Epidemiology and treatment of Adamantiades–Behçet's disease in Germany: A healthcare claims database study
Abstract
Background
Adamantiades–Behçet's disease (ABD) is a rare, chronic, relapsing, multisystem vasculitis, with a reported prevalence of 0.9 out of 100,000 population in Germany in 2012. However, more recent epidemiological data are lacking.
Objectives
To estimate the prevalence and incidence of ABD in Germany and assess associated comorbidities and current treatment patterns.
Methods
This retrospective cohort study used claims data from the anonymized Institute for Applied Health Research Berlin (InGef) research database. Cohorts were formed for the observation years 2016, 2017 and 2018, using data from 2013 to 2018 (allowing for a 3-year baseline period for incidence data). The study population included patients ≥18 years old with a diagnosis of ABD (ICD-10 diagnostic code M35.2), covered under the statutory health insurance system and in the InGef research database, with continuous insurance in the observation year and baseline period. We descriptively evaluated prevalence (≥2 outpatient ABD diagnoses in different quarters/≥1 main inpatient diagnosis in the observation year), incidence (no confirmed outpatient/inpatient diagnosis in the baseline period), comorbidities reported during the study and ABD-related medications.
Results
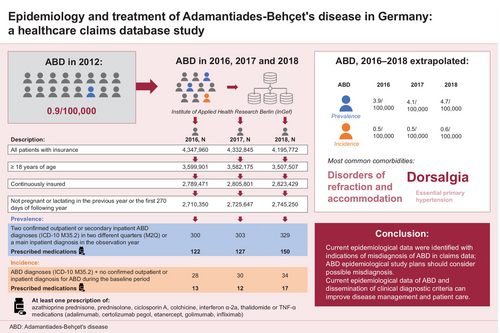
We identified 300, 303 and 329 patients diagnosed with ABD in 2016, 2017 and 2018, respectively, with fewer than half (122/300 [40.7%], 127/303 [41.9%] and 150/329 [45.6%]) prescribed ≥1 disease-related medication. In the treated population, ABD prevalence was 3.9 (2016), 4.1 (2017) and 4.7 (2018) per 100,000 population; annual ABD incidence was 0.5 per 100,000 population in 2016 and 2017 and 0.6 per 100,000 population in 2018. The most commonly reported comorbidities in patients diagnosed with ABD were dorsalgia (an indicator of possible misdiagnosis), disorders of refraction and accommodation, and essential (primary) hypertension. Prednisolone, colchicine and azathioprine were the most commonly prescribed treatments for ABD, with approximately 15% of patients taking >1 medication for ABD.
Conclusions
The reported data provide evidence that ABD remains a rare disease in Germany.
Graphical Abstract
Why was the study undertaken?
Adamantiades–Behçet's disease (ABD) is a rare, chronic disease, affecting multiple organ systems and requiring individualized treatment. Epidemiological data in Germany were last reported in 2012 and current data are needed.
What does this study add?
ABD prevalence in Germany increased from 2016 to 2018, with ≥50% of patients untreated. However, exclusion of patients with certain comorbidities—such as dorsalgia, a potential indicator of misdiagnosis—provides a lower, more consistent prevalence range.
What are the implications of this study for disease understanding and/or clinical care?
ABD is a rare disease in Germany and our data suggest that misdiagnosis may be prevalent in clinical care. Current epidemiological data on ABD and dissemination of clinical diagnostic criteria could improve disease management and patient care.
INTRODUCTION
Adamantiades–Behçet's disease (ABD) is a chronic, relapsing, multisystem, inflammatory vasculitis/perivasculitis that predominantly affects small venous vessels, although it can also affect larger veins and arteries.1-4 ABD can involve the oral and genital mucosa, skin, eyes, joints, nervous system and gastrointestinal system by affecting organ vasculature, which, in some cases, can cause serious organ damage or even death.1, 3, 4 Mucocutaneous manifestations such as oral ulcers are the hallmark of the disease and can significantly impair patient quality of life due to accompanying pain, leading to difficulties with speaking and ingestion.5-9 While the aetiology of ABD is unknown, it may be caused by a combination of genetic and environmental factors.1, 4, 10-15
ABD is a rare disease, with a reported global prevalence of 10.3/100,000 population (95% confidence interval [CI]: 6.1, 17.7).16 There is substantial geographic variation in its prevalence, which is higher along the Silk Road, an ancient trading route (Turkey: 119.8/100,000; Middle East: 31.8/100,000), compared with other regions (Asia: 4.5/100,000; Europe: 3.3/100,000).7, 8, 16 Given the heterogeneous clinical manifestations of ABD, sex-related differences in disease and its relapsing nature, European guidelines (including the European Alliance of Associations for Rheumatology [EULAR]), recommend individualized treatment.17-19 The primary goal of treatment is to rapidly suppress inflammatory exacerbations to prevent irreversible organ damage associated with severe disease.17
Methodological inconsistencies have impeded objective assessment of ABD patient demographics in most countries except Japan, where the ABD registry is managed and regularly maintained by the Ministry of Health.20 The German Registry of ABD (GeR-ABD) collects epidemiological data for Germany; in 2012, ABD prevalence was reported to be 0.9/100,000 population.7, 9 However, recent epidemiological data are lacking. This study estimated the prevalence and incidence of ABD in Germany and evaluated associated comorbidities and current treatment patterns in patients with ABD.
PATIENTS AND METHODS
Source of data and study design
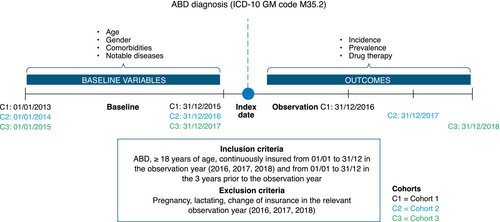
Our retrospective cohort study used health insurance claims data from the anonymized Institute for Applied Health Research Berlin (InGef) research database, which contains longitudinal data on approximately 8 million insured individuals from approximately 60 statutory health insurers (as of February 2020), representing approximately 90.0% of the German population.21 We used a sample subpopulation of approximately 4 million patients per year available for healthcare research projects, representative of the age and sex distribution of health-insured patients in Germany, excluding privately insured patients.22, 23 Cohorts for observation years 2016, 2017 and 2018 were formed using health insurance claims data from 2013 to 2018, allowing for a 3-year baseline period and accuracy in incidence calculations (Figure 1).
Study populations to assess prevalence and incidence
Patients aged ≥18 years with an ABD diagnosis (index event identified by International Classification of Diseases 10th Revision [ICD-10] diagnostic code M35.2), covered under the statutory health insurance (SHI) system and in the InGef research database were included if they met the following criteria: continuous insurance from 1 January to 31 December in the observation year (patients who changed health insurance companies within the year were excluded) and continuous insurance from 1 January to 31 December for a period of 3 years prior to the observation year (baseline period). Patients were excluded if they were pregnant or lactating.
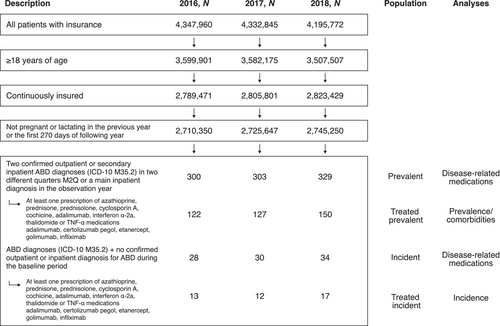
To determine the final analysis population for prevalence and incidence, a step-wise approach to patient selection was undertaken (Figure 2). To evaluate prevalence (prevalent population), ≥2 confirmed outpatient diagnoses in different quarters (minimum 2 quarters) or ≥1 main inpatient diagnosis of ABD in the observation year were required; secondary inpatient diagnoses were treated as outpatient diagnoses (Figure 2). Patients from the prevalent population with no confirmed outpatient or inpatient ABD diagnosis during the baseline period formed the incident population (Figure 2). To improve validity of our study population, we further evaluated the prevalent and incident populations for patients with ABD-relevant medication prescription, referred to as the ‘treated prevalent population’ and ‘treated incident population’, respectively (Figure 2). Patients must have received ≥1 prescription for an ABD-relevant drug therapy in the observation year (Figure 2). Prescriptions represent typical ABD medications and were selected based on observations from clinical practice at the time of study design.
Outcomes
Use of disease-related medications from 2016 to 2018 was evaluated in the overall prevalent and incident populations (Figure 2). Longitudinal analyses covering a period of four quarters over 1 year were used to evaluate drug therapy: incident quarter (quarter in which first diagnosis was documented) and the first, second and third quarters following the incident quarter. Prescriptions were assigned to a quarter based on prescription date. Prevalence/comorbidities and incidence from 2016 to 2018 were evaluated in the treated prevalent and treated incident populations, respectively (Figure 2). Comorbidities were assessed based on ICD-10 codes reported in insured patients with ABD during the observation years and the top 20 comorbidities were examined.
The InGef research database collects claims data according to the German social code (Chapter 10, SGB V) and is anonymized.24 To further ensure data protection, data or any evaluations based on a group size of <5 patients were not shared; only aggregated results (reported as ‘n < 5’) were generated and presented. As this study used anonymized secondary data (i.e. administrative health insurance claims data), ethics approval and consent to participate were not applicable.
Data analyses
Descriptive analyses of prevalence, incidence and disease-related medications were based on the calculation of position (mean, median values) and dispersion parameters (standard deviation). Statistical tests were not performed. Microsoft R Open 3.5.0 was used to perform descriptive analyses and data were extrapolated to the adult German SHI population (approximately 84.4 million people in 2022) using direct standardization by age and sex group and the official KM6 statistics reported by the Federal Ministry of Health in Germany (KM6 contains the number of individuals per age group and sex with SHI on 1 July of any given year).25, 26
Incidence was calculated analogously. For extrapolated data, 95% CIs were calculated according to the Fay and Feuer method.27
RESULTS
Patient baseline characteristics and demographics
From the InGef research database sample of approximately 4 million people, 300, 303 and 329 patients had a diagnosis of ABD in 2016, 2017 and 2018, respectively, and were included in the prevalent population; of these, 9.3% (28/300), 9.9% (30/303) and 10.3% (34/329) were incident patients (Figure 2). In 2016, 2017 and 2018, respectively, 40.7% (122/300), 41.9% (127/303) and 45.6% (150/329) patients in the prevalent population and 46.4% (13/28), 40.0% (12/30) and 50.0% (17/34) in the incident population were prescribed ≥1 relevant disease-related medication and therefore included in the treated prevalent and treated incident populations, respectively. The male:female ratio in the treated prevalent population was 1.03, 1.02 and 1.11 per 100,000 in 2016, 2017 and 2018, respectively (Table 1). The mean age of patients in the treated prevalent population was 50.1, 50.7 and 51.7 years in 2016, 2017 and 2018, respectively (Table 1). The mean age of female patients was higher than that of male patients: 52.1 versus 48.1 years (2016), 52.0 versus 49.4 years (2017) and 52.8 versus 50.7 years (2018). The male:female ratio in the treated incident population was 1.3 per 100,000 in 2018; data for 2016 and 2017 cannot be provided due to data protection rules for sample size <5 (Table 2). Among patients in the treated incident population, the mean age was 49.2, 41.8 and 50.9 years in 2016, 2017 and 2018, respectively (Table 2).
| 2016 | 2017 | 2018 | |
|---|---|---|---|
| Prevalent patients | |||
| N a (M/F) | 122 (62/60) | 127 (64/63) | 150 (79/71) |
| Mean (SD) age, years | |||
| Overall | 50.1 (13.7) | 50.7 (14.3) | 51.7 (13.7) |
| Male | 48.1 (13.4) | 49.4 (13.6) | 50.7 (12.8) |
| Female | 52.1 (13.8) | 52.0 (15.0) | 52.8 (14.6) |
| Prevalence (95% CI), adjusted per 100,000 population | |||
| Overall | 3.9 (3.2, 5.8) | 4.1 (3.4, 5.9) | 4.7 (4.0, 6.4) |
| Male | 4.2 (3.0, 6.2) | 4.2 (3.2, 6.4) | 5.3 (3.9, 7.3) |
| Female | 3.8 (2.9, 7.3) | 3.9 (3.0, 7.2) | 4.4 (3.4, 7.5) |
| M:F ratio | 1.03 | 1.02 | 1.11 |
| Prevalence (95% CI), adjusted per 100,000 population, by age group | |||
| 18–39 years | 4.5 (3.1, 7.8) | 4.2 (2.8, 7.4) | 3.6 (2.3, 6.7) |
| 40–59 years | 4.8 (3.5, 7.1) | 5.3 (4.0, 7.6) | 7.4 (5.8, 10.0) |
| 60+ years | 2.6 (1.7, 7.7) | 2.7 (1.8, 7.5) | 3.1 (2.2, 7.5) |
| Prevalence (95% CI) in German SHI adult population, by age group | |||
| Overall | 2270 (1848, 3352) | 2373 (1954, 3408) | 2779 (2323, 3768) |
| 18–39 years | 769 (519, 1316) | 730 (487, 1271) | 623 (404, 1164) |
| 40–59 years | 985 (725, 1470) | 1089 (826, 1577) | 1511 (1184, 2057) |
| 60+ years | 516 (332, 1551) | 555 (365, 1523) | 645 (446, 1532) |
- Abbreviations: ABD, Adamantiades–Behçet's disease; CI, confidence interval; F, female; M, male; N, total number of patients; SD, standard deviation; SHI, statutory health insurance; TNF, tumour necrosis factor.
- a Number of patients with a diagnosis of ABD who had received ≥1 prescription for one of the following medications: azathioprine, prednisone, prednisolone, cyclosporin A, colchicine, interferon α-2a, thalidomide and TNF-α (adalimumab, certolizumab pegol, etanercept, golimumab and infliximab).
| 2016 | 2017 | 2018 | |
|---|---|---|---|
| Incident patients | |||
| Na-c | 13 | 12 | 17 |
| Mean (SD) age, years | |||
| Overall | 49.2 (12.4) | 41.8 (20.1) | 50.9 (10.8) |
| Male | – d | – d | 49.6 (10.8) |
| Female | 51.8 (12.2) | 36.8 (17.8) | 52.4 (11.3) |
| Incidence (95% CI), adjusted per 100,000 population | |||
| Overall | 0.5 (0.2, 2.3) | 0.5 (0.2, 2.2) | 0.6 (0.3, 2.1) |
| Male | – d | – d | 0.6 (0.3, 2.5) |
| Female | 0.7 (0.3, 4.3) | 0.6 (0.3, 4.0) | 0.5 (0.2, 3.6) |
| M:F ratio | – d | – d | 1.3 |
| Incidence (95% CI), adjusted per 100,000 population, by age group | |||
| 18–39 years | – d | 0.9 (0.3, 3.7) | – d |
| 40–59 years | 0.6 (0.2, 2.6) | – d | 1.2 (0.6, 3.3) |
| 60+ years | – d | – d | – d |
| Incidence (95% CI) in German SHI adult population, by age group | |||
| Overall | 265 (129, 1323) | 265 (137, 1256) | 328 (180, 1230) |
| 18–39 years | – d | 152 (567, 635) | – d |
| 40–59 years | 122 (31, 533) | – d | 252 (121, 678) |
| 60+ years | – d | – d | – d |
- Abbreviations: ABD, Adamantiades–Behçet's disease; CI, confidence interval; F, female; M, male; N, total number of patients; SD, standard deviation; SHI, statutory health insurance; TNF, tumour necrosis factor.
- a Population of patients who had no ABD diagnosis in the previous 3 years (baseline period).
- b Number of patients with a diagnosis of ABD who had received ≥1 prescription for one of the following medications: azathioprine, prednisone, prednisolone, cyclosporin A, colchicine, interferon α-2a, thalidomide and TNF-α (adalimumab, certolizumab pegol, etanercept, golimumab and infliximab).
- c Data corresponding to the number of males and females are not included due to the sample size being <5 in some cases.
- d Sample size <5 in research database; due to data protection rules, the exact numbers for sample size <5 cannot be provided.
Prevalence and incidence of ABD in patients with a prescription for disease-related medication
The prevalence of ABD in the treated prevalent population was 3.9, 4.1 and 4.7 per 100,000 population in 2016, 2017 and 2018, respectively (Table 1). In males, the prevalence was 4.2 per 100,000 in 2016 and 2017, and 5.3 per 100,000 in 2018; corresponding prevalence in females was 3.8, 3.9 and 4.4 per 100,000. Across all observation years, ABD prevalence was highest among patients aged 40–59 years (Table 1).
The incidence of ABD in the treated incident population was 0.5 per 100,000 in 2016 and 2017 and 0.6 per 100,000 in 2018 (Table 2). Incidence in males was 0.6 per 100,000 in 2018; incidence in females was 0.7 in 2016, 0.6 in 2017 and 0.5 in 2018 per 100,000. The incidence of ABD was highest among patients aged 40–59 years in 2018 versus 2016 (Table 2). Incidence could not be calculated for groups with insufficient sample size (n < 5).
Comorbidities reported during observation years
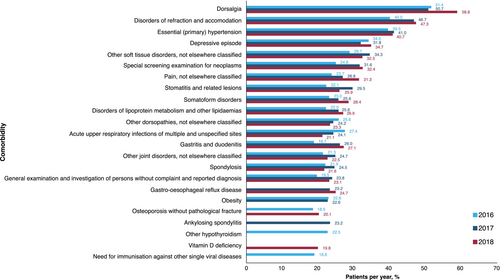
The three most common comorbidities reported during 2016–2018 in the treated prevalent population were dorsalgia (51.4%, 50.7% and 58.8%, in 2016, 2017 and 2018, respectively), disorders of refraction and accommodation (40.0%, 46.7% and 47.3%) and essential (primary) hypertension (39.5%, 41.0% and 40.7%) (Figure 3). These were also the three most common comorbidities reported in men (Table S1). In women, dorsalgia, depressive episode and other soft tissue disorders were the three most common comorbidities reported in 2016, while dorsalgia, other soft tissue disorders and disorders of refraction and accommodation were the three most common comorbidities reported in 2017 and 2018.
Use of disease-related medication
At least half of patients in the prevalent and incident populations did not receive any disease-related medication (Figure 4). The most commonly prescribed disease-related medications in the prevalent population were prednisolone (26.0%, 27.4% and 25.8% in 2016, 2017 and 2018, respectively), colchicine (14.0%, 16.8% and 18.2%) and azathioprine (8.3%, 9.9% and 10.6%) (Figure 4a); 14.3%, 17.2% and 15.2% of the prevalent population received more than one disease-related therapy in 2016, 2017 and 2018, respectively.
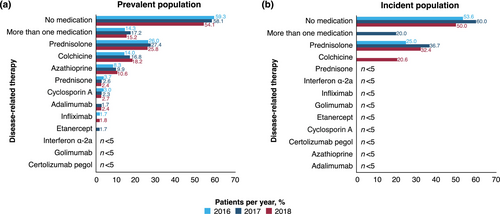
The most commonly prescribed disease-related medication in the incident population was prednisolone (25.0%, 36.7% and 32.4% in 2016, 2017 and 2018, respectively; Figure 4b); overall, 20.0% with incident ABD received more than one disease-related therapy in 2017. Approximately two-thirds to three-quarters of patients did not receive disease-related medication in the incident (65.2%), first (69.6%), second (73.9%) and third (67.4%) quarters after their ABD diagnosis (Table 3). The most common disease-related treatments after diagnosis were prednisolone (22.8% and 15.2%) and colchicine (both 10.9%) in the incident and first quarters, respectively, and prednisolone (12.0% and 15.2%), colchicine (both 10.9%), azathioprine (5.4% and 7.6%) or more than one disease-related therapy (both 6.5%) in the second and third quarters, respectively.
| Quarter 0 | Quarter 1 | Quarter 2 | Quarter 3 | Quarter 0–3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | n SHI | n (%) | n SHI | n (%) | n SHI | n (%) | n SHI | n (%) | n SHI | |
| No medication | 60 (65.2) | 1108.6 | 64 (69.6) | 1216.3 | 68 (73.9) | 1269.0 | 62 (67.4) | 1112.1 | 49 (53.3) | 873.7 |
| Azathioprine | <5 | – | <5 | – | 5 (5.4) | 115.3 | 7 (7.6) | 175.7 | 8 (8.7) | 202.3 |
| Prednisone | <5 | – | <5 | – | <5 | – | <5 | – | <5 | – |
| Prednisolone | 21 (22.8) | 401.7 | 14 (15.2) | 269.2 | 11 (12.0) | 224.2 | 14 (15.2) | 262.4 | 27 (29.4) | 520.5 |
| Cyclosporin A | <5 | – | – | – | – | – | – | – | <5 | – |
| Colchicine | 10 (10.9) | 217.5 | 10 (10.9) | 179.5 | 10 (10.9) | 194.6 | 10 (10.9) | 222.7 | 15 (16.3) | 311.5 |
| Interferon α-2a | <5 | – | <5 | – | <5 | – | <5 | – | <5 | – |
| Infliximab | <5 | – | <5 | – | <5 | – | <5 | – | <5 | – |
| >1 medication | <5 | – | <5 | – | 7 (7.6) | 144.7 | 7 (7.6) | 154.6 | 13 (14.1) | 270.0 |
- N = 92.
- Abbreviations: ABD, Adamantiades–Behçet's disease; SHI, statutory health insurance.
- a Sample size for azathioprine, cyclosporin A, colchicine, infliximab, interferon α-2a and prednisone was n < 5 in the research database; due to data protection rules, the exact numbers for sample size <5 cannot be provided.
DISCUSSION
In our retrospective cohort study evaluating the epidemiology of ABD in Germany, the mean age of patients in the treated prevalent population was similar across the 3-year observation period (50–52 years). Per 100,000 population, ABD prevalence increased from 3.9 in 2016 to 4.7 in 2018. Table 4 provides a summary of published prevalence data for ABD.7, 9, 16, 28-40 A 1997 epidemiology study using data of the GeR-ABD has noted an increasing disease prevalence over previous timepoints (1984, 1989 and 1994).7 Another GeR-ABD study in 2012 reported a prevalence of 0.9 per 100,000 population.9 While the prevalence data from our study are approximately four times higher than the GeR-ABD data, based on the European Union's criteria for rare diseases (defined as a disease affecting fewer than 5 patients per 10,000 population41), ABD remains a rare disease in Germany. Based on a 2018 meta-analysis of 45 reports published from 1974 to 2015, a pooled prevalence of 3.3 per 100,000 population was noted for Europe.16 Differences in study methodology, geographic location and environmental factors may account for observed variations in prevalence data across studies.16
| Author | Prevalence year | Study year | Patient inclusion criteria/analytical methodology | Study region | Study population | Prevalence (cases per 100,000) |
|---|---|---|---|---|---|---|
| Maldini et al.16 |
1974–2015 (pooled data) |
2018 | Meta-analysis |
Turkey, Middle East, Asia and Europe combined |
10.3 | |
| Turkey | 119.8 | |||||
| Middle East | 31.8 | |||||
| Asia | 4.5 | |||||
| Europe | 3.3 | |||||
| Zouboulis et al.7 | 1984 | 1997 | Fulfilment of the Behçet's disease classification tree criteria36 | Berlin-West, Germany | All patients | 0.65 |
| German | 0.16 | |||||
| Non-German | 4.51 | |||||
| 1989 | All patients | 1.68 | ||||
| German | 0.42 | |||||
| Non-German | 10.09 | |||||
| Turkish | 20.75 | |||||
| 1994 | All patients | 2.26 | ||||
| German | 0.55 | |||||
| Non-German | 11.04 | |||||
| Calamia et al.28 | 2000 | 2009 | Fulfilment of the ABD International Study Group criteria37 or clinical diagnosis | Olmsted County, Minnesota, USA | 5.2 | |
| Papoutsis et al.29 | 2005 | 2006 | Fulfilment of various ABD classification criteria b or clinical diagnosis | Germany | 0.72 | |
| Berlin, Germany | 4.87 | |||||
| Riewerts et al.30 | NS | 2010 |
Patient inclusion based on HCP questionnaires |
Baden-Württemberg, Germany | Turkish | 36.0 |
| Southern Württemberg, Germany | Turkish | 44.0 | ||||
| Baden-Württemberg, Germany | German | 0.8 | ||||
| Southern Württemberg, Germany | German | 0.8 | ||||
| Altenburg et al.9 | 1961–2011 c | 2012 | Fulfilment of the ABD International Study Group criteria37, 39 | Germany |
All patients (German and non-German) |
0.9 |
| Mohammad et al.31 | 2011 | 2013 | Fulfilment of the ABD International Study Group criteria37 | Skåne, Sweden | 4.9 | |
| Kappen et al.32 | NS | 2015 | Fulfilment of the ABD International Study Group criteria37 | Rotterdam, the Netherlands | Dutch-Caucasian | 1.4 |
| Turkish | 71.2 | |||||
| Moroccan | 39.0 | |||||
| Dehghan et al.33 | 201635 | 2021 | COPCORD core questionnaire38 | Yazd, Iran | Zoroastrian | 0.0 |
| Davatchi et al.34 | NS | 2017 | COPCORD core questionnaire38 | Iran (Tehran, Zahedan, Sanandaj and Tuyserkan) | All patients | 80 |
| Zouboulis et al. (current study) | 2016 | 2021 | Inpatient or outpatient diagnosis of ABD and prescription of disease-related therapy d | Germany | All patients | 3.9 |
| 2017 | 4.1 | |||||
| 2018 | 4.7 |
- Abbreviations: ABD, Adamantiades–Behçet's disease; COPCORD, Community Oriented Program for Control of Rheumatic Diseases; HCP, healthcare professional; NS, not specified.
- a Studies cannot be compared due to differences in the study methodologies used, geographic location and environmental factors.
- b Behçet's disease classification tree,36 International Study Group for Behçet's Disease criteria37 and Dilsen's criteria.40
- c Period of diagnosis.
- d Azathioprine, prednisone, prednisolone, cyclosporin A, colchicine, interferon α-2a, thalidomide, adalimumab, certolizumab pegol, etanercept, golimumab or infliximab.
The observed prevalence in 2016–2018 was stable in all age groups, except for patients aged 18–39 years, in whom a decreasing trend in ABD prevalence was noted. The prevalence per 100,000 was similar in male and female patients (ratio 1.01–1.11 from 2016 to 2018). This corroborates evaluations of male-to-female ratio in other European countries (1.01:1 in Portugal and 1.38:1 in Spain) and in a 2015 analysis of the 2012 GeR-ABD data (~1.39:17, 18).
In our study, ABD incidence was 0.5–0.6 per 100,000 population between 2016 and 2018. Incidence decreased in females, and male ABD incidence trend could not be reported due to insufficient sample size (n < 5) during 2016–2017. A small number of European studies have reported ABD incidence rates, including 1.0 per 100,000 population in Germany, 0.24 per 100,000 population in Italy and 0.2 per 100,000 population in Sweden.31, 42, 43
Patients in our study most commonly experienced dorsalgia, disorders of refraction and accommodation, and hypertension. Dorsalgia could be indicative of misdiagnosis, as it is a clinical sign for Bechterew's disease and not for ABD.44, 45 This is also true for dorsopathy and spondylosis; hence, some of the comorbidities reported in this study highlight that misdiagnosis between ABD and other diseases could occur in clinical practice. If a coinciding dorsalgia diagnosis were to be taken as an indicator for misdiagnosis, excluding those patients may largely correct for the observed increase in prevalence between 2016 and 2018, indicating a stable prevalence of ABD over the study period and aligning the observed data more closely with those reported from the GeR-ABD (Table S2). This may indicate that the true prevalence rates are between the ones reported in this study with a possible trend for misdiagnosis and the ones reported by the GeR-ABD, which are specific but likely associated with underdiagnosis, as not all patients, but mostly severe cases, with ABD in Germany have been registered.
Additionally, the most common ophthalmological codes reported in this study appear to be preventative consultations or an ordinary symptom diagnosis (ICD H49–H52); the list lacks ocular ABD manifestations such as choroid and retinal disorders (ICD H30–H36); vitreous disorders (H43–H45); optic nerve disorders (H46–H48); and vision disorders and blindness (H53–H54). This could be due to an increased number of routine ophthalmological consultations in patients with ABD, versus the rest of the SHI population, leading to frequent reporting of common ophthalmologic symptoms unrelated to ABD. Among the most common comorbidities, arterial hypertension was the only full diagnosis noted, albeit with a clinical but not with an etiopathological relationship to ABD. Similarly, other studies have reported hypertension as a comorbidity that may be associated with ABD, although further investigation is required to confirm statistical significance.46, 47 In the present study, depressive episodes were noted in >30% of patients, in line with the psychological and neurocognitive sequelae associated with ABD, with depression and anxiety the most frequently researched psychological conditions.48
At least half of patients in the prevalent and incident populations were not prescribed any disease-related medications, which may reflect the rarity of ABD and consequent lack of familiarity among treating physicians, leading to underdiagnosis. Many patients present with one-time symptomatology, mild symptoms of arthropathy and oral aphthosis or permanent orogenital erosions, which can lead to misdiagnosis of ABD. Approximately 10% of patients with ABD do not receive treatment49 and >50% do not adhere to treatment.50 It is plausible that patients with mild ABD do not need treatment. Ineffectiveness/side effects of treatment and lack of disease awareness are potential factors attributed to why patients do not receive, or poorly adhere to, therapy, at least in Germany, where the majority of population receives mandatory health insurance.50, 51
In our study, prednisolone, colchicine and azathioprine were the most commonly prescribed ABD medications; following ABD diagnosis, patients were most commonly prescribed prednisolone. These results reflect the 2018 EULAR recommendations for the use of these drugs to treat specific ABD phenotypes. Subsequent to initiation of our study, new treatment options such as apremilast have been introduced.52 Apremilast was not included in this analysis because it had not been approved for use at study initiation.
We acknowledge that our study has certain limitations. First, as with any health claims data analysis, the data may not capture patients' complete medical history. Thus, extrapolation to the German SHI population may result in bias in prevalence and incidence estimate evaluations if there are gaps in the medical history of patients, and/or if there are deviations in other variables, such as ethnicity and organ involvement. Moreover, GeR-ABD have reported ethnicity-based differences in the prevalence of ABD.7, 53, 54 This study could not report epidemiological data based on ethnicity in Germany, owing to a lack of relevant data in the claims database. Second, as a rare disease, ABD could be misdiagnosed/underdiagnosed, leading to coding errors and the reporting of comorbidities unrelated to or uncommon in ABD.55 This study was designed to minimize the inclusion of false-positive cases, that is, via the assessment of various case definitions, including restrictive definitions of inpatient diagnoses and confirmed outpatient diagnoses, combined with typical ABD prescriptions. However, despite the stringent criteria used in this study, false-positive cases may have been included owing to the multifaceted risk of misdiagnosis. Thirdly, as the analyses in this study are descriptive, statistical comparisons could not be made. In addition, comparisons of prevalence data across studies are difficult to interpret and may be invalid due to differences in study methodologies used, geographic location and environmental factors. Finally, stringent data protection measures meant that data for groups with n < 5 could not be included in this analysis.
CONCLUSIONS
In Germany, ABD remains a rare disease despite the observed increase in prevalence from 2016 to 2018, which may be due to misdiagnosis of ABD. Many patients with ABD were reported to have comorbidities, with dorsalgia, disorders of refraction and accommodation, and hypertension being the most common. While dorsalgia is not a clinical sign of ABD, disorders of refraction and accommodation, and hypertension, are known consequences of the disease. At least half of the patients in our analyses did not receive any ABD-related medication. Although the knowledge and management of ABD have improved dramatically in recent years, additional data from randomized controlled trials and real-world evaluations are needed to improve clinical outcomes. Statistics from SHI systems and real-world evaluations using data from disease registries could help to raise awareness of this rare vascular disease and, ultimately, improve disease management and day-to-day patient care.
AUTHOR CONTRIBUTIONS
JB, RH and TS were involved in the study conception and design of the analyses. All authors contributed to the interpretation of data and the preparation and revision of the manuscript and have given their approval for this version to be published.
ACKNOWLEDGEMENTS
We thank all patients and investigators involved in the study. We also thank Alexandra Stein, MSc (Amgen GmbH, Munich, Germany) for contributions towards interpretation of data and revision of the manuscript. Medical writing support, including development of a draft outline and subsequent drafts in consultation with the authors, assembling tables and figures, collating author comments, copyediting, fact checking and referencing, was provided by Sreerekha Pillai, PhD, CMPP, and Heather Davies, PhD, CMPP, at Aspire Scientific Ltd. (Bollington, UK). Funding for medical writing support for this article was provided by Amgen GmbH (Munich, Germany). The Departments of Dermatology, Venereology, Allergology and Immunology, Staedtisches Klinikum Dessau, Dessau, Germany are healthcare providers of the European Reference Network for Rare and Complex Skin Diseases (ERN Skin-ALLOCATE Skin group).
FUNDING INFORMATION
This study was funded by Amgen GmbH (Munich, Germany). The study sponsor played a role in the study design, data analysis, preparation of the manuscript and decision to publish.
CONFLICT OF INTEREST STATEMENT
C. C. Zouboulis is the President of the Deutsches Register Morbus Adamantiades–Behçet e.V.; a board member of the International Society for Behçet's Disease; and has received lecture honoraria from Amgen. J. Borchert is an employee of WIG2 GmbH which received research funding from Amgen for this study. J. Diesing is an employee of WIG2 GmbH which received research funding from Amgen for this study. R. Heinrich is an employee of WIG2 GmbH which received research funding from Amgen for this study. W. Galetzka was an employee of InGef GmbH at the time of this study. J-P. Medelnik is an Amgen employee and a stockholder of Amgen. A. Altenburg is the General Secretary of the Deutsches Register Morbus Adamantiades–Behçet e.V. and the International Society of Behçet's Disease. A. Feldhus was an employee of Amgen at the time of this study. T. Schönfelder is an employee of WIG2 GmbH which received research funding from Amgen for this study.
ETHICS STATEMENT
As this study used anonymized secondary data (i.e. administrative health insurance claims data), ethics approval and consent to participate were not applicable.
Open Research
DATA AVAILABILITY STATEMENT
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request. The data that support the findings of this study are available from the study sponsor Amgen (GmbH) upon reasonable request. Qualified researchers may request data from Amgen studies. Complete details are available at the following: http://www.amgen.com/datasharing.