcriptional capacity of HY5, such as HYH, BBX protein, PICKLE, COR27, and COR28 (Gangappa and Botto, 2014; Zhang et al., 2017; Li et al., 2020). These factors perform different functions and regulate HY5 via different mechanisms, with some cofactors competing with HY5 for binding sites on DNA and others forming heterodimers to regulate HY5 DNA-binding activity. In the present study, we found that ZmSRO1e is a cofactor of ZmbZIP61 and modulates its transcriptional capacity. ZmSRO1e belongs to the SRO protein family and does not have any known DNA-binding domains, implying that it is unlikely to be a transcription factor. We found that ZmSRO1e and ZmbZIP61 play opposite roles in controlling mesocotyl elongation: ZmbZIP61 represses the expression of cell expansion-related genes and inhibits mesocotyl elongation, whereas ZmSRO1e enhances the expression of cell expansion-related genes and promotes mesocotyl elongation.
ZmSRO1e plays a similar role in regulating plant photomorphogenesis as COP1, both of which are suppressed by exposure to light and negatively regulate photomorphogenesis by suppressing ZmbZIP61/HY5; however, ZmSRO1e regulates the function of ZmbZIP61/HY5 in a different way from that of COP1. It is well known that HY5 is directly regulated at the protein level by ubiquitination via COP1, which leads to HY5 degradation via the 26S proteasome in the dark, whereas light exposure reduces nuclear COP1 to a level that permits the accumulation of HY5 (Deng et al., 1992; Lau and Deng, 2012). We found that ZmSRO1e suppressed the function of ZmbZIP61 mainly by repressing its DNA-binding ability (Figure 7A, B), which may be beneficial for precisely modulating the biochemical ability of ZmbZIP61 to regulate the expression of large downstream genes, which might be a complementary way for COP1 to regulate photomorphogenesis. However, the mechanism by which ZmSRO1e coordinates with COP1 to regulate photomorphogenesis requires further investigation.
ZmSRO1e regulates the activity of ZmbZIP61 not through ADP-ribosylation modification
ADP-ribosylation is an intricate and versatile modification that regulates diverse cellular processes, including DNA repair, chromatin remodeling, and gene transcription, in response to biotic and abiotic stresses (Lüscher et al., 2022). ADP-ribosylation is mediated by ARTs, which catalyze the transfer of ADP-ribose from nicotinamide adenine dinucleotide (NAD+) to substrates to generate MARylation or poly(ADP-ribosyl)ation (PARylation) modifications (Feijs et al., 2013; Cohen and Chang, 2018). In addition to canonical poly(ADP-ribosyl)ation polymerases (PARPs), the plant-specific SRO family also contains the catalytic core of the PARP domain (Jaspers et al., 2010); however, whether the PARP domains in SROs execute the ART function is still under debate. Previously, SROs were suggested to have no ADP-ribosyltransferase activity according to bioinformatic analysis of the PARP domain folding structure and biochemical assays of Arabidopsis RCD1 (Jaspers et al., 2010). Recently, Arabidopsis SRO2 was found to be an ART that MARylates plant immune regulators SZF1/SZF2 at multiple Asp and Glu residues (Kong et al., 2021), and maize ZmSRO1d was shown to MARylate ZmRBOHC, thus promoting the activity of ZmRBOHC (Gao et al., 2022). In this study, we found that ZmSRO1e showed ART activity and that mutation of the catalytic triad Tyr-His-Trp to Leu-His-Asn (similar to Arabidopsis RCD1 with no ART activity) abolished the ART activity of ZmSRO1e (Figure 7G). However, no ADP-ribosylation was detected on ZmbZIP61 when incubated with ZmSRO1e, indicating that ZmSRO1e could not modify ZmbZIP61 via ADP-ribosylation (Figure 7H). Moreover, we found that mutation of the catalytic triad Tyr-His-Trp of ZmSRO1e did not affect its interaction with ZmbZIP61, and the mutated ZmSRO1e also repressed the inhibition of ZmbZIP61 on target genes (Figure 7I–K), indicating that ZmSRO1e influences the transcriptional activity of ZmbZIP61 not through its ART activity. These results were also reasonable, as ZmSRO1e interacted with ZmbZIP61 only through its RST domain and not the PARP domain (Figure 4H). Further investigations are required to determine whether ZmSRO1e interacts with and MARylates other factors that regulate plant development and/or abiotic stress responses.
These results indicate that ZmSRO1e plays an important role in promoting mesocotyl elongation. In the dark, ZmSRO1e transcripts accumulate and inhibit the activity of ZmbZIP61, which promotes the expression of cell expansion-related genes. Light decreases expression of ZmSRO1e and triggers the expression of ZmbZIP61, the activated ZmbZIP61 represses the expression of cell expansion-related genes, thus, mesocotyl elongation was inhibited (Figure 8). Our results provide a new understanding of the mechanism by which SRO regulates mesocotyl elongation, which has potential applications in deep sowing resistance breeding.
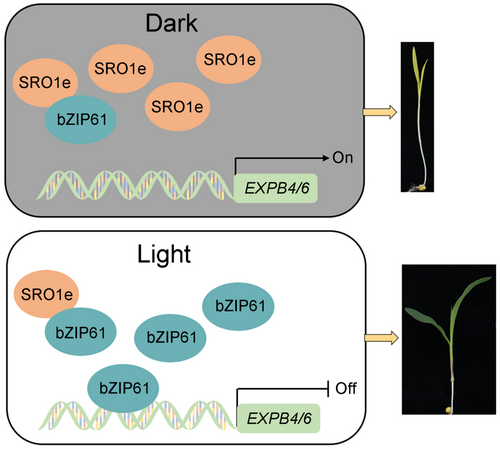
A model of ZmSRO1e in regulating mesocotyl elongation
In the dark, ZmSRO1e accumulates and inhibits the activity of ZmbZIP61, which promotes the expression of cell expansion-related genes. Light decreases expression of ZmSRO1e and triggers the expression of ZmbZIP61, the activated ZmbZIP61 represses the expression of cell expansion-related genes, thus, mesocotyl elongation is inhibited.
MATERIALS AND METHODS
Plant materials and growing conditions
Maize seeds were sterilized by submerging them in a 1% NaClO (w/v) solution for 10 min, rinsing three times with sterile distilled water and imbibed overnight with shaking at room temperature. The imbibed seeds were uniformly germinated into the soil with vermiculite in an artificial climate chamber at 28°C. For transcriptional analysis of SROs in maize, seedlings were grown in constant darkness for 10 d and exposed to white light for 1, 6, and 12 h or under continuous light for 10 d. For phenotypes of OX lines and zmsro1e mutants, imbibed seeds were buried in plastic pots at sowing depths of 2 cm. The same planting density was used for all the experiments. The plastic pots were placed under constant darkness or long day (LD) conditions (16 h light/8 h dark) for 10 d. To characterize the effect of ZmSRO1e on deep sowing tolerance, the imbibed seeds were buried in plastic pots at sowing depths of 5, 10, and 15 cm under LD conditions for 10 d. The A. thaliana hy5-1 (CS71) mutant was grown on a Ler-0 background. Arabidopsis seeds were surface sterilized with 75% alcohol and sown in half Murashige and Skoog (MS) medium supplemented with 1% (w/v) sucrose and 0.8% (w/v) agar. Seeds were stratified for 3 d in the dark at 4°C, and then transferred to white light for 8 h to induce uniform gemination, followed by constant darkness or LD (16 h light/8 h dark) treatments at 22°C for 4–6 d. N. benthamiana seeds were directly sown into the soil and grown in the greenhouse for about 4 weeks.
Plant transformation
The ZmSRO1e-overexpressed lines of maize and Arabidopsis zmsro1e mutants have been previously reported (Qin et al., 2021). The full-length cDNA of ZmbZIP61 was cloned into pENTRTM /SD/DTOPOTM vectors (ThermoFisher, Shanghai, China) and then recombined with the pEarlyGate203 vector (Earley et al., 2006) under the control of the CaMV 35S promoter. For stable transformation, Agrobacterium tumefaciens strain GV3101 carrying the construct was used to transform ZmbZIP61 into Ler and hy5-1 using the flower dip method (Clough and Bent, 1998).
Total RNA extraction and transcriptional analysis
Quantitative RT-PCR expression analyses were performed as described by Qin et al. (2021). Briefly, total RNAs were isolated using the TRIzol reagent (Thermo Fisher, Shanghai, China). Reverse transcription was performed using a FastQuant RT Kit (Tiangen, Beijing, China) according to the manufacturer's instructions. RT-qPCR was performed using the TransStart Green qPCR SuperMix (TransGen, Beijing, China). ZmEF1α and AtACTIN2 were used as internal controls. Primers used for RT-qPCR are listed in Table S2.
Mesocotyl and hypocotyl lengths measurement
To measure the mesocotyl lengths, surface-sterilized maize seeds of various genotypes were sown into different depths of soil (2, 5, 10, and 15 cm) and kept at 28°C for 9–12 d. For hypocotyl length measurements, seeds were surface sterilized, sown on 1/2 MS medium, and kept at 4°C for 3 d. Thereafter, seeds were transferred to white light for 8 h at 22°C to induce uniform germination and exposed to darkness or different light conditions at 22°C for 6 d. Then, the seedlings were photographed by a digital camera, and mesocotyl and hypocotyl lengths were determined with ImageJ software (http://rsbweb.nih.gov/ij/).
Scanning electronic microscopy of hypocotyl epidermal cells
For scanning electron microscopy, 6-d-old hypocotyls of Arabidopsis seedlings were collected and fixed in fixative solution (3% glutaraldehyde in 25 mmol/L phosphate buffer, pH 7.0) at 4°C for overnight. Then, the samples were washed, dehydrated in gradient ethanol, and critical point dried. Finally, the samples were coated with gold and examined under a Quanta 250 FEG scanning electron microscope at an accelerating voltage of 5 kV (FEI, Hillsboro, OR, USA).
Yeast two-hybrid assays
To identify interacting proteins of ZmSRO1e, we performed a Y2H screen using ZmSRO1e as a bait with a library derived from B73 seedlings. ZmSRO1e-BD was first transformed into the yeast strain Y2H gold and plated on synthetic defined (SD)/-Trp medium. Then, the library plasmids were transformed into the yeast strain Y2H gold carrying a ZmSRO1e-BD construct and plated on SD/-Leu-Trp medium with 200 ng/mL Aureobasidin A (AbA) at 30°C for 3–5 d. The colonies were finally tested on SD/-Leu-Trp-His-Ade medium containing 400 ng/mL AbA for further screening. The positive clones are listed in Table S1.
To confirm the interaction between ZmSRO1e and ZmbZIP61/AtHY5, the open reading frames of ZmSRO1e and ZmbZIP61/AtHY5 were amplified and inserted into the EcoR I-BamH I sites of the pGBKT7 and pGADT7 vectors (Clontech, now TaKaRa, https://www.takarabio.com). These pairs of vectors were co-transformed into the yeast strain AH109. Y2H assays were performed as previously described (Qin et al., 2021).
Yeast one-hybrid assays
To prepare constructs for the yeast one-hybrid assay, the promoter fragments of ZmEXPB4 and ZmEXPB6 were amplified from maize B73 genomic DNA and inserted into Kpn I-Sal I sites of pLacZi2μ to generate EXPB4p: LacZ and EXPB6p: LacZ, respectively. Fragments with mutations in the G-boxes of ZmEXPB4 and ZmEXPB6 promoters were ligated into the pLacZi2μ vector between the Kpn I and Sal I sites to produce EXPB4m: LacZ and EXPB6m: LacZ, respectively. To generate the pB42AD-ZmbZIP61 construct, the full-length coding sequence of ZmbZIP61 was amplified and cloned into the EcoR I-Xho I sites of the pB42AD vector (BD Clontech). For the yeast one-hybrid assay, pB42AD-ZmbZIP61 (effector) and various LacZ reporter plasmids were co-transformed into the yeast strain EGY48. Transformants were selected on a medium (SD) lacking Trp and Ura and transferred to SD/-Trp-Ura dropout medium containing X-gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside) for interaction tests.
Co-immunoprecipitation assays
For Co-IP assays, the full-length coding sequences of ZmSRO1e, ZmbZIP61, and AtHY5 were cloned into pEarlyGate103 or pEarlyGate203 plant binary vectors using the Gateway LR Clonase Enzyme Mix (Invitrogen) to generate p35S::ZmSRO1e-MYC, p35S::ZmbZIP61-GFP and p35S::AtHY5-GFP. A. tumefaciens strain GV3101 carried the p35S::ZmSRO1e-MYC, p35S::ZmbZIP61-GFP, or p35S::ZmbZIP61-GFP constructs into N. benthamiana leaves. N. benthamiana tissues were collected after infiltration and grown under LD conditions (16 h light/8 h dark) for 48–60 h. Co-IP assays were performed as previously described (Qin et al., 2021).
Chromatin immunoprecipitation assay
To identify the binding of ZmbZIP61 to the ZmEXPB4 and ZmEXPB6 promoters, ChIP-qPCR was performed as described by Liu et al. (2021). Based on the position of G-boxes and ACE elements on ZmEXPB4/6 promoters, ZmEXPB4/6 promoters were segmented and cloned into the pRI101-AN vector digested with Sca Ⅰ and Xba Ⅰ. N. benthamiana leaves were infiltrated with Agrobacterium harboring these constructs and those expressing ZmbZIP61-GFP or GFP. After 48 h, the samples were harvested and cross-linked with 1% (v/v) formaldehyde for 10 min under a vacuum. Glycine was added and de-crosslinked for another 5 min. Then, samples were ground into powder and sonicated to shear DNA into 250–500 bp fragments at 4°C. The sheared chromatin was incubated with anti-GFP antibody (Cat. No. ab290; Abcam) overnight at 4°C to isolate chromatin complexes. Protein A + G agarose/salmon sperm shear DNA were added to IP the DNA fragments for 60 min at 4°C. After washing and eluting from the beads, the protein-DNA complex was reverse cross-linked by incubation with 5 mol/L NaCl at 65°C overnight. The precipitate DNA samples were analyzed by qPCR with the primers listed in Table S2.
Electrophoretic mobility shift assay
To purify the MBP-ZmbZIP61 recombinant proteins, the full-length ZmbZIP61 coding sequence was inserted into the EcoR I-Sal I sites of the pMAL-c2X vector and expressed in Escherichia coli strain BL21. EMSAs were performed using biotin-labeled probes and a Chemiluminescent EMSA Kit (Beyotime, Beijing, China) according to the manufacturer's instructions. Briefly, 2 μg MBP-ZmbZIP61 fusion protein was incubated together with biotin-labeled probes in the presence or absence of excess unlabeled competitors for 20 min at room temperature and then separated on 6% native polyacrylamide gels in Tris-borate-ethylenediaminetetraacetic acid (TBE) buffer. The bound complexes were electroblotted onto nylon membranes in TBE buffer for 50 min. Finally, protein-DNA signals were detected according to the instructions provided with the EMSA Kit. The probes used for the EMSA are shown in Table S2.
Luciferase imaging
For the SLC assay, fragments containing the full-length ZmSRO1e coding sequence (1–426 aa), ZmSRO1e-M (the Tyr-His-Trp triad at the catalytic core mutated to Leu-His-Asn), truncated ZmSRO1e coding sequence including ΔRST (1–340 aa), and RST (341–426 aa) were inserted into pCAMBIA1300-cLUC and pCAMBIA1300-nLUC by taking advantage of the Kpn I and Sal I cloning sites. Full-length ZmbZIP61 coding sequence (1–170 aa), ZmbZIP61-N78 (1–78 aa), and ZmbZIP61-ΔN78 (79–170 aa) fragments were amplified and cloned into the Kpn I-Sal I sites of the pCAMBIA1300-cLUC and pCAMBIA1300-nLUC vectors. A full-length AtHY5 coding sequence was inserted into the Kpn I-Sal I sites of the pCAMBIA1300-nLUC vector. A suspension of A. tumefaciens strain GV3101 containing the recombinant constructs was infiltrated into N. benthamiana leaves; after 2–3 d of incubation, the leaves were sprayed with 1 mmol/L D-luciferin-free acid (GoldBio, St. Louis, MO, USA) dissolved in 0.01% (v/v) Triton X-100 and incubated for 5 min in the dark. Images were acquired using a charge-coupled device (CCD) camera in the dark for 5 min.
For the transcriptional activity of ZmbZIP61 assay, promoter fragments of ZmEXPB4 and ZmEXPB6 were inserted into pGWB235 vectors (Nakagawa et al., 2007), whereas ZmSRO1e and ZmbZIP61 were inserted into either pEarlyGate203 or pEarlyGate103. To assay the inhibition of ZmbZIP61 on the expression of ZmEXPB4/ZmEXPB6, construct pairs were combined at a 1:2 ratio of EXPB4p-LUC/EXPB6p-LUC: pEarlyGate103/35S-ZmbZIP61. To characterize the effect of ZmSRO1e on the activity of ZmbZIP61, construct pairs were combined in a 1:2:4 ratio for EXPB4p-LUC: pEarlyGate103/35S-ZmbZIP61: pEarlyGate203/35S-ZmSRO1e and EXPB6p-LUC: pEarlyGate103/35S-ZmbZIP61: pEarlyGate203/35S-ZmSRO1e. After infiltration for 2–3 d, N. benthamiana leaves were incubated with 1 mmol/L D-luciferin-free acid and kept in the dark for 5 min. The LUC signal was collected using a CCD camera. Luciferase activity was analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Adenosine diphosphate-ribosylation assays
To purify the MBP-ZmSRO1e recombinant protein, the full-length coding sequences of ZmSRO1e and ZmSRO1e-M (the Tyr-His-Trp triad at the catalytic core mutated to Leu-His-Asn) were inserted into the EcoR I-Sal I sites of the pMAL-c2X vector and expressed in E. coli strain BL21. ADP-ribosylation assays in vitro were performed as previously described (Kong et al., 2021). Briefly, 2 μg purified proteins were incubated with 500 nmol/L activated DNA (TaKaRa) and 0.2 μmol/L NAD+ (R&D Systems, Minneapolis, MN, USA) in the reaction buffer (50 mmol/L Tris-HCl, pH 8.0, 50 mmol/L NaCl) at 23°C for 3 h. The reactions were stopped by adding sodium dodecyl sulfate (SDS) loading buffer and subjected to SDS-polyacrylamide gel electrophoresis for streptavidin-horseradish peroxidase (Thermo Scientific) detection for biotinylated NAD+ or Coomassie blue staining.
Accession numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative, GenBank/EMBL databases or the Maize Genetics and Genomic Database under the following accession numbers: ZmSRO1a (GRMZM5G866843), ZmSRO1b (GRMZM2G145236), ZmSRO1c (GRMZM2G072894), ZmSRO1d (GRMZM2G177878), ZmSRO1e (GRMZM2G122543) and ZmSRO1f (GRMZM2G011469), ZmEF1α (GRMZM2G153541), ZmbZIP61 (GRMZM2G137046), ZmEXPB4 (GRMZM2G154178), ZmEXPB6 (GRMZM2G176595), ZmEXPA2 (GRMZM2G105844), ZmIAA15 (GRMZM2G148188), ACTIN2 (AT3G18780), AtHY5 (AT5G11260), AtDWF4 (AT3G50660), AtEXP2 (AT5G05290), AtEXT3 (AT1G21310), AtIAA19 (AT3G15540).
ACKNOWLEDGEMENTS
This research was supported by grants from the Natural Science Foundation of Shandong Province (ZR2019ZD16 and ZR2022QC007), the National Natural Science Foundation of China (32171935 and 32372039), Agricultural Variety Improvement Project of Shandong Province (2022LZGC002), the National Key Research and Development Program of China (2022YFD1201700), and the Project for Scientific Research Innovation Team of Young Scholars in Colleges and Universities of Shandong Province (2020KJE002). The maize zmsro1e mutant was provided by Prof. Chunyi Zhang from the Biotechnology Research Institute, Chinese Academy of Agricultural Sciences, and Prof. Xiaoduo Lu from Qilu Normal University.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
L.S., X.G., and Q.L. designed the experiments; Q.L., K.F., W.L., C.H., L.J., Z.C., and L.Y. performed the experiments; Q.L., L.S., and X.G. analyzed the data and wrote the manuscript. All authors read and approved of the manuscript.






