From genes to networks: The genetic control of leaf development
Edited by: Zhizhong Gong, China Agricultural University, China
Abstract
Substantial diversity exists for both the size and shape of the leaf, the main photosynthetic organ of flowering plants. The two major forms of leaf are simple leaves, in which the leaf blade is undivided, and compound leaves, which comprise several leaflets. Leaves form at the shoot apical meristem from a group of undifferentiated cells, which first establish polarity, then grow and differentiate. Each of these processes is controlled by a combination of transcriptional regulators, microRNAs and phytohormones. The present review documents recent advances in our understanding of how these various factors modulate the development of both simple leaves (focusing mainly on the model plant Arabidopsis thaliana) and compound leaves (focusing mainly on the model legume species Medicago truncatula).
INTRODUCTION
Leaves are the main photosynthetic organs in plants and leaf morphology varies greatly among species and within different developmental stages and growth conditions (Tsukaya, 2014). Leaves can be simple or compound organs, depending on the leaf blade, which can be entire or dissected into leaflets (Conklin et al., 2019). In most plant species, the leaf is a typically flat lateral organ and differentiates into the upper (adaxial) and the lower (abaxial) surface. The upper surface facilitates light capture, whereas the lower surface facilitates gas exchange and regulation of transpiration.
Leaves are initiated at the shoot apical meristem (SAM) as simple rod-like primordia, which later grow along three-dimensional axes: adaxial–abaxial, proximal–distal, and medio-lateral axes (Satterlee and Scanlon, 2019a). In most dicotyledonous species, such as Arabidopsis thaliana, once the leaf polarity is established, the leaf blade undergoes cell proliferation and expansion to acquire its final size and shape (Yamaguchi et al., 2010; Du et al., 2018; Maugarny-Cales and Laufs, 2018). Here, we review the genetic mechanisms that control leaf development, with an emphasis on the regulatory modules characterized in the simple leaf plant A. thaliana and compound leaf plant Medicago truncatula.
LEAVES ARE INITIATED AT THE SAM
Leaf primordia are initiated from a pool of pluripotent cells lying in the peripheral zone of the SAM (Reddy et al., 2004; Barton, 2010). The WUSCHEL (WUS) homeobox gene, which is expressed in the organizing center (OC), is a key regulator for the maintenance of the stem cell niche in SAM (Laux et al., 1996; Mayer et al., 1998; Baurle and Laux, 2005; Yadav et al., 2010). Mutations in WUS result in the premature termination of the shoot meristem and lack the leaf primordia (Laux et al., 1996; Mayer et al., 1998). In M. truncatula, the WUSCHEL homolog HEADLESS (HDL) plays conserved roles in the SAM and the axillary meristem maintenance (Meng et al., 2019; Wang et al., 2019). However, HDL is expressed in leaf primordia and loss-of-function mutants develop ectopic terminal and lateral leaflets (LTs) (Wang et al., 2019), indicating that HDL have experienced some neofunctionalization over the course of evolution.
Two well conserved mechanisms are known to be involved in the process of leaf primordia initiation: the first depends on the antagonistic relationship between the Class I KNOTTED1-like homeobox proteins (KNOXI) and the ASYMMETRIC LEAVES1 (AS1)/ROUGH SHEATH2 (RS2)/PHANTASTICA (ARP) MYB-domain transcription factors (Tsiantis et al., 1999; Byrne et al., 2000; Lin et al., 2003; Lodha et al., 2013), while the second requires the auxin efflux transporter PIN-FORMED1 (PIN1) polarization, which ensures that auxin is delivered to the sites where leaf primordia initiate (Reinhardt et al., 2003; Jönsson et al., 2006; Smith et al., 2006). In addition to the various transcription factors and phytohormones involved, it has also been shown that specific mechanical cues are also influential (Sampathkumar et al., 2014; Traas, 2017; Jiao, 2019; Du and Jiao, 2020).
The role of transcription factors during leaf initiation
The KNOXI transcription factors SHOOT APICAL MERISTEMLESS (STM), KNOTTED IN ARABIDOPSIS THALIANA (KNAT)1, KNAT2, and KNAT6 are all expressed in the SAM, where they act, in part redundantly, to maintain the SAM (Figure 1) (Barton and Poethig, 1993; Long et al., 1996; Belles-Boix et al., 2006). The loss-of-function mutant stm fails to produce a SAM, and thus remains leafless (Long et al., 1996). During the process of leaf initiation, KNOXI transcription factors are down-regulated at the site of leaf primordium initiation (Jackson et al., 1994; Long et al., 1996). In species with simple leaves, downregulation of KNOXI genes is permanent, whereas in most compound-leafed species, including tomato and Cardamine hirsuta, KNOXI expression is reactivated in leaflet primordia (Hake et al., 2004; Hay and Tsiantis, 2010). Similar to simple leaf species, in legume species belonging to the IRLC, which include M. truncatula and pea, KNOXI genes are permanent down-regulated at the incipient sites of leaf primordia initiation (Hofer et al., 2001; Wojciechowski et al., 2004; Champagne et al., 2007; Zhou et al., 2014). The repression of KNOXI genes in the leaf primordium depends on the presence of ARP transcription factors (Figure 1). AS1 is specifically expressed in cells where a primordium is forming (Byrne et al., 2002). The A. thaliana loss-of-function mutant as1 forms asymmetrically lobed leaf blades with a shortened petiole (Ori et al., 2000; Sun et al., 2002). The AS2 protein, which harbors a LOB domain, interacts with AS1 to form a complex able to repress the two genes KNAT1 and KNAT2 (both encoding KNOXI proteins) by binding to their promoter (Semiarti et al., 2001; Guo et al., 2008; Lodha et al., 2013). Misexpression of KNOXI genes in the leaf primordium results in the formation of both deeply serrated or strongly lobed leaves and ectopic meristems (Ori et al., 2000). Conversely, STM represses AS1 expression in the SAM (Byrne et al., 2000), thus creating two mutually exclusive domains. In M. truncatula, loss of function in KNOX1, KNOX2, or KNOX6 do not lead to obvious defects in SAM maintenance and leaf morphology. Plants with simultaneous disruption of three KNOXI genes show normal leaves, indicating that STM/BP-like KNOXI genes are not involved in compound leaf patterning (Zhou et al., 2014). Furthermore, the repression of STM/BP-like KNOXI genes in vegetative stage leaves is not mediated by PHAN, and no suppression of PHAN by STM/BP-like KNOXI genes, implying that the developmental process of the compound leaf has shifted away from the KNOXI-ARP mediated module in M. truncatula. Interesting, ectopic STM/BP-like KNOXI activity is sufficient for increasing leaf complexity in M. truncatula (Zhou et al., 2014).
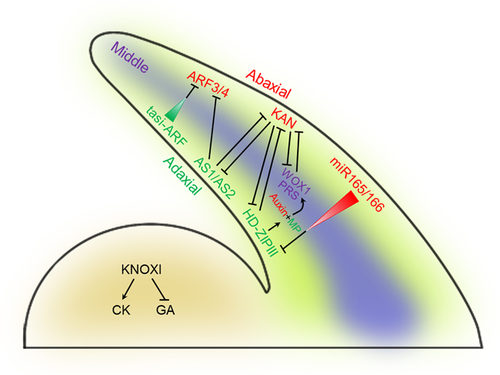
Regulatory network controlling leaf polarity
The developing leaf primordium has three domains which are determined by domain specific transcription factors. AS1/AS2, HD-ZIP III, and tasi-ARF transcripts accumulate in the adaxial domain of the leaf primordium, ARF3/ARF4, KAN, and miR165/166 transcripts accumulate in the abaxial domain, while WOX1/PRS expresses in the middle domain of the leaf primordium. Mature tasi-ARF and miR165/166 RNAs are capable of moving intercellularly and repressing ARF3/ARF4 and HD-ZIP III postranscriptionally. KAN and HD-ZIP III genetically antagonize one another and AS1/AS2 can genetically repress ARF3/ARF4 and KAN. The expression of WOX1 and PRS are repressed by KAN, in turn, KAN are also genetically repressed by WOX1 and PRS. Adaxial-expressed MP and abaxial-enriched auxin together position WOX1 and PRS expression in the middle domain. Furthermore, MP may be a direct target of adaxially expressed HD-ZIPIIIs.
The role of auxin during leaf initiation
The phytohormone auxin regulates many developmental processes. During leaf initiation, local auxin concentration maxima can form in the L1 layer of the SAM, mediated by the polar-localized PIN1 efflux transporter; sites of heightened auxin concentration coincide with where leaf primordia initiate and are thus assumed to promote their formation (Reinhardt et al., 2003; Heisler et al., 2005; de Reuille et al., 2006; Li et al., 2007; Dong and Huang, 2018). In the pin1 mutant, leaf initiation is compromised, but can be restored by treatment with exogenous auxin (Reinhardt et al., 2000; Vernoux et al., 2000; Guenot et al., 2012). When the process of polar auxin transport is disrupted by treating plants with N-1-naphthylphthalamic acid, leaf initiation is blocked (Reinhardt et al., 2000); meanwhile, exposing the SAM periphery with the auxin indole-3-acetic acid induces the formation of a primordium (Reinhardt et al., 2000, 2003). In addition, auxin and AS1 pathway converge to repress KNAT1 expression and promote the initiation of leaf primordia (Hay et al., 2006), indicating that regulatory interactions between auxin, AS1, and KNOX activities play important roles in directing leaf initiation and development. Through their ability to stabilize PIN1-mediated auxin patterning, members of the auxin influx carrier AUXIN1/LIKE-AUX (AUX1/LAX) family are also involved in controlling both the localization and initiation of leaf primordia (Bainbridge et al., 2008; Robert et al., 2015). The product of YUC participates in the determination of phyllotaxy (the arrangement of nascent leaves around the stem) (Cheng et al., 2007). Postembryonic leaf initiation is completely abolished in the yuc1yuc4pin1 triple mutant.
The influence on the process of leaf initiation by mechanical force
In addition to transcription factors and hormones, mechanical forces also influence lateral organ initiation and patterning (Dumais, 2007; Beauzamy et al., 2015; Landrein et al., 2015; Du and Jiao, 2020; Wang and Jiao, 2020a). In the SAM, auxin transport and accumulation, mediated by PIN1, are regulated by mechanical stress (Nakayama et al., 2012). When there is differential auxin gradient and growth, plasma membrane senses the mechanical stress and translates the stress into cellular responses, such as the intracellular localization of membrane-embedded proteins (Nakayama et al., 2012). The mechanical stress can stabilize the amount and localization of PIN1 within the cell and PIN1 localization is also responsive to stress reorientation (Stoma et al., 2008; Heisler et al., 2010; Nakayama et al., 2012; Jiao, 2019). Finally, auxin promotes cell expansion by loosening cell wall, at least partially, through pectin de-methylesterification and isotropic microtubule arrays, which all enable leaf primordium initiation (Hamant et al., 2008; Peaucelle et al., 2011; Braybrook and Peaucelle, 2013; Sassi et al., 2014; Qi et al., 2017; Armezzani et al., 2018). After initiation, the leaf primordium establishes initial bilaterally symmetric structure, which is under the control of mechanical forces and polarity genes. Cells in leaf primordium can sense the main force direction along each wall and orient their cortical microtubules (CMTs) parallel to this direction, leading to directional cell expansion and organ shape deformation (Sampathkumar, 2020; Wang and Jiao, 2020a; Zhao et al., 2020b). Notably, CMT-directed deposition of cellulose microfibrils (CMFs) reduces directional cell expansion (Dyson and Jensen, 2010; Zhao et al., 2020b). Subsequently, microtubule-mediated mechanical feedback amplifies this initial flatness to generate the highly anisotropic growth of the blade (Wang and Jiao, 2020a; Zhao et al., 2020b). Further, margin-expressed genes promote blade outgrowth in concert with the microtubules (Jiao et al., 2020; Zhao et al., 2020b).
THE ESTABLISHMENT AND MAINTENANCE OF LEAF POLARITY
Once a leaf primordium has been initiated, a peg-like structure develops, which expands and differentiates along its adaxial–abaxial, proximal–distal, and medio-lateral axes to finally form a leaf of characteristic shape and size.
Adaxial-abaxial polarity
Leaf adaxial–abaxial polarity is maintained by a series of specific transcription factors and microRNAs (Figure 1). Cell identity on the adaxial surface depends on the activity of a set of functionally redundant class III homeodomain-leucine zipper (HD-ZIPIII) transcription factors, including REVOLUTA (REV), PHABULOSA (PHB), and PHAVOLUTA (PHV) (McConnell et al., 2001; Emery et al., 2003). Gain-of-function mutations to these genes result in the plants forming adaxialized leaves, while their loss-of-function has the opposite effect (McConnell and Barton, 1998; Emery et al., 2003). On the adaxial side of the leaf, the accumulation of transcript generated from these HD-ZIPIII genes is regulated by the degradative action of the two microRNAs miR165 and miR166 (Kidner and Martienssen, 2004; Chitwood et al., 2007). In conjunction with the AS1-AS2 complex, the products of these transcription factors (which are able to interact with REV) act to promote adaxial identity (Husbands et al., 2015; Merelo et al., 2016). In contrast, cell fate on the abaxial side of the leaf depends on the product of members of the KANADI (KAN) family, along with various AUXIN RESPONSE TRANSCRIPTION FACTORs (ARFs). KAN genes encode proteins harboring a GARP domain and are expressed in the abaxial domain (Eshed et al., 2001; Kerstetter et al., 2001). Plants of genotype kan1kan2kan3 produce adaxialized leaves, whereas over-expression of the individual KAN genes induces abaxialization (Eshed et al., 2001, 2004; Emery et al., 2003). Abaxial fate is mediated by a combination of KAN and auxin (Pekker et al., 2005). KAN products promote abaxial fate while those of the key HD-ZIPIII genes promote adaxial fate (Chitwood et al., 2007; Izhaki and Bowman, 2007; Bowman and Floyd, 2008). ETTIN (ETT) (syn. ARF3) and ARF4 are both expressed on the abaxial side of the developing leaf, where their products contribute to determining abaxial identity (Pekker et al., 2005; Guan et al., 2017). The leaf blades formed by arf3arf4 plants produce outgrowths on their abaxial surface, a phenotype which resembles that of kan mutants (Pekker et al., 2005). The expression in abaxial tissue of both ARF3 and ARF4 is negatively regulated by the product of tasiR-ARF (Fahlgren et al., 2006; Garcia et al., 2006; Hunter et al., 2006; Chitwood et al., 2009), as shown by the hyperaccumulation of ARF3 and ARF4 transcript in the leaves of plants in which tasiR-ARF has been inactivated. The repression imposed by the AS1-AS2 complex on a number of abaxially acting genes (including ETT, ARF4, KAN2, and MIR166A) has been taken to indicate that adaxial–abaxial specification depends on an antagonistic interaction (Iwakawa et al., 2002; Ishibashi et al., 2012; Iwasaki et al., 2013; Husbands et al., 2015). Proteins encoded by genes belonging to the YABBY (YAB) family also contribute to leaf adaxial–abaxial polarity and are required for specification of the leaf marginal region: four members—namely YAB1, YAB2, YAB3, and YAB5—are expressed in the abaxial side and marginal domain of the leaf primordium, and their expression is promoted by abaxial factors such as KAN and ARF3/4 (Sawa et al., 1999; Siegfried et al., 1999; Bowman, 2000; Eshed et al., 2004; Garcia et al., 2006; Sarojam et al., 2010). Plants carrying a loss-of-function allele at all four of these YAB genes show reduced lamina growth but only limited polarity defects, indicating that YAB genes act downstream of the network establishing leaf polarity to promote lamina formation (Waites and Hudson, 1995; Stahle et al., 2009; Sarojam et al., 2010). However, YAB expression is restricted to the central domain of leaf primordia in sorghum and Juncus prismatocarpus, implying that these YAB genes function in lamina formation that is independent of polarity establishment (Ishikawa et al., 2009; Yamaguchi et al., 2010).
Proximal-distal polarity
Growth along the proximal–distal axis determines the length of the leaf. The two genes BLADE-ON-PETIOLE1 (BOP1) and BOP2, which both harbor a BTB/POZ domain and several ankyrin repeats, redundantly regulate the leaf's proximal–distal patterning (Ha et al., 2003, 2004; Hepworth et al., 2005). Both the single bop1 and the bop1bop2 double mutants exhibit blade growth on the petiole, diminishing the petiole domain (proximal domain) (Ha et al., 2003; Hepworth et al., 2005). BOP1/2 genes are expressed at the proximal and adaxial side of leaf primordia and promote leaf cell determinacy by inducing AS2 and repressing KNOXI (Ha et al., 2003; Jun et al., 2010). BOP1/2 proteins not only directly activate AS2 but also function redundantly with AS1 and AS2 to pattern the proximal–distal axis (Ha et al., 2007). Changes in the activity of BOP1/2 also affect the expression of the two polarity genes PHV and KAN1 (Ha et al., 2003, 2007). The products of ROTUNDIFOLIA3 (ROT3) and ROT4 also regulate proximal–distal polarity. The former gene encodes a member of the cytochrome P450 family. Its involvement in brassinosteroid (BR) synthesis is significant since this phytohormone regulates leaf length by controlling polar cell expansion in the proximal–distal direction (Kim et al., 1998, 2005). Meanwhile, ROT4 encodes a small peptide. Plants carrying the rot4-1D dominant mutation develop short leaves as a result of the inhibition of cell proliferation along the proximal–distal axis (Narita et al., 2004; Ikeuchi et al., 2010). In addition, two homologous genes, LONGIFOLIA1 (LNG1) and LNG2, are redundantly involved in regulating the leaf's proximal–distal development (Lee et al., 2006). The lng1-1D mutants produce narrow but extremely long leaf blades due to increased cell elongation rather than increased cell proliferation. The lng1 and lng2 mutants shows slightly decreased leaf length, however, the lng1-3 lng2-1 double mutant shows further decreased leaf length. Furthermore, genetic analysis of the lng1-1D rot3-1 double mutant and lng1-3 lng2-1 rot3-1 triple mutant indicating that LNG1 and LNG2 promote proximal–distal axis cell elongation independently of ROT3.
Medio-lateral polarity
Growth along the medio-lateral axis, sometimes referred to as the middle domain, determines the width of the leaf. The two most important regulators of medio-lateral growth are encoded by PRESSED FLOWER/WUSCHEL-LIKE HOMEOBOX3 (PRS/WOX3) and WOX1 (Figure 1) (Vandenbussche et al., 2009; Nakata et al., 2012); their products act redundantly to promote medio-lateral growth while repressing adaxial and abaxial fate (Nakata and Okada, 2012; Nakata et al., 2012). The double mutant wox1prs produces very narrow leaves (Nakata et al., 2012). A functional copy of WOX1 (syn. STENOFOLIA or STF) is required for both the normal outgrowth of the leaf blade and the ordered development of the leaf's vasculature in M. truncatula (Tadege and Mysore, 2011; Tadege et al., 2011a, 2011b). The product of this gene acts primarily as a transcriptional repressor, interacting with TOPLESS family co-repressors to down-regulate various target genes during the morphogenesis of the leaf blade (Lin et al., 2013a, 2013b; Zhang et al., 2014; Zhang and Tadege, 2015). The transcriptional co-repressor MtLUG and the transcriptional co-activator MtAN3 form a complex, and genetically interact with STF to regulate leaf blade outgrowth (Zhang et al., 2019). In addition, ectopic expression of MtWOX9 enhances the stf mutant phenotypes, indicating that WOX9 functions antagonistically to STF during the leaf blade's development (Wolabu et al., 2020). The loss-of-function of WOX3 (syn. LOOSE FLOWER or LFL) does not affect the development of the leaf, but leads to sepal/petal fusion (Niu et al., 2015). Similar to Arabidopsis WOX1-WOX7 repressor genes, LFL acts as a transcriptional repressor able to functionally replace STF during the development of either the flower or the leaf, provided that the gene is driven by the STF promoter (Lin et al., 2013a; Niu et al., 2015). Furthermore, WOX5 acts redundantly with WOX1 and PRS to control leaf growth (Zhang et al., 2020b). Loss of WOX5 in wox1wox3 double mutants led to a further reduction in leaf size and enhanced the narrow leaf defect (Zhang et al., 2020b). WOX1, 3, and 5 promote auxin synthesis and ectopic YUC1 expression partially restores the leaf size, which links the WOX homeobox proteins and the hormone auxin in the context of controlling leaf growth (Zhang et al., 2020b). In addition, WOX1 and WOX3 genes are repressed by KAN and activated by MP (Nakata et al., 2012; Nakata and Okada, 2012; Caggiano et al., 2017). In the kan1kan2 double mutant, WOX1 and PRS are expressed in the abaxial domain (Nakata et al., 2012). WOX1 and PRS are activated in the marginal and middle domains in response to the product of the adaxially expressed gene MONOPTEROS (MP) and abaxially enriched auxin, while the abaxially expressed ARF repressors ETT, ARF2, and ARF4 all act to suppress WOX1 and PRS expression (Qi et al., 2014; Guan et al., 2017). Dominant-negative MP expression plants produce narrow leaves, a phenotype resembling that of the wox1prs double mutant (Pekker et al., 2005; Guan et al., 2017). In plants lacking functional copies of ETT, ARF2 and ARF4, WOX1, and PRS are ectopically expressed in the leaf primordium abaxial domain (Guan et al., 2017). Furthermore, WOX1 and PRS genes are activated by HD-ZIPIII genes. The expression of the HD-ZIPIII gene REV substantially overlaps with the expression of PRS (Guan et al., 2019). PHB, possibly together with other HD-ZIPIII TFs, focus and stabilize the auxin response by directly activating MP, through which activates WOX1 and PRS expression in middle domain (Muller et al., 2016; Guan et al., 2017; Bhatia et al., 2019).
THE REGULATION OF LEAF SIZE AND GROWTH
Following initiation and polarity establishment, the leaf primordium begins to expand until it has acquired its final shape and size. The molecular machinery underlying this progression balances cell proliferation with cell expansion. Initially, cells proliferate throughout the entire leaf primordium, but with time, a transition to cell expansion occurs, following a cell cycle arrest front that migrates towards the leaf base before abruptly disappearing. Several regulators of this process have been identified, including phytohormones, transcription factors and microRNAs.
Phytohormones regulating leaf size
Auxin influences both cell proliferation and cell expansion. Firstly, it induces the gene AUXIN REGULATED GENE INVOLVED IN ORGAN SIZE (ARGOS) along with a group of SMALL AUXIN UP RNA (SAUR) genes, over-expression of which produces plants exhibiting larger than normal leaves (Hu et al., 2003; Spartz et al., 2012). Secondly, it regulates both the AP2-domain transcription factor AINTEGUMENTA (ANT), a controller of cell proliferation, and the D-type cyclin CYCD3;1 (Mizukami and Fischer, 2000). The disturbance to auxin homeostasis and signaling achieved by over-expressing PINOID (PID) results in plants which develop smaller than normal leaves (Saini et al., 2017). Finally, the loss-of-function of ARF2 promotes cell proliferation, thereby increasing leaf size (Okushima et al., 2005; Schruff et al., 2006). The growth of the leaf blade is also promoted by the two phytohormones gibberellin (GA) and BR (Figure 2). GA promote leaf growth by increasing cell proliferation and expansion, since GA synthesis or signaling loss-of-function mutants produce leaves of reduced size, whereas plants over-expressing GA20ox1 produce larger than normal leaves (Phillips et al., 1995; Huang et al., 1998; Eriksson et al., 2000; Richards et al., 2001; Ueguchi-Tanaka et al., 2005, 2007; Mitchum et al., 2006). The positive effect of GA on cell proliferation likely involves the repression of KIP-RELATED PROTEIN 2 (KRP2) and SIAMESE (SIM) (Achard et al., 2009). Similarity, in M. truncatula, loss of DWARF AND INCREASED BRANCHING 1 (DIB1) (syn. MtGA3OX1) function also leads plants to produce small leaves due to decreased cell length, whereas plants over-expressing MtGA20ox1 produce larger leaves, implying the mechanism by which GA regulates leaf size is conserved among different species (Wang et al., 2020; Zhang et al., 2020a). Meanwhile, BR promotes leaf growth via its positive effect on both cell proliferation and cell expansion. BR-deficient or -insensitive mutants form small leaves with short petioles, whereas the leaves of both BR gain-of-function mutants and BR receptor BR INSENSITIVE 1 (BRI1) over-expressors are larger than normal (Clouse et al., 1996; Li et al., 2001; Cheon et al., 2010; Gonzalez et al., 2010; Oh et al., 2011; Shang et al., 2011; Zhiponova et al., 2013). Finally, the phytohormone cytokinin (CK) is also involved in the regulation of leaf size (Figure 2), since genetically altering the leaf's CK content by manipulating the expression of genes such as IPT or HvCKX2 demonstrates that this phytohormone maintains cells in proliferation mode by blocking their transition to cell expansion (Werner et al., 2001; Černý et al., 2013; Skalák et al., 2019).
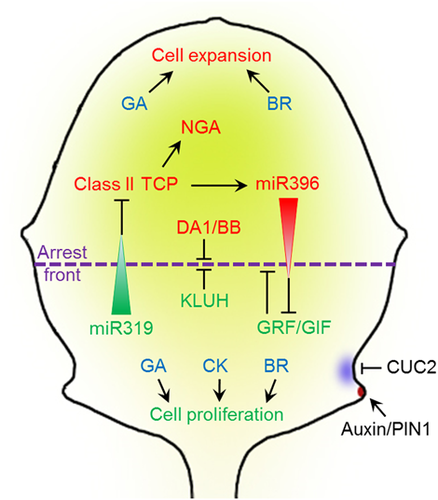
Regulation of leaf growth and leaf margin development
Two main cellular processes control final leaf size: cell proliferation and cell expansion. The switch from cell proliferation to differentiation follows a basipetal gradient during leaf development. TCP transcription factors are negatively regulated by miR319 and promote cell expansion. Meanwhile, GRF/GIF transcription factors also promote cell proliferation and are negatively regulated by miR396. TCP activates miR396 expression and thus forms a feedback loop to regulate the shift of the cell cycle arrest front. The NGA genes are activated by TCP to promote cell expansion, while DA1 and BB control the timing of cell proliferation. Additionally, KLUH and cytokinin (CK) promote cell proliferation. Other phytohormones, such as gibberellin (GA) and brassinosteroid (BR) have a positive influence on both cell proliferation and cell expansion. During leaf margin development, CUC2 promotes the establishment of PIN1 convergence points which in turn generates auxin maxima at the tip of serrations along leaf margin. The auxin maximum represses CUC2 at the serration tip and promotes tooth growth.
The miR319-TEOSINTE BRANCHED, CYCLOIDEA and PCF1/2 (TCP) module
Class II CINCINNATA-TCPs, which belong to the TCP family of plant-specific transcription factors (Martin-Trillo and Cubas, 2010), are involved in switching cells from the proliferation to the differentiation mode. In plants carrying a loss-of-function mutation for some of the encoding genes, the mitotic arrest front is delayed, leading to the formation of larger leaves with over-proliferation at their margin (Figure 2) (Nath et al., 2003; Efroni et al., 2008; Bresso et al., 2018). The five genes TCP2, TCP3, TCP4, TCP10, and TCP24, which all encode proteins involved in the regulation of cell proliferation, are each targeted by miR319 (Palatnik et al., 2003). Plants in which the expression of MIR319a is enhanced form large, crinkled leaves (Palatnik et al., 2003). The phenotype of many tcp mutants features an increase in both the size and curvature of the leaf, achieved via the up-regulation of genes encoding cyclins (Schommer et al., 2008; Bresso et al., 2018). The effect of miR319-targeted TCPs on leaf size regulation is enhanced by the other three TCP5-like CIN-related genes (Efroni et al., 2008). Meanwhile, plants in which the miR319-insensitive gene TCP4 is over-expressed form relatively small, narrow leaves, as a result of a reduction in cell number (Schommer et al., 2008, 2014; Sarvepalli and Nath, 2011). TCP4 also directs cell fate from proliferation to differentiation, both through its promotion of the auxin response and its activation of HAT2 (Challa et al., 2019).
Leaf expansion in A. thaliana is controlled by a TCP-NGA regulatory module
Leaf expansion is also under the control of a TCP-NGATHA (NGA) regulatory module (Figure 2). The functionally redundant NGA genes positively regulate the localized synthesis of auxin. Their loss-of-function results in the formation of wider than normal leaves (Ballester et al., 2015; Alvarez et al., 2016), while their over-expression results in plants forming both smaller and narrower leaves (Kwon et al., 2009; Trigueros et al., 2009). Both TCP2 and TCP3 are able to activate the expression of NGA genes by binding to a conserved region within their promoter sequences. Mutants which lack both TCP and NGA functionality form deeply lobed leaves with a crinkled margin (Ballester et al., 2015; Alvarez et al., 2016).
The miR396-GROWTH-REGULATING FACTOR (GRF) and GRF-INTERACTING FACTOR (GIF) (GRF-GIF) regulatory module
GRFs, which share both a QLQ and a WRC domain, represent a further class of proteins involved in the regulation of leaf growth (Figure 2). These proteins are highly abundant in meristematic tissue, where they positively regulate leaf size by promoting cell proliferation (Kim et al., 2003a). Plants carrying a grf mutation form small, narrow leaves characterized by a reduced number of cells, whereas GRF over-expressors tend to form over-sized leaves (Kim et al., 2003a; Horiguchi et al., 2005; Kim and Lee, 2006). Exceptionally, GRF9 functions as a negative regulator of leaf growth by controlling the gene ORG3 and hence restricting cell proliferation (Omidbakhshfard et al., 2018). Complexes between GRF and a GIF transcriptional co-activator allow the recruitment of the SWI2/SNF2 complex into the GRF regulatory region (Kim and Kende, 2004; Horiguchi et al., 2005). The expression pattern of GIFs (which promote cell proliferation) resembles that of GRFs. GIF1 over-expressors exhibit enlarged leaves, whereas gif loss-of-function mutants produce small leaves containing a reduced number of cells (Kim and Kende, 2004; Lee et al., 2009). The triple gif1gif2gif3 mutant is severely compromised with respect to overall growth (Lee et al., 2009). The expression of several GRF genes is post-transcriptionally targeted by miR396 (Rodriguez et al., 2010; Debernardi et al., 2012, 2014). The over-expression of miR396 suppresses GRF, resulting in a reduced leaf size and cell number, whereas either the inactivation of miR396 or the over-expression of miR396-resistant GRF genes induce the plant to develop larger than normal leaves (Liu et al., 2009; Rodriguez et al., 2010; Wang et al., 2010). TCP4 directly activates the expression of MIR396B, and thus is antagonistic to GRF transcription; this interaction links the miR319/TCP and miR396/GRF modules in the context of controlling cell proliferation and leaf shape (Rodriguez et al., 2010; Schommer et al., 2014).
The DA1 and PEAPOD pathway
An additional source of control over leaf size is provided by the ubiquitin receptor DA1, the E3 ubiquitin ligase DA2, and ENHANCER OF DA1-1 (EOD1) (syn. BIG BROTHER or BB), which act to limit the duration of cell proliferation (Figure 2). The da1-1 allele encodes a mutant DA1 protein (DA1R358K) that has a negative effect on DA1 and DA1-related (DAR1) and plants carrying the da1-1 mutation or simultaneous disruption of DA1 and DAR1 form out-sized leaves (Li et al., 2008; Dong et al., 2017). Both DA2 and EOD1 are able to monoubiquitinate DA1, which activates the peptidase activity of DA1. The active DA1 peptidase can then cleave diverse growth regulators, including DA2 and EOD1, to regulate organ growth. Both eod1 and da2-1 mutants have large leaves, indicating that EOD1 and DA2 are negative regulators of organ size (Li et al., 2008; Xia et al., 2013; Dong et al., 2017). The deubiquitinase SUPPRESSOR2 OF DA1 (SOD2) (syn. UBIQUITIN-SPECIFIC PROTEASE15 or UBP15) acts downstream of DA1; the over-expression of UBP15 results in an enlarged leaf phenotype which resembles that of the da1-1 mutant (Du et al., 2014). KLUH encodes yet another regulator of leaf size (Figure 2); its product, a cytochrome P450 monooxygenase accumulates at the base of a developing organ (Anastasiou et al., 2007; Adamski et al., 2009; Kazama et al., 2010; Stransfeld et al., 2010). Mutations in KLUH result in the premature arrest of cell proliferation, leading to the formation of leaves of reduced size; plants over-expressing KLUH form larger than normal leaves harboring more than the usual cell number.
BIG SEEDS1 (BS1) encodes a plant-specific transcription regulator that plays a critical role in seed and leaf size determination in M. truncatula (Ge et al., 2016). BS1 is homologous to Arabidopsis PEAPOD1 (PPD1) and PPD2, which regulate organ size by promoting meristemoid cell proliferation (White, 2006). PPD proteins form a repressor complex with KINASE-INDUCIBLE DOMAIN INTERACTING (KIX) proteins and TOPLESS, and plants mutation in PPD or KIX8/9 display larger and dome-shaped leaves resulting from increased meristemoid cell proliferation (Gonzalez et al., 2015). The PPD/KIX complex is polyubiquitinated and degraded by the F-box protein STERILE APETALA (SAP) containing SCF-complex, in M. truncatula by SMALL LEAF AND BUSHY 1 (SLB1), leading to activation of downstream target gene, such as CYCD3;2 and CYCD3;3 (Wang et al., 2016; Baekelandt et al., 2018; Li et al., 2018; Yin et al., 2020). Interestingly, SAP was identified as SUPPRESSOR OF DA1 (SOD3), indicating an interaction between the DA1 and PPD pathways (Li et al., 2008; Wang et al., 2016).
THE DEVELOPMENT OF THE COMPOUND LEAF
Two major types of leaf (simple and compound) have long been recognized. While simple leaves comprise a single, undivided surface, compound ones are formed by a number of discrete leaflets. The two most frequent forms of compound leaves are pinnate (leaflets lying on either side of a common axis) and palmate (lobes radiating from a common point), although other higher-order structures also exist. Both developmental and environmental cues influence the morphogenesis of compound leaves.
The KNOXI-APR module
Compound leaf primordia are initiated from the periphery of the SAM. In some compound leaf plants, KNOXI is reactivated in developing primordia after its early down-regulation. Thus, in both tomato and the A. thaliana relative C. hirsuta, the mutation or suppression of KNOXI results in a reduction in the number of leaflets per leaf formed (Hay and Tsiantis, 2006; Shani et al., 2009), whereas in both the dominant Mouse-ear and Curl tomato mutants, as well as in C. hirsuta plants constitutively expressing KN1, leaflet number is much larger than normal; the latter observation is consistent with the hypothesis that KNOXI activity is not only necessary but also sufficient for leaflet initiation (Hareven et al., 1996; Parnis et al., 1997; Janssen et al., 1998; Hay and Tsiantis, 2006, 2009, 2010). The antagonism between ARP and KNOXI is also important for the development of the compound leaf. In C. hirsuta, mutation of the AS1 homolog results in an altered pattern of KNOXI expression, leading to the formation of a higher than normal leaflet number (Rast-Somssich et al., 2015). The pea (Pisum sativum) crispa mutant (affecting the PHAN protein) develops stipules on its petiole-rachis axis, a site also associated with ectopic KNOXI expression (Tattersall et al., 2005; DeMason and Chetty, 2014). The down-regulation of the tomato homolog of PHAN results in a switch from pinnate into palmate compound leaves, and is accompanied by a reduced leaflet number (Kim et al., 2003b; Zoulias et al., 2012). The compound leaf formed by the Medicago truncatula phan mutant is characterized by a shortened petiole and a loss of leaflet symmetry; however, the repression of STM/BP-like KNOXI in the leaf is not mediated by PHAN (Ge et al., 2014; Zhou et al., 2014).
Auxin is required for leaflet initiation and marginal patterning
Auxin coordinates phyllotaxis and regulates the process of leaflet initiation (Bilsborough et al., 2011; Xiong and Jiao, 2019). In M. truncatula, the product of LATERAL LEAFLET SUPPRESSION 1 (LLS1) participates in auxin synthesis (Zhao et al., 2020a). The lls1 mutant is compromised with respect to the outgrowth of LTs, an observation which has been taken to imply that LLS1 is required for the development of the lateral but not the terminal leaflets (TLs) (Zhao et al., 2020a). In tomato, the effect of treating with exogenous auxin or of over-expressing a bacterial auxin synthesis gene is to revert the leaf from compound to simple (Ben-Gera et al., 2012). PIN1-mediated auxin transport is an important controlling factor for the morphogenesis of compound leaves. The formation of the leaf as a whole, as well as of individual leaflets is inhibited in C. hirsuta pin1 mutants (Barkoulas et al., 2008). Meanwhile, in M. truncatula, the leaves of the slm1 mutant exhibit an increase in complexity and a decrease in marginal patterning, suggesting a manifold effect of auxin on leaf patterning (Peng and Chen, 2011; Zhou et al., 2011). The plant's response to exogenous auxin is typically mediated by ARF and Aux/IAA proteins. Mutation of the tomato ENTIRE (E) gene, which encodes an Aux/IAA family repressor, results in the formation of simple leaves (Wang et al., 2005; Zhang et al., 2007; Koenig et al., 2009). Mutants carrying disabled alleles of the ARF encoding genes SlMP, SlARF19A, and SlARF19B all develop smaller leaf blades while suppressing the e phenotype in a dosage dependent manner, leading to a continuum of leaf shape (Israeli et al., 2019). Furthermore, the over-expression of SlARF10A, SlARF10B, or SlARF17 induces an increase in leaf complexity, which suggests that the various auxin signal antagonists act cooperatively to ensure leaflet separation (Hendelman et al., 2012; Ben-Gera et al., 2016). Ta-siRNAs are known to regulate some ARFs, and so are also involved in leaf patterning. In M. truncatula, disrupting the TAS3 pathway induces lobing at the leaf margin but does not affect the level of leaf complexity (Zhou et al., 2013). In contrast, the failure of the tomato ta-siRNA program induces the formation of narrow simple leaves (Yifhar et al., 2012). Although disrupting auxin distribution affects leaflet formation and marginal patterning, the mechanistic basis of this response clearly varies from species to species.
CK and GA regulate the development of the compound leaf
In addition to auxin, both CK and GA strongly influence the development of compound leaves (Bar and Ori, 2015; Shwartz et al., 2016). The former phytohormone helps to maintain the activity of the leaf's marginal blastozone. In tomato, altering tissue CK content by ectopic expressing AtIPT7 induces an increase in leaf complexity, while the opposite is the case in transgenic plants expressing AtCKX3 (Shani et al., 2010). Conversely, expression of AtCKX3 in tomato leaves led to the production of simplified leaves that made only primary leaflets. Furthermore, ectopic AtIPT7 expression is able to partially rescue the phenotype of the Tkn2-SRDX transgenic plant, suggesting that this phytohormone acts downstream of KNOXI during the elaboration of compound leaves (Shani et al., 2010). GA negatively regulates leaf complexity in tomato by shortening the morphogenetic stage. The leaf form of tomato plants either exposed to exogenous GA or lacking a functional DELLA protein is to block the formation of intercalary leaflets; this observation has been taken to imply that an excessive GA content or a heightened GA response delays leaflet initiation (Bassel et al., 2008; Jasinski et al., 2008; Fleishon et al., 2011; Yanai et al., 2011; Shwartz et al., 2016). Moreover, the super-compound leaf phenotype of the Mouse-ear mutant is suppressed by GA treatment, supporting the notion that GA signaling acts downstream of KNOXI (Hay et al., 2002).
Molecular genetics of compound leaf development
A forward genetic screen directed at leaflet formation in C. hirsuta succeeded in identifying RCO (Sicard et al., 2014; Vlad et al., 2014), a gene which has no homolog in A. thaliana. The gene is thought to have evolved from a duplicate copy of LATE MERISTEM IDENTITY 1 (LMI1). Unlike LMI1, however, RCO is specifically expressed at the leaflet base, representing an example of neofunctionalization. The effect of expressing RCO in A. thaliana is to increase leaf complexity, implying that its loss during speciation was key to the simple leaf phenotype of this species. An analysis of the upstream sequences of RCO and LMI1 has demonstrated that a specific enhancer element in RCO altered leaf shape by changing gene expression from the distal leaf blade to its base (Vuolo et al., 2016; Nikolov et al., 2019). Moreover, a single residue substitution appears to reduce the stability of RCO, thereby minimizing the pleiotropic effect of its altered expression (Vuolo et al., 2016). In addition to RCO, the formation of C. hirsute compound leaves also requires the presence of KNOXI (Hay and Tsiantis, 2006; Rast-Somssich et al., 2015). STM promotes the growth of leaf primordia and thus the emergence of leaflets, while RCO inhibits growth at the base of a developing leaflet, thereby accentuating the growth differences created by marginal patterning (Kierzkowski et al., 2019). Like STM, RCO regulates growth through its orchestration of CK homeostasis (Hajheidari et al., 2019; Hudson, 2019). Modulating both local and global growth by co-expressing RCO and STM in the A. thaliana leaf is sufficient to convert a simple, serrate A. thaliana leaf into a compound C. hirsuta leaf, demonstrating the impact of growth and patterning on the diversity of leaf shape (Kierzkowski et al., 2019; Satterlee and Scanlon, 2019b).
In legume species belonging to the IRLC, the function of KNOXI is carried out by homologs of LEAFY (Hofer et al., 1997, 2001; Wojciechowski et al., 2004; Champagne et al., 2007). The compound leaves formed by the pea mutant uni, which lacks a functional copy of the LEAFY homolog UNIFOLIATA, are simplified (Hofer et al., 1997). Similarly, the loss-of-function of SINGLE LEAFLET1 (SGL1) results in the conversion of adult leaves from trifoliate to single blade, indicating that LEAFY orthologs take over roles in compound leaf patterning in IRLC (Wang et al., 2008). However, constitutive expression of SGL1 fails to increase leaf complexity, indicating that KNOXI and SGL1 are functionally unequal, and SGL1 may regulate parallel pathways with KNOXI in compound development (Zhou et al., 2014).
A forward genetic screen carried out in M. truncatula identified a mutant, named palmate-like pentafoliata1 (palm1), which develops a compound leaf with five leaflets, a TL and two pairs of LLs (Chen et al., 2010). The mutagenized gene PALM1 encodes a Cys(2)His(2) zinc finger transcription factor harboring an EAR motif, suggesting that PALM1 functions as a repressor. A detailed analysis showed that in the mutant, a pair of extra leaflet primordia is initiated at an early stage of primordium development, indicating that PALM1 suppresses morphogenetic activity in the proximal region of the compound leaf primordium and is required to ensure the formation of a trifoliate leaf. While the abundance of SGL1 transcript is higher in the palm1 mutant than in wild type, the palm1sgl1 double mutant produces simple rather than compound leaves; the implication is that SGL1 is epistatic over PALM1 and that the supernumerary leaflets formed by the palm1 mutant is due to an upregulation of LL primordium morphogenetic activity. The presence of REV1 (syn. PINNATE PENTAFOLIATA1 or PPF1) is necessary for ensuring both adaxial–abaxial polarity and compound leaf patterning (Zhou et al., 2019). About 85% of the leaves formed by plants lacking a functional copy of this gene develop four or five leaflets and exhibit a partial loss in adaxial identity. The over-expression of an miR166-resistant allele of REV1 results in adaxialized leaves and the formation of supernumerary leaflets along the dorsoventral axis. The conclusion of a micro-array based comparison between the transcriptomes of this genotype and a rev1 mutant was that REV1 is involved in auxin homeostasis and functions in a number of pathways modulated by auxin. In the pinnate-like pentafoliata1 (pinna1) mutant, around 64% of the adult leaves form five leaflets (two pairs of pinnately arranged LLs and a TL); the additional pair of LLs appears to be initiated from TL primordium (He et al., 2020; Wang and Jiao, 2020b). The indication is that PINNA1 encodes a BELL-like homeodomain transcription factor which harbors an EAR motif. Since the gene product functions solely in the TL, the suggestion is that it acts to suppress TL morphogenetic activity during leaf morphogenesis.
CONCLUSIONS AND PERSPECTIVES
A great deal of progress has been made towards identifying the genes underlying the regulation of leaf development, largely based on the use of mutants. The coverage here of the development of the simple (rather than the compound) leaf has focused mostly on the model species A. thaliana. An important determinant of leaf patterning is the establishment of a PIN1-mediated auxin concentration gradient, the formation of which precedes the initiation of leaf primordia in the SAM and leaflet primordia in leaf marginal blastozones. A major genetic determinant is represented by Class I KNOX genes. KNOXI proteins regulate the balance between GA and CK in the meristem and in leaf founder cells, while the repression of KNOXI in lateral organs is required to drive the development of the leaf. A critical priority remains to elucidate how spatiotemporal activation and/or reiteration of conserved gene regulatory networks has led to the diversification of leaf shape. Many key regulators of compound leaf development have also been identified, but a number of important issues as yet remain unresolved. It is uncertain, for example, how the FLO/LEAFY and KNOXI pathways evolved in the legumes. The identification of upstream regulators, functional protein complexes and downstream effectors will be required to shed more light on the determination of species-specific leaf developmental events. The search for enhancers and repressors in mutants such as palm1 and sgl1 will represent an important means of further expanding our understanding of the regulatory network underlying the development of compound leaves.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Natural Science Foundation of China (31871459 and 31671507) and Shandong Province (ZR2020KC018 and ZR2019MC013), China Postdoctoral Science Foundation (2019M662836), Project for Scientific Research Innovation Team of Young Scholar in Colleges and Universities of Shandong Province (2019KJE008), and Project for innovation and entrepreneurship leader of Qingdao (19-3-2-3-zhc).
AUTHOR CONTRIBUTIONS
H.W. drafted the manuscript. F.K. and C.Z. revised the manuscript. All authors read and approved of its content.






