Harpephyllum caffrum fruit (wild plum) facilitates glucose uptake and modulates metabolic activities linked to neurodegeneration in isolated rat brain: An in vitro and in silico approach
Abstract
Alteration in brain glucose metabolism due to glucose uptake reduction has been described in the onset of certain neurodegenerative disorders. This study determined Harpephyllum caffrum fruit's potential ability to improve glucose uptake and its modulatory effects on intrinsic antioxidant, glucogenic, cholinergic, and nucleotide-hydrolyzing enzyme activities in isolated rat brain. Consequently, the bioactive compounds of the fruits were identified with LC–MS. The fruit significantly improved brain glucose uptake following coincubation with glucose and brain tissue. The fruit extract also elevated GSH level, SOD, catalase, glycogen phosphorylase, and ENTPDase activities while simultaneously suppressing NO and malonaldehyde levels and fructose-1,6-bisphosphatase, ATPase, acetylcholinesterase and butyrylcholinesterase activities. LC–MS analysis revealed S-methylcysteine sulfoxide, dihydroquercetin, 3,4-dimethyl-2,5-bis(3,4,5-trimethoxyphenyl) tetrahydrofuran (MTHF), nobiletin, puerarin, quercetin 3-rutinoside, 8-D-glucosyl-4′,5,7-trihydroxyflavone, asperulosidic acid, 1,2,4,6-tetragalloylglucose, and phellamurin. This study suggests the neuroprotective effects of H. caffrum fruit due to its ability to enhance glucose uptake, attenuate glucose-induced oxidative stress while modulating glucogenic, cholinergic, and nucleotide-hydrolyzing enzyme activities in normal brain tissues.
Practical applications
Available scientific evidence describes oxidative stress as one of the physiological processes contributing to aging-associated neurodegeneration in humans. In this regard, commonly consumed natural products from plants have attracted much interest due to their ability to mitigate redox imbalance-related pathologies that affect various organs in the body such as the brain. Harpephyllum caffrum or bush mango is an evergreen plant native to the South African vegetation. The fruit from the plant is consumed locally as food or specifically for improving the nutritional quality of meals as deserts or condiments. While previous findings described the high antioxidant properties of the fruits, this study reported possible mechanisms via which the plant may exhibit ameliorative effects against oxidative stress-related neurological disorders in the brain. Hence, findings from the current work present another justification for the significance of fruits as a safer nutraceutical alternative for therapy in neurological disease management.
1 INTRODUCTION
Glucose is an obligatory energy substrate for the brain as it is required for implementing its vital functions, which include neuronal signaling, basal brain activities, and nonsignaling conducting functions (Dienel, 2019; Han et al., 2021). Thus, impaired glucose metabolism is detrimental to neuronal function. Studies have shown that disturbances in glucose metabolism arising from impaired brain glucose uptake have been implicated in the pathophysiology of neurodegenerative diseases such as Alzheimer's disease and Parkinson's disease (Han et al., 2021; Mergenthaler et al., 2013). These conditions have been ascribed to the suppressed glucose transporters activities at the blood–brain barrier (BBB), causing altered glucose transportation across the BBB and subsequently resulting in impaired glucose uptake and metabolism (Han et al., 2021; Salau, Erukainure, Bharuth, & Islam, 2021).
One of the factors implicated to increase human predisposition to certain neurodegenerative diseases in the brain is hypometabolism, resulting from impaired glucose uptake and utilization (Zilberter & Zilberter, 2017).
Glucose hypometabolism in the brain is associated with the elevated generation of radical species and ultimately oxidative stress, thus contributing to neuronal dysfunction and metabolic imbalance (Salau, Erukainure, Bharuth, & Islam, 2021; Zilberter & Zilberter, 2017). Efficient glucose metabolism is, therefore, critical for proper neuronal function and the health of the brain.
Since ancient times, fruits have been indispensable in the human diet owing to their high vitamin, minerals, and fiber nutritional composition. Their consumption has been widely correlated with numerous therapeutic benefits (Amao, 2018) such as low risks of diabetes, cardiovascular diseases, neurodegeneration, short life span, obesity, and cancer (Boffetta et al., 2010; Erukainure et al., 2020; Oyebode et al., 2014).
Harpephyllum caffrum, also commonly known as wild plum or bush mango, is a deciduous evergreen tree belonging to the Anacardiaceae family and the fourth largest tree family in Southern Africa (Dlamini, 2004). It is a famous tree plant widely distributed throughout the eastern part of Southern Africa (Moodley et al., 2013). The plum-like tasty fruits of H. caffrum containing only one seed are consumed by humans, animals, birds, and insects. The fruit's pulp is commercially used to produce jam, jellies, and rosé wine due to their sweet–sour taste (Moodley et al., 2013; Nawwar et al., 2011). H. caffrum fruits have been reported to boost human health and nutritional needs due to their moderate elemental composition (Moodley et al., 2013). Although the therapeutic activities of the leaves, bark, and roots of H. caffrum have been documented (Buwa & Van Staden, 2006; Nawwar et al., 2011), there is little information concerning the potential properties of the fruits in managing brain-related dysfunction.
Thus, the present work investigated the glucose uptake facilitative property of H. caffrum fruit and its modulatory effect on antioxidant, cholinergic, glucogenic, and purinergic enzyme activities in brain tissues. The phytochemical constituents of the fruit were investigated while their pharmacokinetic properties, toxicity, and molecular interactions with the studied brain enzymes were determined in silico.
2 MATERIALS AND METHODS
2.1 Plant material
Harpephyllum caffrum fresh fruits were obtained in January 2021 from tree plants growing at the University of KwaZulu-Natal, South Africa. The fruit authentication was done at the Ward Herbarium of the University of KwaZulu-Natal, Durban, South Africa, where it was assigned the voucher no.: K. Olofinsan & F. Olawale 4.
2.2 Preparation of fruit extract
The fruits were washed in running water before their pulps were separated from the seeds. About 250 g of the fruit pulps were homogenized with 500 ml distilled water in an electric blender (Philips, South Africa). The puree obtained was centrifuged at 10,000 g for 20 min, and the recovered supernatant was freeze-dried.
2.3 Estimation of polyphenol concentration
The concentration of the fruit polyphenols was estimated as gallic acid equivalent via a slightly modified spectrophotometric method of McDonald et al. (2001). Briefly, 700 μl of 1: 10 v/v Folin–Ciocalteu reagent was added to 0.6 ml of sodium carbonate (700 mM) and 100 μl solutions of extract (1 mg/ml) or gallic acid standards. Incubation was done at 25°C for 30 min, and absorbance was measured at 765 nm.
2.4 Ferric reducing antioxidant power (FRAP)
This was evaluated with the reported protocol of Oyaizu (1986) with slight modifications. Briefly, 500 μl of 50–200 μg/ml extract or ascorbic acid was added to 250 μl phosphate buffer (0.2 M, pH 6.6) and 125 μl of 1% K₃[Fe(CN)₆]. A 30-min incubation period was followed this step at 50°C. Then 150 μl of trichloroacetic acid (10%), 250 μl of distilled water, and 250 μl of ferric (III) chloride (0.1%) were added in sequence. Absorbance was read at 700 nm, and the extract reducing power was expressed as a percentage equivalence of 200 μg/ml ascorbic acid.
2.5 2,2′-Diphenyl-1-picrylhydrazyl (DPPH) scavenging activity
This antioxidant activity of the fruit was evaluated with a modified procedure of Ak and Gülçin (2008). Briefly, 0.5 ml solution of DPPH powder in methanol (300 μM in) was added to 0.5 ml of 50–200 μg/ml concentrations of the fruit extract or ascorbic acid. The solution was transferred to a chamber for a 30-min incubation period at 25°C. Then the intensity of the color change in the test sample was read against a blank solution at 517 nm.
2.6 Animals
Five Sprague–Dawley male rats with body weights ranging from 180 to 240 g were obtained from the biomedical resource unit, University of KwaZulu-Natal, Durban, South Africa. The night before the organ sampling, the rats were denied food for 12 hrs when the water was supplied ad libitum. In the morning, the animals were euthanized by Isofor followed by cardiac puncture, and their brain tissues were harvested immediately for in vitro glucose uptake assay. The animal maintenance and processing of all biological tissue samples were done following the University of KwaZulu-Natal, Animal Research Ethics Committee guidelines (Protocol no: AREC/00002325/2021).
2.7 Glucose uptake in isolated rat brain
The effect of the fruit extract on glucose uptake in freshly excised brain tissues was evaluated using the protocol of Chukwuma and Islam (2015). Briefly, 500 mg of brain tissue was transferred into an 8 ml solution of 0.0111 M glucose and 50–200 μg/ml of the extract in Krebs's buffer. Experimental set-up with metformin (200 μg/ml) and a similar glucose concentration in Kreb's buffer served as the control. The solution with the tissue was incubated for 2 hrs at 37°C and 5% CO2. A 2 ml aliquote of the incubating solution was collected before and after the incubation to measure glucose concentrations.
2.8 Preparation of brain homogenate
The tissue homogenate was obtained by homogenizing 400 mg of incubated brain sample with 4,000 μl of a physiological buffer system. The mixture was subjected to cold centrifugation (4°C) at 20,000 g for 15 min. The supernatant recovered there after was utilized for in vitro biochemical studies.
2.9 Reduced glutathione (GSH) concentration
The GSH protein concentration in the brain homogenates was determined using Ellman (1959) spectrophotometric method. Briefly, 0.25 ml of sample was added to 0.75 ml trichloroacetic acid (10%), and the solution was subjected to centrifugation for 5 min at 3,500 g. Then, 0.05 ml of Ellman's reagent was added to 100 μl of the resulting supernatant. After 30 min of incubation at 25°C, absorbance was taken at 415 nm, and the protein concentration was estimated from a plotted graph of glutathione standard.
2.10 Superoxide dismutase activity
The SOD activity in brain samples was determined by employing Kakkar et al. (1984) method with slight modifications. Briefly, 25 μl homogenate was added to 0.17 ml diethylenetriaminepentaacetic acid (100 μM) in a 96-well microplate. A total of 0.002 ml 6-hydroxydopamine (1.6 mM) was added to the mixture, and enzyme activity was measured thrice at 492 nm in a spectrophotometer set to take readings at 1-min intervals.
2.11 Catalase activity
This enzyme assay was evaluated with the procedure described by Hadwan and Abed (2016) with slight modifications. A total of 0.05 ml of the sample was added to 0.4 μl H2O2 (0.065 mM). The solution was incubated for 2 min at 37°C before 100 μl solution of ammonium molybdate (32.4 mM) was added to stop the reaction. Absorbance was measured at 347 nm against a blank solution with H2O2 only.
2.12 Determination of malondialdehyde (MDA) level
The extent of lipid peroxidation in brain homogenate was estimated as MDA equivalent using Fraga et al.'s (1988) method with slight modification. Briefly, 0.05 ml SDS (8.1%), 0.188 ml of 20% pure acetic acid, 500 μl thiobarbituric acid (0.25%), and 125 μl Milli-Q water were mixed with 50 μl of tissue homogenate in Eppendorf tubes. After heating in a water bath for 60 min, the absorbance of cooled samples was read for MDA estimation at 532 nm.
2.13 Determination of nitric oxide (NO) concentration
Spectrophotometric estimation of NO in brain samples was done using a hybrid procedure of Erukainure et al. (2019) and Tsikas (2005). A mixture containing 0.1 ml of the homogenate and 0.1 ml Griess reagent was incubated in the dark at 25°C for 30 min. Then, NO concentration was determined at 546 nm.
2.14 Determination of fructose-1-6-bisphosphatase (FBPase) activity
This assay was done using a modified procedure of Balogun and Ashafa (2017). A total of 0.1 ml brain homogenate was added to 600 μl Tris–HCl buffer (100 mM, pH 7.0), 0.05 ml MgCl2 (0.1 M), 0.05 ml fructose solution (0.05 M), 0.125 ml EDTA (1 mM), and 125 μl of KCl (100 mM). The solution was incubated at 37°C for 5 min before 0.05 ml trichloroacetic acid (10%) was added. Reaction tubes containing the reacting solution was subjected to centrifugation at 5,000 g for 10 min. The upper layers were transferred to new tubes containing freshly prepared ascorbic acid (9%) before another incubation at 37°C for 30 min. FBPase activity was estimated at 680 nm.
2.15 Determination of glycogen phosphorylase (GlyP) activity
Brain homogenates were analyzed for GlyP activity using a modified protocol of Balogun and Ashafa (2017). Briefly, 100 μl of glycogen solution (4%) and 0.1 ml of glucose-1-phosphate (64 mM) were incubated with 200 μl of the brain homogenate in Eppendorf tubes for 37°C for 10 min. A total of 2,500 μl ammonium molybdate (20%) was added before another 45-min incubation period at the previous temperature. Then, Elon reducer and distilled water were added, and absorbance was measured at 600 nm.
2.16 Determination of adenosine triphosphatase (ATPase) activity
Spectrophotometric assay for ATPase enzyme was done by adopting the method of Salau, Erukainure, and Islam (2021). Briefly, 200 μl of brain homogenate was transferred to a mixture constituting 200 μl KCl (5 mM), 1.3 ml of 100 mM Tris–HCl buffer, and 0.04 ml of adenosine triphosphate (50 mM). After incubation at 37°C for 30 min, ammonium molybdate solution (1,000 μl) was added. This step was followed by adding 1,000 μl of ascorbic acid (9%) before further incubation for 30 min at 25°C. ATPase enzyme activity was determined at 660 nm.
2.17 Determination of ecto-nucleoside triphosphate diphosphohydrolase (ENTPdase) activity
This assay was done using the protocol detailed by Salau, Erukainure, and Islam (2021). A solution containing 0.025 ml of the brain homogenate and 0.2 ml of a buffer solution (0.222 M sucrose, 1.5 mM CaCl2, 5 mM KCl, 0.045 M Tris–HCl, 0.1 mM EDTA, and 10 mM glucose) was incubated at 37°C for 10 min. A total of 0.02 ml ATP (0.05 M) was added and then a further incubation in a shaker for 20 min at 37°C was carried out. After terminating the reaction with 0.2 ml of 10% TCA, 0.2 ml of 1.25% ammonium molybdate and 9% ascorbic acid were added to the reaction mixture. The solution was kept on ice for 10 min before readings were taken at 600 nm.
2.18 Determination of cholinesterase activities
The acetylcholinesterase (ACTh) activity of the fruit extract on the brain samples was determined using the method described by Ellman et al. (1961) with slight modification. Briefly, 0.1 ml of the brain homogenate was mixed with 0.050 ml of Ellman's reagent (0.0033 M, pH 7.0) and 0.25 ml of phosphate buffer (0.1 mM, pH 8). After 20 min of incubation at room temperature, 0.050 ml of acetylcholine iodide 0.05 M was added to the reaction mixture. For the butyrylcholinesterase (BUTh) activity assay, 50 μl of butyrylcholine iodide was used while other reagents were the same. Subsequently, absorbance was read at 412 nm three times at 3-min intervals.
2.19 Liquid chromatography-mass spectrometry (LC–MS) analysis
The LC–MS characterization of the fruit extract was done using a Shimadzu LC/MS-2020 Single Quadrupole Mass Spectrometer. The mobile phase consists of A (0.1% formic acid), B (methanol), C (acetonitrile), and D (water). Data acquisition was set at LC stop time of 50 min and with a photodiode array (PDA) sampling frequency of 1.5625 Hz. The oven temperature was programmed as 40°C and with a detectable maximum of 50°C. Other operating conditions are described thus: Pump A mode was set at low operating gradient with a flowrate of 300 μl/min, B Conc.: 30.0%; C Conc.: 0.0%; D Conc.: 70.0%; Start Wavelength: 220 nm; End Wavelength: 400 nm; Cell Temp.: 40°C; Start Time: 0.0 min; End Time: 40.0 min; Acquisition Mode: Scan Polarity: Positive; Event Time: 0.25 s; Detector Voltage: +0.00 kV; Threshold: 0; Start m/z: 100.0; End m/z: 1,000.0; Scan Speed: 5,000 u/s. The result was analyzed with MZmine software V 2.5, and compound identification was made by comparing mass spectral data generated with those in the online NIST library.
2.20 In silico docking analysis
The free binding energy between chemical compounds in the fruit extract and the studied brain enzymes was calculated via virtual docking simulation. The 3D chimeric structures of ACTh, ATPase, BCTh, CAT, ENTPDase, FBPase, GlyP, and SOD were downloaded from the protein data bank (PDB). The PDB ID of the proteins was 1EVE, 4HYT, 6ESJ, 1F4J, 6YE2,5ZWK 3DD1, and 2C9V, respectively. The catalytic pocket of the proteins was determined using the automated algorithm of the CASTp web server. The proteins were prepared using the dock prep tool of UCFS Chimera software (V. 1.14), which removed co-crystallized water molecules before adding hydrogen atoms and Gasteiger charges (Pettersen et al., 2021). The 3D chemical structures of the compounds in the fruit extract were also obtained from the PubChem database and prepared with previously described procedures adopted for the protein molecules. Molecular docking of the compounds with the brain protein targets was done, employing the Lamarckian genetic algorithm of Autodock Vina software (Trott & Olson, 2010). The 2D images displaying the interactions between protein–ligand complex with the best binding pose for each compound was visualized with the Dassault Studio client (Version 21.1.0).
2.21 In silico pharmacokinetic property, ADME, and oral toxicity prediction
Virtual prediction of the absorption, distribution, metabolism, and excretion (ADME) and pharmacokinetic attributes of phytochemicals identified in the fruit extract was carried out on the SwissADME website. Potential oral toxicity of the natural compounds depicted by lethal dose (LD50) classes was predicted using the ProTox-II online database available at https://tox-new.charite.de/protox_II.
2.22 Statistical analysis
Each experiment was carried out in triplicate, and the results were presented as mean ± standard error of the mean. The level of significance was set at p < .05, while the difference between treatment groups was determined with one-way analysis of variance and Tukey's multiple range post-hoc test. All statistical analyses were computed with windows IBM SPPS version 26 (IBM Corp., Armonk, NY, USA).
3 RESULTS
The estimated total phenolic content of H. caffrum fruit extract was 493.9 ± 2.5 mg/g gallic acid equivalent.
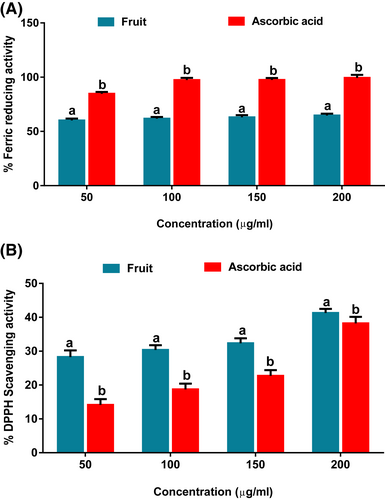
As shown in Figure 1A, the fruit extract exhibited potent FRAP activity but at a significantly (p < .05) lower capacity than the standard compound, ascorbic acid. This is further depicted by its IC50 value (43.3 μg/ml), which is higher than that of ascorbic acid (11.6 μg/ml). As shown in Figure 1B, the extract portrayed a significant (p < .05) DPPH radical scavenging activity compared with ascorbic acid. The activity was dose-dependent with an inhibitory concentration-50 value of 40.0 μg/ml relative to 396.1 μg/ml of ascorbic acid.
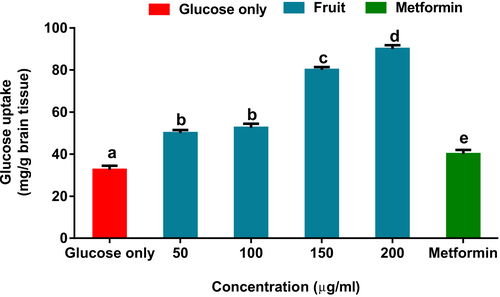
Glucose uptake in brains incubated with the fruit extract increased significantly, as shown in Figure 2. Interestingly, the uptake was higher than brains incubated with metformin.
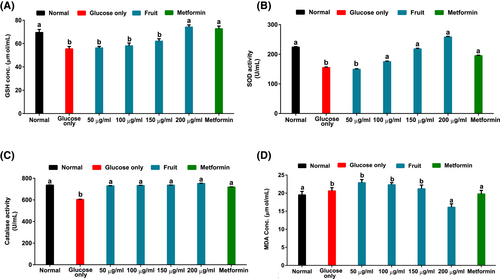
As indicated in Figure 3A–D, there was a significant (p < .05) reduction in brain glutathione concentration, superoxide dismutase, and catalase activities in tissues incubated in glucose only solution, whereas MDA increased. However, treatment with the H. caffrum fruit extract increased glutathione level, superoxide dismutase, and catalase activities with simultaneous reduction of MDA concentration.
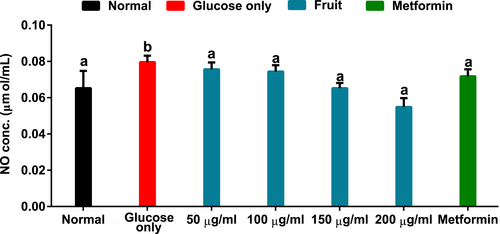
Data in Figure 4 showed that brain tissue incubated with glucose only had elevated NO concentration. However, incubation with the fruit extract lowered the NO level dose-dependently to levels comparable to normal and metformin treatment groups.
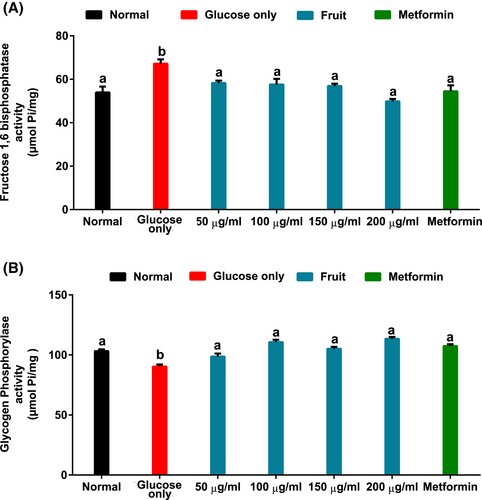
As indicated in Figure 5A,B, brain tissues incubated in solution with only glucose had significantly (p < .05) elevated FBPase activity and suppressed GlyP activity (Figure 5B). Interestingly, the fruit extract significantly (p < .05) reversed the glucogenic enzyme activities.
As shown in Figure 6A,B, the untreated brain tissues incubated with only glucose had significantly (p < .05) elevated ATPase and depleted ENTPDase activities. These activities were significantly (p < .05) reversed following treatment with the fruit extract.
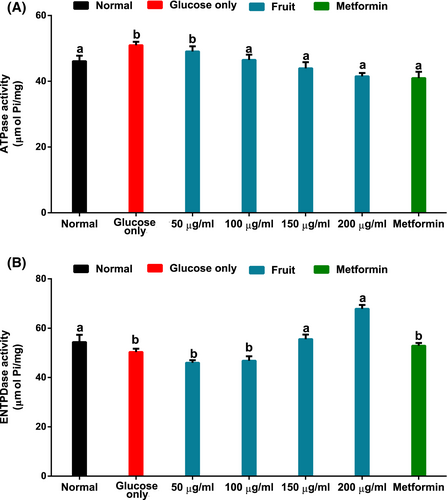
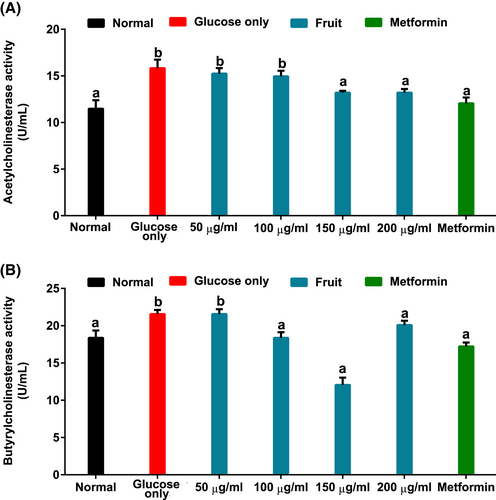
Figure 7A,B indicated, respectively, a significant (p < .05) increase in ACTh and BUTh activities of brain tissues incubated with only glucose. However, the presence of the fruit extract caused a reversal of cholinesterase enzyme activities, with ACTh, more importantly, being dose dependent.
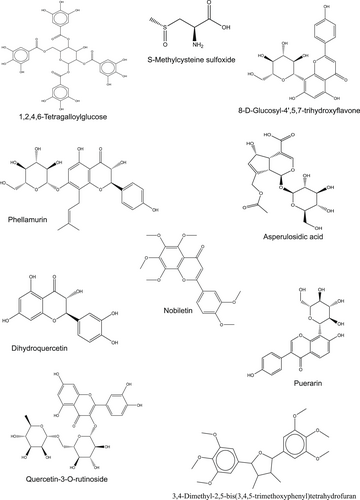
Chemical characterization of H. caffrum extract showed the presence of dihydroquercetin, phellamurin, nobiletin, puerarin, 8-D-glucosyl-4′,5,7-trihydroxyflavone, quercetin 3-rutinoside, asperulosidic acid, S-methylcysteine sulfoxide, 3,4-dimethyl-2,5-bis(3,4,5-trimethoxyphenyl) tetrahydrofuran, and 1,2,4,6-tetragalloylglucose presented in Table 1 and Figure 8.
| Phytochemical | Retention time (min.) | [M + H] +(m/z) | MS2 (m/z) |
|---|---|---|---|
| S-Methylcysteine sulfoxide | 4.49 | 151.03 | 110.40 |
| Asperulosidic acid | 5.12 | 432.37 | 175.20 |
| Dihydroquercetin | 5.68 | 304.24 | 287.21 |
| Puerarin | 7.80 | 416.41 | 268.10 |
| 1,2,4,6-Tetragalloylglucose | 8.57 | 778.59 | 287.00 |
| 8-D-Glucosyl-4′,5,7-trihydroxyflavone | 9.58 | 432.36 | 367.15 |
| Phellamurin | 10.82 | 518.47 | 207.20 |
| 3,4-Dimethyl-2,5-bis(3,4,5-trimethoxyphenyl)tetrahydrofuran | 11.40 | 432.48 | 217.23 |
| Nobiletin | 23.01 | 402.31 | 301.24 |
| Quercetin 3-rutinoside | 28.23 | 610.53 | 413.25 |
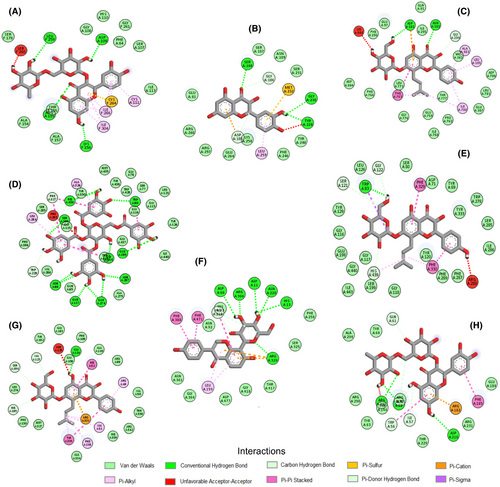
In Table 2, root mean square deviation (RMSD) values less than or around 2.0 Å for each protein, and their co-crystallized ligands indicate a high accuracy in the selected proteins' active sites. The LC–MS-identified phytochemicals' molecular docking analysis data displayed favorable interactions with negative binding free energy for the different protein targets. Phellamurin had the highest binding affinity with three protein targets, namely ACTh (10.9 Kcal/mol), ATPase (9.6 Kcal/mol), and catalase (11.0 Kcal/mol). Figure 9 depicted that the compound's interaction with these proteins' active site amino acid residues was stabilized by hydrogen bonds, pi bonds, carbon-hydrogen bonds, and other hydrophobic interactions. Additionally, quercetin 3-rutinoside with 7.2 Kcal/mol and 7.9 Kcal/mol binding energies (Table 2) formed strong hydrogen bonds with LEU 259, ASP 109, ARG 115, LYS 156 (Figure 9A) and ARG 291, ASP 216, GLN 60 (Figure 9H) amino residues of SOD and glycogen phosphorylase, respectively.
| Compound | Acetyl-cholinesterase | Butyryl-cholinesterase | ENTPDase | ATPase | Fructose −1,6-bisphosphatase | Glycogen phosphorylase | Superoxide dismutase | Catalase |
|---|---|---|---|---|---|---|---|---|
| S-Methylcysteine sulfoxide | −4.7 | −4.7 | −5.0 | −3.9 | −4.2 | −4.1 | −3.6 | −5.0 |
| Asperulosidic acid | −8.9 | −8.8 | −6.8 | −7.8 | −6.8 | −7.2 | −5.4 | −9.4 |
| Dihydroquercetin | −9.9 | −8.3 | −9.6 | −8.4 | −7.2 | −7.2 | −7.0 | −10.1 |
| Puerarin | −10.0 | −10.1 | −11.4 | −8.4 | −5.2 | −7.4 | −6.0 | −9.9 |
| 1,2,4,6-Tetragalloylglucose | −9.6 | −11.5 | −7.4 | −9.0 | −6.4 | −7.7 | −6.0 | −10.3 |
| 8-D-Glucosyl-4′,5,7-trihydroxyflavone | −9.7 | −9.2 | −9.7 | −8.7 | −6.3 | −7.8 | −5.9 | −9.8 |
| Phellamurin | −10.9 | −10.4 | −7.7 | −9.6 | −6.7 | −6.9 | −7.0 | 11.0 |
| 3,4-Dimethyl-2,5-bis(3,4,5-trimethoxyphenyl)tetrahydrofuran | −9.5 | −7.9 | −7.1 | −8.0 | −3.8 | −6.4 | −6.0 | 8.1 |
| Nobiletin | −9.3 | −7.6 | −8.8 | −7.8 | −4.4 | −6.7 | −6.9 | −9.1 |
| Quercetin 3-rutinoside | −10.7 | −10.2 | −7.4 | −9.1 | −6.5 | −7.9 | −7.2 | 10.6 |
| Metformin | −5.3 | −5.2 | −5.2 | −4.5 | −4.9 | −3.8 | −3.9 | −5.2 |
| aRMSD of Co-crystallized Ligand | 2.256 | 0.356 | 2.382 | 0.808 | 2.061 | 2.616 | 0.109 | 1.461 |
- a Root mean square deviation (RMSD) calculated value of protein cocrystallized ligand docked at the catalytic pocket of their corresponding protein.
The predicted pharmacokinetic properties of the LC–MS-identified compounds indicate that S-methylcysteine sulfoxide, dihydroquercetin 3,4-dimethyl-2,5-bis(3,4,5-trimethoxyphenyl) tetrahydrofuran (MTHF), and nobiletin have high gastrointestinal absorption (Table 3). MTHF was predicted to be BBB permeable. Moreover, the compounds were indicated to have no inhibitory effects on CYP1A2 and CYP2C19. Apart from dihydroquercetin, tetragalloylglucose, and quercetin 3-rutinoside, the other compounds were described to have no PAIN alert.
| A | B | C | D | E | F | G | H | I | J | |
|---|---|---|---|---|---|---|---|---|---|---|
| GI absorption | High | Low | High | Low | Low | Low | Low | High | High | Low |
| BBB permeant | No | No | No | No | No | No | No | Yes | No | No |
| CYP1A2 inhibitor | No | No | No | No | No | No | No | No | No | No |
| CYP2C19 inhibitor | No | No | No | No | No | No | No | No | No | No |
| Bioavailability Score | 0.55 | 0.11 | 0.55 | 0.55 | 0.17 | 0.55 | 0.17 | 0.55 | 0.55 | 0.17 |
- Note: A = S-methylcysteine sulfoxide; B = asperulosidic acid; C = dihydroquercetin; D = puerarin; E = 1,2,4,6-tetragalloylglucose; F = 8-D-glucosyl-4′,5,7-trihydroxyflavone; G = phellamurin; H = 3,4-dimethyl-2,5-bis(3,4,5-trimethoxyphenyl) tetrahydrofuran; I = nobiletin; J = quercetin 3-rutinoside.
The Protox II online virtual laboratory results suggest that most of the compounds in the fruit extract are in toxicity class 4 (Table 4). Notably, tetragalloylglucose, phellamurin, nobiletin, and quercetin 3-rutinoside belong to class 5 with lesser acute toxicity.
| Compound | LD 50 (mg/kg) | Toxicity class |
|---|---|---|
| S-Methylcysteine sulfoxide | 660 | 4 |
| Asperulosidic acid | 2,000 | 4 |
| Dihydroquercetin | 2,000 | 4 |
| Puerarin | 832 | 4 |
| 1,2,4,6-Tetragalloylglucose | 2,260 | 5 |
| 8-D-Glucosyl-4′,5,7-trihydroxyflavone | 832 | 4 |
| Phellamurin | 2,300 | 5 |
| 3,4-Dimethyl-2,5-bis(3,4,5-trimethoxyphenyl)tetrahydrofuran | 1,500 | 4 |
| Nobiletin | 5,000 | 5 |
| Quercetin 3-rutinoside | 5,000 | 5 |
4 DISCUSSION
There are indications from previous findings that impaired glucose uptake in the brain is related to dysregulated metabolisms. This condition has been associated with processes that include redox imbalance, altered cholinergic signaling, and dysregulated glucose metabolism, which are similarly involved in the progression of neurodegenerative diseases such as Alzheimer's and Parkison's. The present study investigated the effects H. caffrum fruit pulps on glucose uptake and altered biochemical parameters in glucose-induced oxidative stress in isolated rat brains.
The ferric reducing potential and DPPH-free radical scavenging activities (Figure 1) displayed by the fruit suggest potential antioxidant properties. Antioxidants are molecules capable of terminating free radical-propagated reactions that could otherwise result in damage to cellular macromolecules (Kurutas, 2015). Hence, antioxidants play a vital role in health. Plants and their natural products have been documented for their potent antioxidant capacity, associated with their intrinsic bioactive chemical constituents (Cui et al., 2020; Olofinsan et al., 2021; Sultana et al., 2021). Therefore, in vitro antioxidant properties displayed by the H. caffrum fruit may be attributed to its bioactive components (Table 1). These results correlate with previous reports by Moodley et al. (2014) on the DPPH-mopping and iron III-reducing properties of methanolic extract of H. caffrum fruit, whose antioxidant biological activity was associated with the presence of catechin and other bioactive compounds isolated from the fruit.
According to Wang et al. (2016), one of the earlier diagnostic criteria for certain neurodegenerative disorders is the suppression of glucose uptake in different brain regions. Studies have also reported that the enhanced movement of glucose into the brain improves cognitive functions (Nilsson et al., 2012). The observed increased uptake in tissue kept in solution containing the fruit extract and the glucose (Figure 2) may suggest the fruit's potential in improving neuronal glucose consumption and utilization. Lignan polyphenol and its derivatives, such as MTHF present in the fruit (Table 1), have been reported to cross the BBB and elicit physiological responses (Rom et al., 2018; Wu et al., 2016).
The ability of several phytochemicals to facilitate glucose transport via activation of BBB bound receptors have also been evidenced in previous findings (Baron et al., 2021; Erukainure et al., 2019). Thus, the enhanced glucose uptake after treatment with the fruit extract may be associated with MTHF, which in the current study was predicted to have BBB permeability in silico (Table 3).
Compared with other body tissues, the brain has a lower endogenous antioxidant defense capacity that increases its risk of oxidative attack (Cobley et al., 2018). Consequently, redox imbalance has been described as one of the mechanisms underlying the onset and progression of degenerative neurological disorders (Carvalho et al., 2017; Floyd, 1999). In the current study, the decrease in brain GSH level with simultaneous reduction in the enzymatic antioxidant (SOD and CAT) activities (Figure 3A–C) indicate the occurrence of an oxidative state due to incubation with glucose. This biochemical dysregulation could result in lipid peroxidation, which is evident in the untreated brain by the high MDA concentration (Figure 3D). However, the elevated concentration of GSH and the increased SOD and CAT activities with simultaneous reduction of MDA after treatment with the fruit may indicate its potential neuroprotective efficacy against redox imbalance due to impaired glucose uptake in the brain. This study supports Erukainure et al.'s (2019) findings which demonstrated the ability of plant products to ameliorate oxidative parameters in rat brain glucose uptake.
Under normal conditions, NO performs various essential roles in the brain. Some of these include induction of dendritic spine growth, immunoregulation, neurotransmission, vascular tone control, among others (Picón-Pagès et al., 2019). However, elevated levels of this signaling molecule have been implicated in the etiology of many neurodegenerative diseases (Yuste et al., 2015). When NO interacts with superoxide, it produces peroxynitrite (ONOO−), a reactive radical capable of initiating reaction cascades leading to proinflammation that encourages pathophysiology of several brain tissue-associated disorders (Pacher et al., 2007; Picón-Pagès et al., 2019). Hence, a significantly high NO level in brain tissue incubated with glucose (Figure 4) coupled with the diminished SOD activity (Figure 3B) may suggest proinflammation. The ability of H. caffrum fruit to reduce NO concentration in the treatment groups may insinuate its therapeutic potential in managing nitrosative stress in the brain.
Elevated gluconeogenesis in the brain via the targeted increase in fructose-1,6-bisphosphatase activity has been reported as a mechanism to meet the brain's glucose demand in episodes of short supply and/or utilization (Cloix & Hévor, 2009). In the current work, this condition is indicated by the high fructose-1,6-bisphosphatase activity of tissues incubated with glucose with suppressed sugar uptake activity (Figure 5A). The reduction in FBPase activity with varying fruit concentrations thus suggests the deactivation of gluconeogenesis and simultaneous activation of the glycolytic pathway. This is also supported by the decreased glycogen phosphorylase activity in brain tissues incubated with the fruit sample (Figure 5B). The reduced glycogen phosphorylase activity further suggests activation of glycogenesis. These activities can be ascribed to the phytochemical constituents of the fruit as depicted by the strong binding affinity of dihydroquercetin and quercetin 3-rutinoside with the glucogenic enzymes (Table 2 and Figure 9).
Adenosine nucleoside and ATP nucleotide molecules carry out crucial purinergic signaling roles in the brain. While adenosine is involved in homeostatic modulation of synaptic plasticity, immature neural cell differentiation, and proliferation, ATP is associated with neurotransmission and other vital brain functions. (Grković et al., 2019). However, in the findings by Pathak et al. (2015), depletion of cellular ATP levels was identified as one of the features characteristics of Leigh's model of neurodegenerative disorder. The increased ATPase (Figure 6A) with suppressed ENTPDase (Figure 6B) in brain tissue incubated with glucose suggests suppressed levels of these signaling molecules. These observations corroborate the previous report of Salau, Erukainure, and Islam (2021), where a derangement in glucose transport into isolated rat brain results in dysregulated purinergic enzymes' activity. Interestingly, the reversal of the nucleotide-hydrolyzing enzyme activities in the presence of fruit extract may thus indicate its ability to improve brain purinergic activities by exacerbating the availability of adenosine and intracellular ATP levels. This correlates with previous reports on improved purinergic metabolism linked to enhanced brain glucose uptake (Salau, Erukainure, Bharuth, & Islam, 2021). Interestingly, a study by Haas and Selbach (2000) suggested enhanced adenosine regulation as an explorable therapeutic means in managing schizophrenia, epilepsy, Alzheimer dementia, and other neurological disorders. Thus, further indicating the neuroprotective potentials of H. caffrum.
In the progression of Alzheimer's and several other brain disorders, physiological elevated ACTh and BUTh activities have been well reported (Stanciu et al., 2020). The increased cholinesterase activities in brain samples kept in a glucose-only solution may suggest the occurrence of cholinergic dysfunction due to impaired glucose uptake (Figures 7A,B). Thereby, this observation may depict reduced neuronal levels of choline. Thus, the ability of H. caffrum fruit extract to lower ACTh and BUTh activities in the treatment groups may suggest the plant's potential to increase choline esters' availability for improved neurological activities. The negative binding energy between the fruit characterized phytochemicals and the cholinergic enzymes (Table 2) may also indicate strong interaction that could have led to the observed biological activity of the plant. Interestingly, previous in vitro studies using other parts of the plant have demonstrated the acetylcholinesterase inhibitory activity of H. caffrum (Moyo et al., 2010; Olofinsan et al., 2022.).
Computational methods are increasingly being adopted in screening phytochemicals with opportunities for drug development. This predicted high gastrointestinal absorption of S-methylcysteine sulfoxide, dihydroquercetin, 3,4-dimethyl-2,5-bis(3,4,5-trimethoxyphenyl) tetrahydrofuran, and nobiletin insinuates these fruits' phytochemicals can easily permeate the intestine and be transported to the bloodstream to exert their therapeutic effect. The predicted noninhibitory effect on CYP1A2 and CYP2C19 indicates that the LC–MS-identified compounds might not cause drug–drug interactions when taken with other drugs metabolized by these enzymes. Thus, suggesting their safety. Moreover, the fruit's high lethal dose-50 values and toxicity group classification (Table 4) further indicates its potential nontoxic effect when ingested as food.
5 CONCLUSION
Based on results from the present study, H. caffrum fruit possesses the ability to stimulate brain glucose uptake with concomitant neuroprotective effects. These are evident in the fruit's ability to improve brain antioxidant capacity and modulate glucogenic, cholinergic, and nucleotide-hydrolyzing enzyme activities. This observation is also supported by the molecular interactions of the identified phytochemicals of the fruits with the studied biomarker enzymes. However, further in-depth studies in animal models are required to elucidate possible biochemical mechanisms explaining the fruit's biological activities under human physiological conditions.
ACKNOWLEDGMENTS
The first author acknowledges the Research Office, University of KwaZulu-Natal and the National Research Foundation—The World Academy of Science (NRF-TWAS), South Africa, under unique grant no. 116093, for funding this research.
CONFLICT OF INTEREST
The authors have declared no conflicts of interest for this article.
AUTHOR CONTRIBUTIONS
Kolawole A. Olofinsan: Conceptualization; formal analysis; investigation; methodology; writing – original draft. Veronica F. Salau: Formal analysis; investigation; methodology; writing – review and editing. Ochuko L. Erukainure: Formal analysis; methodology; validation; writing – review and editing. Md. Shahidul Islam: Funding acquisition; project administration; resources; supervision; validation; writing – review and editing.
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the article




