Resveratrol inhibits hepatic stellate cell activation by regulating autophagy and apoptosis through the SIRT1 and JNK signaling pathways
Jing Zhang and Jian Ping contributed equally.
Abstract
Resveratrol, which is a natural polyphenol found in grapes, berries, peanuts, and medicinal plants, has previously been reported to perform several biological functions, including inhibition of hepatic fibrosis. Activated hepatic stellate cells (HSCs) are the major cellular source of matrix protein-secreting myofibroblasts, which are the major drivers of liver fibrogenesis. Numerous studies on the protective effects of resveratrol against liver fibrosis have focused on the inhibition of HSC activation. Although the underlying mechanisms remain to be fully elucidated, the regulation of autophagy and apoptosis might be intimately related. The mouse HSC line JS1 was stimulated with resveratrol to assess the mechanism and relationship between autophagy and apoptosis. Resveratrol modulated JS1 cell viability in a dose-dependent manner. Moreover, resveratrol inhibited JS1 cell activation and induced autophagy and apoptosis. This antifibrotic effect was attenuated when autophagy was inhibited using chloroquine (CQ) or 3-methyladenine (3-MA) or when apoptosis was inhibited using Z-VAD-FMK. Furthermore, whether the Sirtuin1 (SIRT1) and c-Jun N-terminal kinase (JNK) signaling pathways were associated with the resveratrol-mediated induction of autophagy and apoptosis in JS1 cells was examined. The SIRT1 inhibitor EX527 reversed autophagy, and the JNK inhibitor SP600125 reversed both autophagy and apoptosis induced by resveratrol. These findings suggest that the SIRT1 and JNK signaling pathways may be involved in the resveratrol-mediated inhibition of HSC activation by regulating autophagy and apoptosis. SIRT1 may be responsible for inducing autophagy, while JNK affects both autophagy and apoptosis. This study highlighted autophagy and apoptosis as therapeutic targets by which resveratrol can attenuate fibrosis.
Practical applications
Resveratrol, which is a natural polyphenol found in grapes, berries, peanuts, and medicinal plants, has previously been reported to inhibit hepatic fibrosis. Since activated HSCs are the major drivers of liver fibrogenesis, many studies on the anti-hepatic fibrosis effects of resveratrol have focused on inhibiting HSC activation. The objective of this study was to evaluate the inhibitory effect of resveratrol on HSC activation and focused on the mechanism by which resveratrol modulated autophagy and apoptosis in JS1 cells, a mouse immortalized HSC line. It was shown that resveratrol inhibited HSC activation by inducing autophagy and apoptosis in a dose-dependent manner, and the mechanism may be associated with the SIRT1 and JNK signaling pathways. This study highlighted autophagy and apoptosis as therapeutic targets by which resveratrol can attenuate fibrosis. These findings may provide a new framework for understanding the mechanism by which resveratrol inhibits HSC activation.
1 INTRODUCTION
Hepatic fibrosis is a dynamic process characterized by the net accumulation of extracellular matrix (ECM), and it results from chronic liver injury of any etiology, including viral infection, alcoholic liver disease, and nonalcoholic steatohepatitis (Tsuchida & Friedman, 2017). A key discovery in understanding fibrosis has been that hepatic stellate cells (HSCs) are the primary effector cells that orchestrate the deposition of ECM in normal and fibrotic livers (Lee & Friedman, 2011). HSCs are localized in the subendothelial space of Disse, interposed between liver sinusoidal endothelial cells and hepatocytes. Following liver injury or liver cell culture in vitro, HSCs become activated, transdifferentiating from vitamin Astoring cells to myofibroblasts, which are proliferative, contractile, inflammatory, and chemotactic cells that are characterized by enhanced ECM production (Tsuchida & Friedman, 2017). In normal liver, laminins, type IV collagen, and a mixture of proteoglycans are scattered within the hepatic ECM. In contrast, fibrilforming type I and III collagens are abundant in fibrotic liver. HSCs express two types of collagen receptors, each of which receives signals from ECM components to regulate cell adhesion, differentiation, proliferation, and migration (Tsuchida & Friedman, 2017). Therefore, activated HSCs are the major drivers of liver fibrogenesis (Higashi, Friedman, & Hoshida, 2017). Inhibiting the proliferation and clearance of activated HSCs remains the major therapeutic strategy for liver fibrosis (Meira Martins et al., 2015).
Resveratrol (3,4′,5-trihydroxy-trans-stilbene), a natural polyphenol found in grapes, berries, peanuts, and medicinal plants, has several biological activities, including anticarcinogenic, antiaging, antioxidant, anti-inflammatory, and lipid modulating activities (Ahmadi, Hayes, & Karimi, 2021; Chupradit, Bokov, Zamanian, Heidari, & Hakimizadeh, 2021). Moreover, numerous studies have shown that resveratrol has a dose-dependent effect and can exert opposite effects in a variety of diseases depending on the concentration, time of exposure, and cell type (Lee, Kim, Lee, & Kim, 2020; Lieben Louis et al., 2019; Martins et al., 2014). Recent reports have shown that resveratrol can prevent or slow the progression of various fibrotic diseases, including cardiac fibrosis (Zivarpour, Reiner, Hallajzadeh, & Mirsafaei, 2022), pulmonary fibrosis (Wang et al., 2021), renal fibrosis (Liu et al., 2019), prostate fibrosis (Vicari et al., 2020), and systemic sclerosis (Yao et al., 2020). In liver fibrosis, resveratrol provides significant protection against fibrosis induced by carbon tetrachloride (CCl4) (Li, Zhang, Zhan, & Zheng, 2021; Ma et al., 2022), dimethylnitrosamine (Abdu & Al-Bogami, 2019), bile duct ligation (ShamsEldeen et al., 2021), and Schistosoma japonicum (Chen et al., 2019). In fact, resveratrol reduces portal pressure, HSC activation, inflammatory cell infiltration of liver tissue, and fibrosis and improves hepatic endothelial function in cirrhotic rats (Izzo et al., 2021). Numerous in vitro studies on the effects of resveratrol against liver fibrosis have focused on the inhibition of HSC activation. Although the underlying mechanisms remain to be fully elucidated, autophagy and apoptosis regulation might be intimately related.
Autophagy and apoptosis are functionally distinct mechanisms of cytoplasmic and cellular turnover (Bata & Cosford, 2021). Autophagy is an essential catabolic degradation process in which cellular proteins, organelles, and invading microbes are engulfed by double-membraned autophagosomes and degraded in lysosomes (Mizushima, Levine, Cuervo, & Klionsky, 2008). Basal autophagy occurs in all types of cells; however, uncontrolled autophagy leads to programmed cell death (Mizushima et al., 2008). Apoptosis is widely appreciated as a major mechanism of regulated death, which may be triggered by extrinsic stimuli through cell surface death receptors or by intrinsic stimuli via the mitochondrial signaling pathway. Caspase activation results in mitochondrial membrane permeabilization, chromatin condensation, and DNA fragmentation, thereby leading to the destruction of the cell (Nikoletopoulou, Markaki, Palikaras, & Tavernarakis, 2013). The two pathways are regulated by common factors, and each can regulate and modify the activity of the other. The crosstalk between autophagy and apoptosis is complex and determines cell death or survival and the progression of liver disease (Wang, 2015). Recently, it has been reported that resveratrol could modulate autophagy (Zhu, Mou, Wang, Zhu, & Cheng, 2020) and apoptosis (Li et al., 2021) as the main forms of HSC death through various signaling pathways.
SIRT1 is a member of the class III histone deacetylase family that is distinctively dependent on nicotinamide adenine dinucleotide (NAD+) for catalytic reactions (Yousafzai, Jin, Ullah, & Wang, 2021); SIRT1 modulates gene expression, apoptosis, energy homeostasis, autophagy, acute stress responses, and mitochondrial biogenesis (Tovar-Palacio, Noriega, & Mercado, 2022). Resveratrol is an agonist of SIRT1, so the activation of SIRT1 signaling is considered a key mechanism for its effects on a variety of diseases (Chen et al., 2022; Gomes et al., 2018; Nishigaki, Tsubokura, Tsuzuki-Nakao, & Okada, 2022). Additionally, studies have also shown that the JNK pathway is a potential target of resveratrol (Shati, 2019; Wang et al., 2018). JNKs are among the most crucial mitogen-activated protein kinases (MAPKs), and they have been shown to play roles in apoptotic and nonapoptotic programmed cell death, including necroptosis, ferroptosis, pyroptosis, and autophagy (Dhanasekaran & Reddy, 2017).
The therapeutic effect of resveratrol on hepatic fibrosis and the possible roles of autophagy and apoptosis induction in this process are not yet clear. In the present study, we aimed to evaluate the inhibitory effect of resveratrol on HSC activation and focused on the mechanism by which resveratrol modulated autophagy and apoptosis by activating the SIRT1 and JNK signaling pathways in JS1 cells, a mouse immortalized HSC line. These findings may provide a new framework for understanding the mechanism by which resveratrol inhibits HSC activation.
2 MATERIALS AND METHODS
2.1 Cells and cell culture
The immortalized mouse HSC line JS1 was kindly provided by Professor Scott L. Friedman (Mt. Sinai School of Medicine). JS1 cells were cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin at 5% CO2 and 37°C. The medium was replaced with media supplemented with 0.5% FBS for 12 hr prior to treatment. Resveratrol (cat. no. R5010; Sigma–Aldrich; Merck KGaA) was dissolved in ethanol (cat. no. 459836; Sigma–Aldrich; Merck KGaA) to a stock concentration of 100 mM and diluted sequentially in DMEM. Different concentrations of resveratrol were added to the medium and incubated for different durations, according to the experimental design. 3-Methyladenine (3-MA; cat. no. M9281; Sigma–Aldrich; Merck KGaA) was dissolved in DMEM and used at a concentration of 5 mM. Chloroquine (CQ) diphosphate salt (cat. no. C6628; Sigma–Aldrich; Merck KGaA) was dissolved in DMSO to a stock concentration of 30 mM. Z-VAD-FMK (cat. no. C1202; Beyotime Institute of Biotechnology) dissolved in DMSO to a stock concentration of 20 mM. JS1 cells were pretreated with 5 mM 3-MA, 30 μM CQ, or 20 μM Z-VAD-FMK for 2 hr and 10 μM EX527 (cat. no. ab141506; Abcam) or 10 μM SP600125 (cat. no. ab120065; Abcam) for 1 hr, followed by treatment with or without 50 μM resveratrol for 24 hr. Each in vitro experiment was repeated three times.
2.2 Measurement of JS1 cell viability
Cells were plated in plastic 96-well plates and incubated with various concentrations of resveratrol for 24 hr, with or without pretreatment with 3-MA (5 mM) or Z-VAD-FMK (20 μM) for 2 hr. Subsequently, cell viability was assessed using an MTT Cell Proliferation and Cytotoxicity Assay kit (cat. no. C0009; Beyotime Institute of Biotechnology) according to the manufacturer's protocol. The supernatant was removed after the cells were treated, and 90 μL of fresh culture medium and 10 μL of MTT solution were added and incubated for 4 hr at 37°C. The cell supernatant was then discarded, and 100 μL of formazan was added with gentle shaking for 10 min. The absorbance was recorded at a wavelength of 490 nm with a microplate reader (SpectraMax M5; Molecular Devices LLC).
2.3 Western blotting
JS1 cells were prepared for electrophoresis as previously described (Lv et al., 2010). After being resolved, the protein concentration was measured using a bicinchoninic acid protein assay kit (cat. no. P0012; Beyotime Institute of Biotechnology). Proteins were separated by SDS–PAGE (5% stacking gel, 10 or 15% separating gel; 30 μg of protein in 15 μL of volume per lane) and subsequently transferred to a PVDF membrane. The membrane was blocked in blocking buffer (cat. no. 927-40,000; LI-COR Biosciences) for 1 hr at room temperature and incubated with primary antibodies overnight at 4°C. Subsequently, the membrane was incubated with a fluorescently tagged secondary antibody in the dark at room temperature for 45 min and measured at a wavelength of 800 nm (780 nm excitation, 820 nm detection) or 700 nm (680 nm excitation, 720 nm detection) with an Odyssey Infrared Imaging System (Odyssey; LI-COR Biosciences) according to the manufacturer's instructions. ImageJ 6.0 (National Institutes of Health) was used for quantification. The following antibodies were used: rabbit anti-collagen type I (Col. I; cat. no. ab292; Abcam), rabbit anti-microtubule-associated protein 1 light chain 3B (LC3B; cat. no. L7543; Sigma–Aldrich; Merck KGaA), rabbit anti-p62 (cat. no. 5114; Cell Signaling Technology, Inc.), rabbit anti-cleaved caspase3 (cat. no. 9664; Cell Signaling Technology, Inc.), rabbit anti-c-Jun N-terminal kinase (JNK) (cat. no. 9258; Cell Signaling Technology, Inc.), rabbit anti-phosphorylated (p)-JNK (cat. no. 4671; Cell Signaling Technology, Inc.), rabbit anti-Sirtuin1 (SIRT1) (cat. no. sc-15,404, Santa Cruz) (all diluted 1:500) and mouse anti-GAPDH (cat. no. KC-5G4; 1:10,000; KangChen BioTech Co., Ltd.). The secondary antibodies used were as follows: IRDye 680RD goat anti-rabbit (cat. no. 926–68,071; LI-COR Biosciences) and IRDye 800CW donkey anti-mouse (cat. no. 926–32,212; LI-COR Biosciences) (1:10,000 dilution).
2.4 RFP-GFP-LC3B transduction
JS1 cells were transfected with RFP-GFP-LC3B viral particles using a Premo Autophagy Tandem Sensor RFP-GFP-LC3B kit (Invitrogen; Thermo Fisher Scientific, Inc.) at a multiplicity of infection (MOI) of 30 for 24 hr at 37°C and subsequently treated with resveratrol (10 or 50 μM) for 12 hr. Treated cells were fixed with 4% paraformaldehyde for 30 min at room temperature, and the autophagic flux was observed using a confocal microscope (FV10C-PSU; Olympus Corporation).
2.5 Reverse transcription-quantitative polymerase chain reaction (RT–qPCR)
RT–qPCR was performed as previously described to determine the gene expression levels in the cultured cells (Liu, Hu, Wang, Liu, & Xu, 2000). Total RNA was extracted from the cells using a nucleic acid purification kit (cat. no. NPK-201; Toyobo Life Science), and cDNA was synthesized using a RevertAid First Strand cDNA Synthesis kit (cat. no. K1622; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. Total cDNA was mixed with PCR master mix (cat. no. DRR041A; Takara Bio, Inc.) and predesigned primers. The following primer pairs (synthesized by Sangon Biotech Co., Ltd.) were used for qPCR: Atg7 forward, 5′-GGAGAAGAACCAGAAAGGAGGC-3′ and reverse, 5′-GCAGGCACTTGACAGACACGAC-3′; Beclin1 forward, 5′-CTTACCACAGCCCAGGCGAA-3′ and reverse, 5′-AGATGCCTCCCCGATCAGAG-3′; Bcl-2 forward, 5′-TGTGGAGAGCGTCAACAGGG-3′ and reverse, 5′-AGACAGCCAGGAGAAATCAAACAGA-3′; and GAPDH forward, 5′-AAGGTCATCCATGACAACTTTGGC-3′ and reverse, 5′-ACAGTCTTCTGGGTGGCAGTGAT-3′. GAPDH was used as the internal reference gene. The thermocycling conditions used for qPCR were as follows: initial denaturation for 1 min at 95°C, followed by 40 cycles of 15 s at 95°C and 30 s at 60°C. Amplification was performed on a ViiA7 system (Thermo Fisher Scientific, Inc.). Gene expression was normalized to that of GAPDH using the 2−ΔΔCq method (Livak & Schmittgen, 2001).
2.6 Statistical analysis
Data were collected in triplicate from at least three separate cell cultures. The results are expressed as the mean ± SEM of three repeats. Differences among groups were compared using one-way anova followed by Tukey's post-hoc test or a t test. SPSS 17.0 (SPSS, Inc.) was used to perform statistical analyses. p < .05 was considered to indicate a statistically significant difference.
3 RESULTS
3.1 Resveratrol inhibits JS1 cell activation
Resveratrol is well known to exert cytotoxic effects by inducing death in various cell types. JS1 cells were used in our study, and we examined the effect of various concentrations of resveratrol on JS1 cells. As shown in Figure 1a, cell viability was significantly reduced after 24 hr of treatment with higher doses (50, 100 μM) of resveratrol. Conversely, lower doses (0.1, 1, and 10 μM) slightly promoted cell viability. These findings indicated that resveratrol dose-dependently affected the viability of JS1 cells and even exerted opposing effects at different doses.
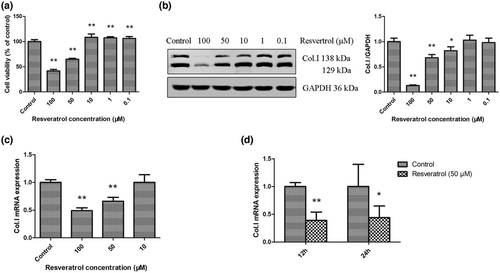
Moreover, we observed that higher doses (50, 100 μM) of resveratrol significantly inhibited the protein and mRNA expression of Col. I in JS1 cells compared with controls (Figure 1b–d), and these results were consistent with previous studies showing that higher doses of resveratrol inhibited cell viability. However, these results were inconsistent when the concentration was 10 μM. As shown in Figure 1b,c, 10 μM resveratrol significantly inhibited the protein expression of Col. I but had no effect on Col. I mRNA, which may be attributed to resveratrol promoting the degradation of intracellular Col. Taken together, these findings were consistent with those of previous reports (de Oliveira et al., 2021; Martins et al., 2014; Zhang et al., 2016), showing that resveratrol had a dose-dependent effect on HSC viability and inhibited HSC activation at higher doses.
3.2 Resveratrol inhibits HSC activation by inducing autophagy and apoptosis
Studies have shown that resveratrol-induced autophagy or apoptosis regulates fibrosis in multiple types of fibroblasts, including fibroblast-like synovial cells (Cao et al., 2018), primary mouse embryonic fibroblasts (Ulakcsai, Bagaméry, Szökő, & Tábi, 2018), human dermal fibroblasts (Choi et al., 2013), keloid fibroblasts (Si et al., 2020) and rat cardiac fibroblasts (Lieben Louis et al., 2019). We sought to determine whether resveratrol also induced autophagy and apoptosis in JS1 cells. As shown in Figure 2a, the expression of the autophagy marker LC3BII was significantly increased in JS1 cells treated with lower doses (0.1, 1, 10 μM) of resveratrol for 12 hr. Moreover, the expression of the apoptotic marker cleaved caspase3 was increased in response to higher doses (50, 100 μM) of resveratrol. Interestingly, higher and lower concentrations induced the opposite effects on LC3BII and cleaved caspase3. Subsequently, whether there was an association between autophagy and apoptosis in HSCs was assessed.
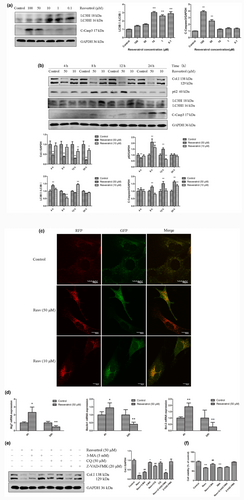
JS1 cells were treated with 50 and 10 μM resveratrol for 4 and 12 hr, which decreased in Col. I expression (Figure 2b). Furthermore, we observed that compared with the control, 50 μM resveratrol significantly increased LC3BII and inhibited p62 expression at 4 hr and increased cleaved caspase 3 expression after 8 hr. Resveratrol (10 μM) significantly increased LC3BII and inhibited p62 expression at 12 hr and increased cleaved caspase 3 expression at 24 hr. These results suggested that resveratrol induced autophagy and apoptosis successively at different times, autophagy preceded apoptosis, and the effect of initiating autophagy and apoptosis was postponed by decreasing the resveratrol concentration. Moreover, JS1 cells were transfected with a viral vector containing RFP-GFP-LC3B to confirm that treatment with resveratrol increased the expression of LC3BII. The immunofluorescence data indicated a significant increase in punctate LC3BII staining in JS1 cells treated with resveratrol. Under physiological conditions, autophagic activity remains at a very low level, leading to yellow puncta being diffusely distributed in the cytoplasm. However, treatment with resveratrol increased the immunofluorescence staining of autophagosomes (yellow) and autolysosomes (red) compared with the control (Figure 2c). The presence of yellow and red puncta in the cytoplasm indicated that resveratrol increased autophagosome synthesis. Collectively, these data support the notion that resveratrol induces autophagy in JS1 cells.
In addition, JS1 cells were treated with 50 μM resveratrol for 4 and 24 hr, and the PCR results are shown in Figure 2d. Compared with those in the controls, the mRNA expression levels of autophagy-related genes (Atg7, Beclin1) and an antiapoptotic gene (Bcl-2) were significantly upregulated at 4 hr and downregulated at 24 hr. Taken together, these results suggested that resveratrol induced autophagy prior to apoptosis in JS1 cells. Autophagy is upstream of apoptosis because autophagy may be an adaptive stress response prior to apoptotic cell death (Wang, 2015).
It was hypothesized that resveratrol inhibits HSC activation by inducing autophagy and apoptosis. Therefore, blocking autophagy or apoptosis may attenuate the antifibrotic effects of resveratrol. JS1 cells were stimulated with 50 μM resveratrol in the presence of autophagy or apoptosis inhibitors, and the effects were assessed. The results suggested that treatment with 3-MA, CQ (pharmacological inhibitors of autophagy), or Z-VAD-FMK (pancaspase inhibitor) significantly attenuated the inhibitory effect of resveratrol on Col. I expression (Figure 2e). Moreover, 3-MA significantly attenuated the inhibitory effect of resveratrol on JS1 cell viability, while Z-VAD-FMK had no significant effect (Figure 2f). Therefore, inhibiting autophagy or apoptosis may reduce resveratrol-mediated inhibition of JS1 cell activation, suggesting that the induction of autophagy and apoptosis might be required for resveratrol-mediated inhibition of hepatic fibrosis.
3.3 Resveratrol regulates autophagy and apoptosis by activating the SIRT1 and JNK signaling pathways
The mechanisms involved in autophagy and apoptosis were next investigated. We hypothesized that the SIRT1 and JNK signaling pathways may be involved in resveratrol-induced autophagy and apoptosis in JS1 cells. JS1 cells were stimulated with resveratrol in the presence of the SIRT1 inhibitor EX527 and JNK inhibitor SP600125, and the effects were assessed to investigate the role of resveratrol-induced autophagy and apoptosis. Subsequently, the expression of Col. I, LC3BII, and cleaved caspase3 were examined by western blotting. As shown in Figure 3a, resveratrol treatment resulted in a significant decrease in the expression of the fibrosis marker Col. I, and the effect was partially reversed by treatment with the SIRT1 inhibitor EX527. Moreover, EX527 abrogated SIRT1 and LC3BII expression in the presence of resveratrol at 24 hr. However, the expression of cleaved caspase3 induced by resveratrol was increased by EX527. This finding suggested that resveratrol may partly induce autophagy to inhibit HSC activation via the SIRT1 pathway.
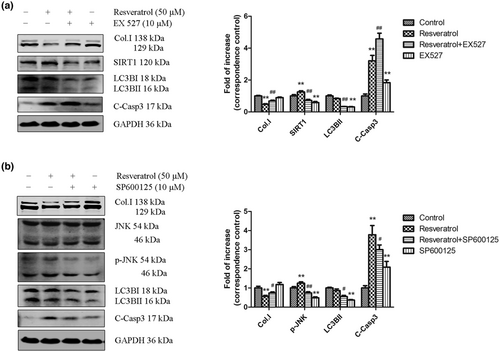
As shown in Figure 3b, the JNK inhibitor SP600125 reversed JNK phosphorylation and significantly decreased the expression of Col. I, LC3BII, and cleaved caspase3 in the presence of resveratrol. Resveratrol-induced autophagy and apoptosis were reduced by inhibition of the JNK signaling pathway. In conclusion, these findings suggest that SIRT1 may induce autophagy, while JNK affects both autophagy and apoptosis during resveratrol-mediated inhibition of HSC activation.
4 DISCUSSION
In the present study, it was shown that resveratrol induced both autophagy and apoptosis while inhibiting HSC activation. Furthermore, inhibiting autophagy with CQ and 3-MA or inhibiting apoptosis with Z-VAD-FMK reversed the antifibrotic effects of resveratrol. These results showed that resveratrol inhibited HSC activation by inducing autophagy and apoptosis in a dose-dependent manner, and the mechanism may be associated with the SIRT1 and JNK signaling pathways.
A large amount of evidence suggests that resveratrol exerts dose-dependent effects and even contradictory effects on various cell types or diseases. For example, in renal fibrosis, Liu et al. (2019) showed that resveratrol activated antifibrotic or profibrotic effects on kidneys depending on the dose. In vivo, low-dose treatment with resveratrol (≤25 mg/kg) partly improved renal function, whereas high-dose treatment with resveratrol (≥50 mg/kg) had no antifibrotic effect and even exacerbated renal fibrosis. In addition, resveratrol at concentrations >25–50 μM typically triggers growth arrest, senescence, and/or apoptosis in different cell types. In contrast, concentrations <10 μM enhance the growth of log phase cell cultures and can rescue senescence in multiple types of human fibroblasts (Birar, Sheerin, Ostler, & Faragher, 2020).
There are also conflicting reports on HSC activation due to different concentrations of resveratrol. Resveratrol (15 μM) upregulated the mRNA expression of α-smooth muscle actin (α-SMA) and amplified the profibrogenic effects of free fatty acids on LX-2 cells (human HSC line) (Bechmann et al., 2009). In contrast, resveratrol decreased t-HSC/Cl-6 cell (rat HSC line) viability at very low concentrations (1.56 μM) at 24 hr (Zhang et al., 2016). de Oliveira et al. (2021) suggested that resveratrol at 1 and 10 μM did not alter the protein levels of α-SMA, collagen I, and glial fibrillary acidic protein (GFAP) in GRX cells (mouse HSC line). Resveratrol at 10 and 50 μM decreased GRX cell migration and collagen I contraction. Consistent with previous studies, the results of the present study showed that resveratrol dose-dependently supported or inhibited JS1 cell proliferation (Figure 1a). Furthermore, the effect of resveratrol on HSCs was not only dose-dependent but also time dependent. Martins et al. (2014) reported that treating GRX cells with 50 μM resveratrol for 24 hr induced oxidative stress-related damage, drastically reducing cell viability, but this cytotoxicity seemed to be attenuated after 120 hr. The complex effects of resveratrol on promoting or preventing HSC activation may be dependent on different cell types, concentrations, and durations of treatment.
In the present study, resveratrol inhibited the mRNA and protein expression of Col. I. However, the results were inconsistent when JS1 cells were treated with 10 μM resveratrol. As shown in Figures 1a–c and 2a, 10 μM resveratrol promoted cell viability, increased LC3BII expression and significantly inhibited Col. I protein expression but had no effect on Col. I mRNA expression. Therefore, we concluded that resveratrol promoted intracellular Col. I degradation through autophagy rather than inhibiting its production in JS1 cells. Autophagy is an important protein degradation pathway. A large amount of research has shown that Col. I, which is the major component of the ECM, is degraded by the autophagy–lysosome system (Ishida et al., 2009; Kawano et al., 2017). For example, Kawano et al. (2017) showed that internalized collagen accumulated in autophagy-deficient murine embryonic fibroblasts. Kim et al. (2012) also found that reduced Beclin1 expression, through genetic disruption of Beclin1 or knockdown by specific siRNA in primary mouse mesangial cells, resulted in increased protein levels of Col.I. Moreover, autophagy exerts antifibrotic effects and enhances collagen degradation in intestinal fibroblasts (Cosin-Roger et al., 2019), cardiac fibroblasts (Liu et al., 2016), and lung fibroblasts (Ghavami et al., 2018). However, other studies reported contrasting results. Nakamura et al. (2021) showed that autophagy facilitated type I collagen synthesis in periodontal ligament cells. Additionally, inhibiting autophagy promoted collagen degradation in pancreatic stellate cells (Li et al., 2018). The complex role of autophagy in promoting or inhibiting collagen degradation may be dependent on different cell types.
Autophagy and apoptosis are well-controlled biological processes that determine cell death or survival and the development of diseases. Accumulating evidence reveals that autophagy and apoptosis can cooperate, antagonize, or assist each other, and the crosstalk between these processes is complex (Nikoletopoulou et al., 2013). As shown in Figure 2b, both high and low concentrations of resveratrol induced autophagy and apoptosis successively at different times. The autophagic response occurs earlier than apoptotic cell death, placing autophagy upstream of apoptosis. Autophagy may be an adaptive stress response prior to apoptotic cell death. Furthermore, autophagy can induce or antagonize apoptosis (Wang, 2015). Autophagy antagonizes apoptosis and promotes cell survival via a series of responses to damaged organelles, endoplasmic reticulum stress, DNA stability, or loss of nutrient and growth factor signaling pathways. Conversely, autophagic activation beyond a certain threshold may result in the collapse of cellular function, directly resulting in autophagic cell death or the execution of apoptotic cell death via common regulators (Wang, 2015). In conclusion, resveratrol induced autophagy and apoptosis in JS1 cells, and crosstalk may occur between these processes. Resveratrol increased autophagic flux and may directly induce autophagic cell death, while the overactivation of autophagy contributes to apoptotic cell death.
As previously reported, the SIRT1 signaling pathway is involved in the regulation of autophagy and apoptosis and has been studied as part of the mechanism of resveratrol in various diseases (Chen et al., 2022; Gomes et al., 2018; Nishigaki et al., 2022). Previous studies have shown that SIRT1 increases autophagic flux, and this effect may be mediated by the class III PI3K/Beclin1 and mTOR signaling pathways (Ghosh, McBurney, & Robbins, 2010; Yang, Jiang, Wang, & Guo, 2019; Yousafzai et al., 2021). Moreover, p53 deacetylation by SIRT1 has been confirmed to play a significant role in inhibiting apoptosis (Zhang et al., 2011). As shown in Figure 3a, our findings suggested that SIRT1 signaling induced autophagy to participate in the antifibrotic effects of resveratrol, which was probably attributable to autophagy-mediated promotion of Col. I degradation in HSCs. In addition, we observed that the SIRT1 inhibitor EX527 upregulated the expression of cleaved caspase3 induced by resveratrol in JS1 cells (Figure 3a). That is, resveratrol inhibited apoptosis by inducing autophagy through the SIRT1 signaling pathway, which may be attributed to autophagy antagonizing apoptosis. Autophagy may partially counteract apoptotic cell death via the cell survival pathway at an earlier stage. Similar results were recently reported in which resveratrol induced SIRT1-dependent autophagy to prevent apoptosis in VSC4.1 motoneurons (Tian et al., 2021), intestinal epithelial cells (Qin et al., 2021) and human trophoblasts (Wang et al., 2020).
JNK signaling may play critical roles in many biological diseases by regulating various cellular processes, including inflammatory responses, differentiation, proliferation, death, and survival (Chen et al., 2021). JNKs are among the most crucial MAPKs and include JNK1, JNK2, and JNK3. All three JNKs have been shown to be involved in stimulating apoptotic signaling (Dhanasekaran & Reddy, 2017). A general mechanism through which JNKs modulate the apoptotic pathway involves stimulating the expression of proapoptotic genes and decreasing the expression of prosurvival genes via multiple transcription factors. In addition to their effect on gene expression, JNKs actively regulate both the intrinsic and extrinsic apoptotic pathways (Dhanasekaran & Reddy, 2017). Furthermore, the JNK signaling pathway has also been reported to participate in autophagy regulation in response to various stress signals. JNK induces autophagic cell death by enhancing the expression of autophagy-related genes, including Atg5, Atg7, LC3, and Beclin1 (Dhanasekaran & Reddy, 2017). In the present study, the role of the JNK pathway in resveratrol-induced autophagy and apoptosis was assessed. We observed that the JNK inhibitor SP600125 could attenuate autophagy and apoptosis in JS1 cells induced by resveratrol. Resveratrol exerted antifibrotic effects by mediating the JNK pathway and inducing apoptosis and autophagy in HSCs. A similar result was reported in cardiac cells. Xu et al. (2018) reported that resveratrol protected cardiac cells by regulating the switch between autophagy and apoptotic machinery under diabetic conditions, and this effect was associated with JNK-mediated Beclin1-Bcl-2 dissociation. Bcl-2 is an important mediator of JNK-mediated autophagy and apoptosis activation. Activated JNK phosphorylates Bcl-2/Bcl-xL to release Beclin1 from the Beclin1-Bcl-2/Bcl-xL complex and promote autophagy. Sustained Bcl-2 phosphorylation not only leads to Beclin1-mediated autophagic cell death but also antagonizes the antiapoptotic activity of Bcl-2/Bcl-xL via a Bid-Bax-dependent mechanism (Chen et al., 2021; Fan & Zong, 2013; Wu et al., 2019).
5 CONCLUSION
In conclusion, the present study showed that resveratrol inhibited HSC activation by regulating autophagy and apoptosis by activating the SIRT1 and JNK signaling pathways. SIRT1 may be responsible for inducing autophagy, while JNK affects both autophagy and apoptosis. This study highlighted autophagy and apoptosis as therapeutic targets by which resveratrol can attenuate fibrosis. Additionally, these findings may provide a new framework for understanding the mechanism by which resveratrol inhibits HSC activation. However, autophagy and apoptosis may act independently on the SIRT1 and JNK pathways or may influence one another, which requires further study.
AUTHOR CONTRIBUTIONS
Jing Zhang: Investigation; Formal analysis; Validation; Visualization; Writing-original draft; Writing-review & editing. Jian Ping: Investigation; Project administration; Supervision; Validation; Writing-review & editing. Na Jiang: Formal analysis. Lieming Xu: Conceptualization; Funding acquisition; Project administration; Resources; Supervision.
ACKNOWLEDGMENTS
We would like to thank Professor Scott L. Friedman (Icahn School of Medicine at Mount Sinai, USA) for providing the immortalized mouse HSC line JS1.
FUNDING INFORMATION
This study was supported by the General Program of the National Natural Science Foundation of China (no. 81373859), the Youth Program of Shanghai Municipal Health Commission (no. 20204Y0046), and the Traditional Chinese Medicine Project of Shanghai Jiading District Health Commission (no. 2018-QN-ZYY-01).
CONFLICT OF INTEREST
The authors have no relevant financial or nonfinancial interests to disclose.
Open Research
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.




