Application of CRISPR/Cas12i.3 for targeted mutagenesis in broomcorn millet (Panicum miliaceum L.)
Edited by: Lanqin Xia, Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, China
Graphical Abstract
As the global human population continues to grow and is predicted to reach 10 billion by 2050, the demand for food volume and quality raises a tremendous challenge (OECD/FAO, 2017). The worldwide arable land area is decreasing, posing a major threat to crop production. To address these problems, we need to improve the endurance of major crops. This can be accomplished by a meaningful exploration of germplasm resources for pioneer crops that can grow in marginal areas. Broomcorn millet (Panicum miliaceum L.), a C4 monocot Poaceae grass, is one of the earliest domesticated crops in China (Lu et al., 2009). It has a short life cycle (60–90 d), has high photosynthetic efficiency and harvest index, can grow in arid and saline-alkali soil, and has desirable nutritional traits for combating hidden hunger caused by micronutrient deficiency (Saleh et al., 2013; Shi et al., 2022). Broomcorn millet is a recent allotetraploid (2n = 4× = 36) the genome of which has been sequenced and annotated (Hunt et al., 2014; Shi et al., 2019). However, a genetic transformation protocol for broomcorn millet has not been established until now.
To establish a genetic transformation system for broomcorn millet, we surveyed existing genetic transformation methods for other crops. Considering that broomcorn millet seeds are small caryopses enclosed by the pericarp and seed coat (Figure 1A), we decided to use mature seeds without lemmas or paleas to induce callus cells as transformation recipient tissues (Figure 1B). To this end, we cultured surface-sterilized and bare mature seeds in the dark for 18 d on the medium containing 2,4-dichlorophenoxyacetic acid (2, 4-D) and kinetin to induce callus formation. Due to the accumulation of secondary metabolites in medium, we subcultured the calli on fresh medium once. After about 2 weeks, the calli were divided into 5 mm pieces as recipients (Figure 1C). The calli were then infected with a fresh Agrobacterium suspension harboring the binary vector with the green fluorescent protein (GFP) reporter gene. We transferred all of the infected calli to co-culture medium for 3 d to allow for T-DNA integration (Figures S1A, 1D), after which we transferred the infected calli to screening medium containing the appropriate concentrations of hygromycin for selection of resistant calli. We examined the resulting resistant calli for GFP fluorescence, which we observed after 10 d of cultivation on selection medium, indicating that the integration of the T-DNA in the genome and expression of transgenes can be obtained by Agrobacterium-mediated transformation of broomcorn millet calli (Figure 1E, F). We placed resistant calli onto pre-regeneration medium for culture under light conditions for 10 d. We then transferred calli with buds to regeneration medium for about 20 d of culture, when we started to observed the development of regenerated shoots from buds (Figure 1G). After rooting these regenerated plantlets for 10–14 d in rooting medium and transplanting them into nutrient soil, we obtained fertile regenerated T0 plants that produced T1-generation seeds with detectable GFP fluorescence (Figure 1H–J). These results suggest that we have successfully established a genetic transformation method for broomcorn millet mediated via co-culture of calli with Agrobacterium.
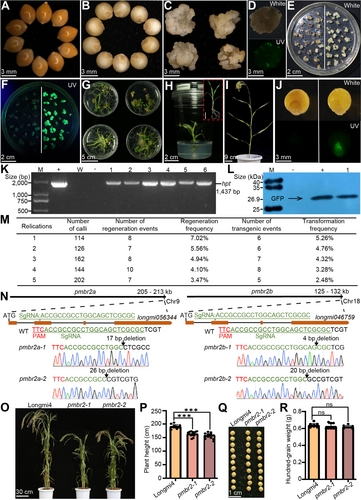
An efficient Agrobacterium tumefaciens-mediated genetic transformation protocol and clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated nuclease 12.3 (Cas12i.3) gene editing system in broomcorn millet
(A) Broomcorn millet mature seeds. (B) Broomcorn millet mature seeds without paleas. (C) Induced calli used as the recipient for transformation. (D) Infected calli after co-culture with Agrobacterium for 3 d. (E and F) Calli cultured on screening medium for 20 d. Uninfected calli and infected hygromycin-resistant calli are located on the left and right side of the white line, respectively. (G) Shoots regenerated from transformed calli following culture on regeneration medium for 20 d. (H) Regenerated plantlet in rooting medium. The red dashed box indicates the regenerated plant after 14 d of culture in rooting medium. Scale bar, 2 cm. (I) T0 plant transplanted into nutrient soil at the heading stage. (J) T1-generation seeds. The non-transgenic seeds and transgenic seeds of the T1 generation are located on the left and right side of the solid white line, respectively. (K) Polymerase chain reaction genotyping of resistant T0 plantlets. M, molecular marker; +, plasmid DNA; W, water control; −, non-transformed regenerated plantlets; 1–6, independent T0 plantlets. (L) Immunoblot analysis of green fluorescent protein (GFP) accumulation. M, protein marker; −, non-transformed regenerated plant; +, GFP protein; 1, a randomly selected transgenic T0 plantlet. (M) Regeneration and transformation frequency of broomcorn millet. (N) Targeted mutagenesis of PmBR2. (O) Morphological characterization of wild type and pmbr2 in Longmi4. (P) Statistical analysis of plant height. (Q) Seed morphology of wild type and pmbr2 in Longmi4. (R) Statistical analysis of 100-grain weight of the indicated genotypes. Each black circle represents the height of an independent plant in (P) and (R), as measured from the ground to the top of the plant. Values are means ± SEM (n ≥ 3). Statistical differences were analyzed using Student's t-test; ns: P > 0.05; ***P < 0.001.
To evaluate the efficiency of this system, we counted the number of infected calli that formed shoots: we reached a regeneration frequency of 3.5%–10% (Figure 1M). Genotyping polymerase chain reaction (PCR) and immunoblot assays identified hpt-positive T0 events among all infected calli to calculate the transformation frequency of our system (Figure 1K, L). We determined that the transformation frequency was 2.5%–5.3%, with an average transformation frequency of 4.0% (Figure 1M). Thus, the Agrobacterium-mediated genetic transformation system of broomcorn millet presented in this study is stable and efficient.
Dwarfism or semidwarfism can make crops more resistant to lodging and allow denser planting, ultimately achieving higher harvest indices (Hedden, 2003; Salamini, 2003; Wang et al., 2018). Broomcorn millet plants can usually reach 2 m or more in height, making them prone to lodging, which will affect yield. The loss of BRACHYTIC2 (BR2) function in maize (Zea mays L.) is characterized by compact lower stalk internodes, leading to shorter plant stature (Multani et al., 2003). Therefore, we used the clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated nuclease 12.3 (Cas12i.3)-based gene editing system with the binary vector pBUE411 to generate dwarf broomcorn millet genotypes via de novo domestication (Figure S1B). We identified two genes homologous to ZmBR2 in broomcorn millet: PmBR2-a (longmi056344) and PmBR2-b (longmi046759), located on chromosome 9 and chromosome 18, respectively.
We designed one single guide RNA (sgRNA) in the homologous domain of PmBR2-a and PmBR2-b, targeting both the genes, and cloned it into the CRISPR/Cas12i.3 vector. We infected 123 calli with Agrobacterium cultures harboring each binary vector. We obtained five hpt-positive calli, of which four were edited at both target sites. We selected T2 homozygous and free mutants for phenotypic investigation (Figure 1N). A phenotypic investigation of plant height in wild type and pmbr2 mutants indicated that the average plant height of pmbr2-1 (pmbr2-a pmbr2-b-1) and pmbr2-2 (pmbr2-a pmbr2-b-2) mutant lines is 163.3 and 161.4 cm, respectively, thus significantly smaller than the average plant height of wild type at 192.1 cm (Figure 1O, P). Moreover, consistent with the published roles of ZmBR2, pmbr2 mutants mainly affected plant height by shortening the length of their internodes, while the number of stems did not change (Figure S2A, B). In addition, we examined grain size and measured 100-grain weight. Excitingly, grain size and 100-grain weight were comparable between the wild type and the pmbr2 mutant lines (Figure 1Q, R).
In our present study, we established a stable and effective Agrobacterium-mediated genetic transformation system and CRISPR/Cas12i.3 gene editing platform for broomcorn millet. Their implementation allowed the successful generation of the first gene-edited germplasm in broomcorn millet with significantly lower plant height, which will greatly contribute to the improvement of agronomic traits and the cultivation of new broomcorn millet varieties. Moreover, this system will simultaneously support the editing of multiple genes to produce new varieties containing multiple excellent traits, a direction that needs more investments in the future. We hope to optimize our genetic transformation method of weedy broomcorn millet and to use the gene editing system established in this study to generate de novo domesticated broomcorn millet germplasm with excellent characteristics and suitable for modern agricultural production.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (32271541), the National Key Research and Development Program of China (2021YFD1200701), Key Project of Maize Germplasm Improvement (2022010202, B21HJ0509). This research is supported by Pinduoduo-China Agricultural University Research Fund (PC2023A01004).
CONFLICTS OF INTEREST
There are no conflict of interest to declare relevant to the contents of this article.
AUTHOR CONTRIBUTIONS
W.S., Y.L., and S.L. conceived and designed the study. Y.B.H. and Y.B. performed most of the research. S.L. drafted the manuscript. H.Z.N., H.Z.M., and J.L. guided the experiments. Y.B.H. carried out the genetic transformation of broomcorn millet, and phenotypic investigation. Y.B. and Z.X. performed the data analysis. S.L., Y.L., and W.S. revised the manuscript. All of the authors read and approved the manuscript.