Repigmentation by body region in patients with vitiligo treated with ruxolitinib cream over 52 weeks
Vitiligo is a chronic autoimmune disease that targets melanocytes, resulting in patches of skin depigmentation.1, 2 Repigmentation is an important treatment goal; however, the likelihood of repigmentation is affected by the location of vitiligo lesions.3, 4 The face, trunk and proximal extremities are generally more responsive to repigmentation than bony prominences, hands and feet.4
Ruxolitinib cream, a topical Janus kinase (JAK) 1/JAK2 inhibitor5 and the first approved repigmentation therapy for vitiligo, was statistically superior to vehicle at Week 24 and was well tolerated in two Phase 3 studies of adults and adolescents ≥12 years with nonsegmental vitiligo (TRuE-V1/TRuE-V2, NCT04052425/NCT04057573).5 In subanalyses from an earlier Phase 2 study (NCT03099304), ruxolitinib cream produced clinically meaningful repigmentation of all body areas, including hands and feet.6
We evaluated the effect of ruxolitinib cream on achievement of ≥50% improvement in Vitiligo Area Scoring Index (VASI) from baseline (VASI50) by body region (head/neck [excluding face], upper extremities [excluding hands], hands, trunk [including genitals], lower extremities [excluding feet] and feet; and all body regions combined [excluding face]) using pooled data from the TRuE-V studies.
Detailed study methodology and baseline characteristics of the 674 patients randomized in the TRuE-V studies were previously published.5 The 24-week, double-blind, vehicle-controlled period included 661 patients (ruxolitinib cream, n = 443; vehicle, n = 218); 569 patients continued in the 28-week open-label extension (ruxolitinib cream only, n = 385; crossover from vehicle, n = 184). Baseline mean facial and total VASI scores were 0.92 and 6.66, respectively.
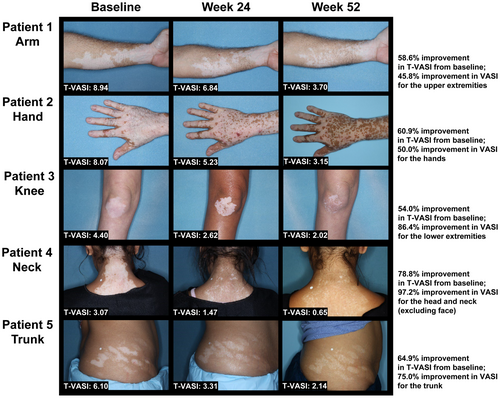
For all body regions (excluding face), VASI50 response rates increased steadily through 52 weeks among patients treated with ruxolitinib cream only (Weeks 12/24/40/52: 8.7%/20.8%/37.0%/47.7%, respectively; Figure 1a). A similar response pattern was observed among patients who crossed over from vehicle after Week 24 (Figure 1a). Among patients who applied ruxolitinib cream only, VASI50 response rates rose steadily through Week 52 for both the head/neck (excluding face; Weeks 12/24/40/52: 28.3%/45.3%/60.0%/68.1%) and trunk (Weeks 12/24/40/52: 15.5%/26.4%/38.8%/48.4%; Figure 1b). VASI50 patterns of response were similar for upper extremities (Weeks 12/24/40/52: 14.1%/33.2%/50.2%/56.7%) and lower extremities (Weeks 12/24/40/52: 15.1%/29.5%/46.1%/54.5%; Figure 1c). Response rates for hands and feet were lower overall, peaking at Week 46 (hands, 40.5%; feet, 30.7%) and tapering off slightly thereafter. Nevertheless, at Week 52, 38.2% and 29.3% achieved VASI50 for hands and feet, respectively (Figure 1d). Regimentation mostly occurred on the dorsal areas of hands and feet versus the tips of the fingers and toes. Representative clinical images are shown in Figure 2.
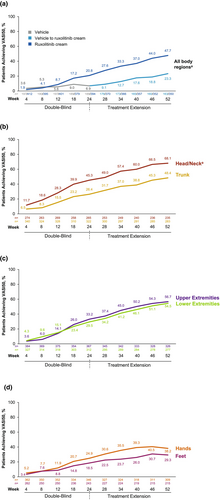
These clinical data demonstrate that although repigmentation is markedly affected by lesion location, repigmentation outcomes typically improve with longer treatment duration. In our analysis of ruxolitinib cream clinical data, head/neck regions (excluding the face, which is most responsive to repigmentation) repigmented best, followed by upper and lower extremities and the trunk. Hands and feet were most difficult to repigment, although approximately one-third of patients achieved VASI50 in these areas at Week 52. Interestingly, with the exception of hands and feet, no body areas reached a response plateau over 52 weeks, demonstrating the benefit of longer-term treatment. Extremities of the fingers and toes are notably more resistant to repigmentation.4, 6, 7 The causes of repigmentation resistance are likely complex, and further research to identify additional targets for achieving optimal repigmentation is warranted. Furthermore, studies assessing the efficacy of combination therapy (e.g. ruxolitinib cream and phototherapy) or newer topical drugs targeting alternative pathways are warranted, particularly for patients with vitiligo in areas most difficult to repigment.
In summary, based on pooled analyses from the TRuE-V1/TRuE-V2 Phase 3 studies of ruxolitinib cream for treatment of vitiligo, a substantial percentage of patients applying ruxolitinib cream achieved meaningful repigmentation across body regions after 52 weeks of treatment.
ACKNOWLEDGEMENTS
Writing assistance was provided by Samantha Locke, PhD, a medical writer and employee of ICON (Blue Bell, PA, USA) and was funded by Incyte Corporation (Wilmington, DE, USA).
FUNDING INFORMATION
This study was funded by Incyte Corporation (Wilmington, DE, USA).
CONFLICT OF INTEREST STATEMENT
TP has received grants and/or honoraria from AbbVie, ACM Pharma, Almirall, Amgen, Astellas, Bristol Myers Squibb, Calypso, Celgene, Galderma, Genzyme/Sanofi, GlaxoSmithKline, Incyte, Janssen, LEO Pharma, Eli Lilly, Novartis, Pfizer, Sun Pharmaceuticals, UCB and Vyne Therapeutics; is the cofounder of NIKAIA Pharmaceuticals and has patents on WNT agonists or GSK3β antagonist for repigmentation of vitiligo and on the use of CXCR3B blockers in vitiligo. JEH has served as a consultant for AbbVie, Aclaris Therapeutics, BiologicsMD, EMD Serono, Genzyme/Sanofi, Janssen, Pfizer, Rheos Medicines, Sun Pharmaceuticals, TeVido BioDevices, The Expert Institute, 3rd Rock Ventures and Villaris Therapeutics; has served as an investigator for Aclaris Therapeutics, Celgene, Dermira, EMD Serono, Genzyme/Sanofi, Incyte, LEO Pharma, Pfizer, Rheos Medicines, Stiefel/GlaxoSmithKline, Sun Pharmaceuticals, TeVido BioDevices and Villaris Therapeutics; holds equity in Aldena Therapeutics, NIRA Biosciences, Rheos Medicines, TeVido BioDevices and Villaris Therapeutics; is a scientific founder of Aldena Therapeutics, NIRA Biosciences and Villaris Therapeutics; and has patents pending for IL-15 blockade for treatment of vitiligo, JAK inhibition with light therapy for vitiligo and CXCR3 antibody depletion for treatment of vitiligo. AGP has served as an investigator for Aclaris Therapeutics, Immune Tolerance Network, Incyte and Pfizer; a consultant for AbbVie, Arcutis, Avita Medical, Immune Tolerance Network, Incyte, Pfizer, Thalocan, TWi, Viela Bio, Vimela, Villaris, Vyne and WCG/Trifecta; and holds stock options for Tara Medical and Zerigo Health. JS has received grants and/or honoraria from AbbVie, Bristol Myers Squibb, Calypso Biotech, Eli Lilly, Incyte, LEO Pharma, Novartis, Pfizer, Pierre Fabre, Sanofi, Sun Pharmaceuticals and Viela Bio; and has patents on MMP9 inhibitors and uses thereof in the prevention or treatment of a depigmenting disorder and three-dimensional model of depigmenting disorder. PG has served as a consultant for Aclaris Therapeutics, Clarify Medical, DermaForce, Incyte, Proctor & Gamble and Versicolor Technologies; and a principal investigator for Aclaris Therapeutics, Allergan/SkinMedica, Clinuvel Pharmaceuticals, Incyte, Johnson & Johnson, L'Oreal, Merz Pharma, Pfizer, Thync Global Inc. and VT Cosmetics. DK is an employee and shareholder of Incyte. MW was an employee and shareholder of Incyte at the time of the study. KE is a consultant for AbbVie, Incyte, La Roche-Posay, Pfizer, Pierre Fabre, Sanofi and Viela Bio. DR has received honoraria as a consultant for AbbVie, Abcuro, AltruBio, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Concert, Dermavant Sciences, Dermira, Incyte, Janssen, Kyowa Kirin, Lilly, Novartis, Pfizer, Regeneron Pharmaceuticals, Revolo Biotherapeutics, Sanofi, Sun Pharmaceuticals, UCB and Viela Bio; has received research support from AbbVie, Amgen, Bristol Myers Squibb, Celgene, Dermira, Galderma, Incyte, Janssen, Lilly, Merck, Novartis, Pfizer and Regeneron Pharmaceuticals; and has served as a paid speaker for AbbVie, Amgen, Celgene, Janssen, Lilly, Novartis, Pfizer, Regeneron Pharmaceuticals and Sanofi.
ETHICS STATEMENT
The trial protocols were reviewed and approved by an institutional review board or ethics committee at participating centres. The trials were conducted in accordance with the Declaration of Helsinki and adhered to Good Clinical Practice guidelines and applicable country-specific laws and regulations. Written informed consent or assent was provided by all patients. The patients in this article have given written informed consent or assent for publication of their case details.
Open Research
DATA AVAILABILITY STATEMENT
Incyte Corporation (Wilmington, DE, USA) is committed to data sharing that advances science and medicine while protecting patient privacy. Qualified external scientific researchers may request anonymized datasets owned by Incyte for the purpose of conducting legitimate scientific research. Researchers may request anonymized datasets from any interventional study (except Phase 1 studies) for which the product and indication have been approved on or after 1 January 2020 in at least one major market (e.g. US, EU and JPN). Data will be available for request after the primary publication or 2 years after the study has ended. Information on Incyte's clinical trial data sharing policy and instructions for submitting clinical trial data requests are available at: https://www.incyte.com/Portals/0/Assets/Compliance%20and%20Transparency/clinical-trial-data-sharing.pdf?ver=2020-05-21-132838-960.




