Overnutrition in the early postnatal period influences lifetime metabolic risk: Evidence for impact on pancreatic β-cell mass and function
Abstract
Overconsumption of energy-rich foods that disrupt caloric balance is a fundamental cause of overweight, obesity and diabetes. Dysglycemia and the resulting cardiovascular disease cause substantial morbidity and mortality worldwide, as well as high societal cost. The prevalence of obesity in childhood and adolescence is increasing, leading to younger diabetes diagnosis, and higher severity of microvascular and macrovascular complications. An important goal is to identify early life conditions that increase future metabolic risk, toward the goal of preventing diabetes and cardiovascular disease. An ample body of evidence implicates prenatal and postnatal childhood growth trajectories in the programming of adult metabolic disease. Human epidemiological data show that accelerated childhood growth increases risk of type 2 diabetes in adulthood. Type 2 diabetes results from the combination of insulin resistance and pancreatic β-cell failure, but specific mechanisms by which accelerated postnatal growth impact one or both of these processes remain uncertain. This review explores the metabolic impact of overnutrition during postnatal life in humans and in rodent models, with specific attention to the connection between accelerated childhood growth and future adiposity, insulin resistance, β-cell mass and β-cell dysfunction. With improved knowledge in this area, we might one day be able to modulate nutrition and growth in the critical postnatal window to maximize lifelong metabolic health.
INTRODUCTION
Metabolic diseases, such as obesity and type 2 diabetes, afflict a significant proportion of adults worldwide, leading to preventable deaths, reduced health and productivity losses.1 The prevalence of obesity in childhood and adolescence has also increased, escalating risk for metabolic disease in adulthood.2 Concerningly, youth with impaired glucose tolerance (IGT) or type 2 diabetes have an accelerated course of diabetes progression and complications.3 There is an urgent need to understand the early life causes of metabolic disease. Type 2 diabetes is characterized by insulin resistance combined with insufficient insulin secretory capacity. Loss of pancreatic β-cell mass and function are key contributors to the progression to type 2 diabetes, but molecular mechanisms are not yet sufficiently understood to support development of rational therapeutic approaches.
Fetal and early postnatal life represent two important developmental programming windows that impact adult metabolic health.4-7 Surprisingly, both nutritional overabundance and deficiency have deleterious impacts on future metabolism. Roles played by the in utero nutrition environment on offspring metabolism have been comprehensively reviewed.6, 7 Less attention has been paid to the early postnatal period, even though rapid growth after birth is a major contributor to worsened adult cardiometabolic outcomes.8, 9
Nutrients are key regulators of adult β-cell mass and function,10-12 but whether the early postnatal nutritional environment impacts β-cell development during this key window in which functional β-cell mass is established is not well understood. This review summarizes human and rodent studies illuminating the harmful impact of postnatal overnutrition on long-term metabolic health, with a focus on the incomplete current understanding of how postnatal nutrition influences development of pancreatic β-cell mass and function. We highlight the many areas in which more investigation is needed.
HUMAN CHILDHOOD GROWTH TRAJECTORY IMPACTS ADULT CARDIOMETABOLIC RISK
The remarkable long-term studies summarized here outline a critical pre- and postnatal human developmental window during which future metabolic health is determined. The impact of early life nutrition across the human lifespan underlines the need for improved understanding of the process by which a healthy individual develops, and the mechanisms of disruption by nutritional influences.
Prenatal period: Both low and high birthweight increase lifelong cardiometabolic risk
Evidence connecting fetal undernutrition with childhood and adult cardiometabolic parameters, a relationship termed the Developmental Origins of Health and Disease, has been reviewed6, 7 and is briefly summarized here. In 1991, a landmark study was published linking low birthweight with IGT and type 2 diabetes. A total of 468 men in Hertfordshire, the UK, underwent an oral glucose tolerance test at age 59–70 years. The men who had the lowest birthweight had a higher prevalence of IGT, type 2 diabetes, blood pressure and death from cardiovascular disease.13, 14 After this publication, studies examined intrauterine undernutrition due to famine in the Netherlands, Ukraine or China, finding increased risk of IGT, hyperinsulinemia and type 2 diabetes in offspring exposed to famine during gestation.15-17 In utero growth itself seems to be a critical factor; of genetically identical twins born from the same in utero environment, the twins that developed type 2 diabetes later in life had significantly lower birthweight than the twins who did not.18 Intriguingly, studies have also linked high birthweight with adult risk of type 2 diabetes, possibly due to the influence of maternal hyperglycemia or diabetes propensity.19 These studies and others show that the human fetal nutritional environment influences metabolic health across the lifespan.
Postnatal period: Accelerated childhood growth is associated with worse metabolic health
After birth, human studies from around the globe show that rapid childhood growth, defined generally as growth that exceeds the mean rate of growth of the population studied, leads to unhealthy metabolic parameters in childhood, adolescence and adulthood. Compelling data come from India. In one study of 477 8-year-olds, children with higher current bodyweight and adiposity had higher systolic blood pressure, plasma insulin, proinsulin, insulin resistance, low-density lipoprotein and triglycerides. The strongest trends were seen for children with accelerated growth.20 A second study analyzing 1,492 Indian young adults age 26–32 years found those with IGT or type 2 diabetes had accelerated body mass index (BMI) accrual during ages 2–12 years relative to their peers.21 Only 0.5% of the IGT/type 2 diabetes group met International Obesity Task Force criteria for obesity at 16 years-of-age, suggesting the weight trajectory might be more important than the absolute weight.
Studies carried out in Helsinki, Finland, found a similar relationship between postnatal growth and adult metabolic parameters.22-24 A total of 7,086 men and women born between 1924 and 1933 who developed type 2 diabetes as adults had faster growth between the ages of 7 and 15 years than their peers.22 A second cohort born at the same hospital between 1934 and 1944, found that rapid BMI increase over years 2–11 was associated with increased risk of type 2 diabetes regardless of birthweight.23 The risk of coronary events in adulthood was strongly related to the rate of childhood growth after 2 years-of-age; the data did not support a harmful effect of rapid growth before age 2 years.24
Rapid postnatal weight gain correlated with impaired metabolic health in black children in South Africa at 7 years-of-age.25-27 Children with the highest weight velocity between birth and 7 years had elevated insulin levels and insulin resistance at age 7 years, as well as increased adiposity.25
There is uncertainty whether rapid growth in the first 1–2 years after birth is as detrimental as rapid growth later in childhood. In India, future IGT or type 2 diabetes was associated with accelerated growth only after age 2 years.21 In South African children, accelerated growth between birth and 1 year did not adversely impact metabolism at age 7 years, but excess growth after 1 year did.27 In contrast, the Avon Longitudinal Study of Pregnancy and Childhood found that more weight gain between ages 0–3 years predicted higher BMI and waist circumference, and lower insulin sensitivity at age 8 years.28 In a low birthweight cohort in the Netherlands, weight gain from 0 to 3 months was associated with reduced insulin sensitivity and increased acute insulin response in adulthood.29 Intriguingly, a Helsinki cohort suggested that rapid gain in BMI after age 2 years increased future risk most acutely in individuals who had slow growth in length between birth and 3 months of age.23
Accelerated childhood growth is particularly detrimental in the setting of low birthweight
The combination of low birthweight, indicating poor prenatal growth, with accelerated postnatal growth, also termed catch-up growth, leads to poor metabolic outcomes in later childhood and adulthood. In Indian children, the highest levels of insulin resistance were found in children with low birthweight, but high childhood fat mass.20 Intriguingly, that study found a relationship with intergenerational height comparisons: the most insulin resistant were tall children who had short parents. A second Indian study found that the combination of low BMI in infancy with accelerated BMI increase after age 2 years was associated with IGT or type 2 diabetes at age 26–32 years.21 Young adults with low birthweight in the Netherlands with catch-up growth had worse insulin sensitivity than individuals with low birthweight who remained small.30, 31 Childhood insulin resistance in the Avon cohort was highest in those who were born small and had catch-up growth.28, 32 An analysis of factors influencing prenatal and postnatal growth found that maternal smoking and primiparous pregnancy were both associated with low birthweight with vigorous catch-up growth, whereas breastfeeding was associated with normal birth size, but slower growth in infancy.33 Catch-up growth is common in low birthweight children.29, 34
In sum, data from multiple populations show that low birthweight and accelerated childhood growth independently increase metabolic risk in adulthood, and the effect is especially prominent in individuals with both conditions (Figure 1). Studies testing whether this increased metabolic risk is related to insulin resistance or impaired or β-cell mass or function will be examined next.
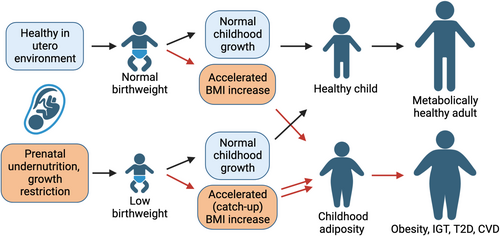
MECHANISMS BY WHICH HUMAN POSTNATAL GROWTH TRAJECTORY IMPACTS FUTURE GLUCOSE TOLERANCE
Accelerated early childhood growth increases future risk of IGT and type 2 diabetes, even decades later as adults. Understanding the metabolic mechanisms could guide future childhood interventions to prevent type 2 diabetes, as well as potentially inform clinical decision-making in the adolescent and adult. Here, we review evidence from human studies that accelerated postnatal growth impacts future adiposity, insulin resistance and β-cell mass or function.
Accelerated postnatal growth increases total and central adiposity
Rapid growth even very early in life leads to a relative increase in fat mass, and specifically abdominal fat, over lean mass. In the UK, greater weight gain between 0 and 3 years-of-age increased BMI and waist circumference.28 Accelerated growth between 0 and 2 years-of-age resulted in 5-year-olds with higher BMI, body fat percent by skinfold and waist circumference.32 Catch up growth in the first year of life resulted in more fat mass and trunk mass in early adulthood, whereas lean mass was unaffected.31 South African children with accelerated weight gain between 0 and 7 years-of-age had increased subscapular and triceps skinfold thickness, especially if they also had low birthweight.25 In Indian children, subscapular to triceps skinfold ratios were highest in low birthweight children who had high bodyweight at 8 years-of-age. This same group also had the highest total cholesterol, homeostasis model assessment of insulin resistance (HOMA-IR) and systolic blood pressure.20 In another study of Indian children, the earlier the date of ‘adiposity rebound’, defined as the age after infancy at which the BMI begins to rise, the higher the BMI in later childhood, as well as higher prevalence of IGT and type 2 diabetes at age 30 years.21 These studies show that accelerated postnatal growth leads to body composition changes including increased total and truncal adiposity.
Accelerated postnatal growth leads to insulin resistance
Strong evidence indicates that rapid growth in childhood increases the risk of IGT and type 2 diabetes by increasing insulin resistance. Indian children with low birthweight, but high fat mass at 8 years-of-age had higher HOMA-IR; current fat mass increased HOMA-IR across all birthweight strata, but the effect was most pronounced in the children with lower birthweight.20 In another Indian cohort, the adults with IGT and type 2 diabetes, which were associated with accelerated childhood BMI increase, had increased fasting and 120 min plasma insulin levels and HOMA-IR.21 In Helsinki, adult fasting plasma insulin and proinsulin were positively associated with BMI change between 2 and 11 years, with the highest levels in the individuals with the largest childhood increase in BMI.24 In the Netherlands, accelerated postnatal growth increased adult insulin resistance, even after adjusting for adult adiposity.29 In 8-year-olds in the UK, HOMA-IR was positively associated with increased weight gain between 0–3 years-of-age.28 Children in China who were born small for gestational age and then experienced catch-up growth had higher HOMA-IR and fasting insulin after age 6 years.35
A few studies have evaluated the impact of postnatal growth pattern on insulin sensitivity using dynamic techniques, including the frequently sampled intravenous glucose tolerance test (FSIGT) or hyperinsulinemic euglycemic clamp. These data also show that accelerated childhood growth leads to insulin resistance. In the Netherlands, insulin sensitivity was assessed in 9-year-old children born small for gestational age (SGA) using hyperinsulinemic clamp. SGA children without catch-up growth had similar insulin sensitivity to controls born normal size, but SGA children with catch-up growth were more insulin resistant, especially those with BMI >17 kg/m2 at the time of study.36 A study using FSIGT found that 21-year-olds who were SGA with catch-up growth had lower insulin sensitivity and higher insulin secretion than participants who were SGA without catch-up growth.30 When the same individuals were studied at 32 years-of-age by FSIGT, participants with SGA without catch-up growth still had similar insulin sensitivity as control participants, but those with SGA with catch-up growth showed persistent insulin resistance compared with other groups.31 Taken together, many studies in different populations support the concept that accelerated postnatal growth leads to insulin resistance later in childhood and in early adulthood. The data are most pronounced in individuals with reduced prenatal growth who subsequently increase weight quickly relative to their peers.
Does accelerated postnatal growth impair development of β-cell function or mass?
As the pathogenesis of type 2 diabetes requires both insulin resistance and β-cell insufficiency,37 and β-cell mass is established in early childhood,38 the field has speculated that early life insults such as impaired prenatal or accelerated postnatal growth might lead to reductions in β-cell mass, function or both. Human data supporting this hypothesis are less strong than for insulin resistance. As aforementioned, many studies have identified increased, not decreased, fasting and post-challenge insulin levels in individuals with rapid postnatal growth.15, 20, 21, 24-28, 39 Complicating progress are the dual challenges of dissecting β-cell function from insulin sensitivity in intact physiological systems, and the lack of tools to quantify β-cell mass in living people.
Accelerated postnatal growth lead insulin secretory function to be increased or unchanged. Some studies assessing β-cell function by static HOMA-β report insulin secretion to be unaffected by childhood growth pattern.20, 26 Oral glucose tolerance test-based dynamic insulin secretion estimated β-cell function to be increased25-28 or unchanged36 in individuals with rapid postnatal growth. FSIGT showed insulin secretion to be increased in infants39 or adults29 with accelerated postnatal weight gain; however, other studies also using FSIGT reported insulin secretion to be unchanged.30, 31 A study applying hyperglycemic clamp in young adults with a history of low birthweight and normal current glucose tolerance found insulin secretion was not impaired.40 In contrast, proinsulin levels were higher in children who were heavier20 and children with rapid growth, and were generally associated with increased insulin levels.24, 26, 27
Intriguingly, height might be important. One study found that the highest risk of type 2 diabetes later in life was in individuals with slow increase in length between 0 and 3 months-of-age, but rapid BMI gain after age 2 years.23 Rapid increase in length over the first year of life was associated with increased insulin secretion by FSIGT at 1 year.39 In contrast, 8-year-olds in the UK with short stature were noted to have lower insulin secretion after an oral glucose load. The reduced insulin secretion in shorter children persisted even after correcting for current BMI and insulin resistance.28 Note that in children, accelerated height accrual also suggests the onset of puberty.
Taken together, at the ages measured and using currently available techniques, accelerated childhood growth in weight is associated with increased or unchanged insulin secretion capacity, and possibly with impairment in insulin processing. These results are congruent with the Restoring Insulin Secretion (RISE) trial observation that adolescents with IGT or early type 2 diabetes have increased rather than decreased β-cell function.41, 42 How these observations relate to the future insulin secretion deficit that occurs with established type 2 diabetes remains unclear.
Although increased circulating insulin has traditionally been thought to reflect an appropriate secondary β-cell response to insulin resistance, a vigorously defended counter-hypothesis argues for early β-cell hyperfunction as a primary defect leading to insulin resistance.43-48 If the former hypothesis is correct, early β-cell hyperfunction might represent a secondary response to insulin resistance, eventually leading to β-cell fatigue, dysfunction and loss. In contrast, if the latter hypothesis is correct, early β-cell hyperfunction might represent an addressable primary defect that causes early insulin resistance as well as late β-cell fatigue, dysfunction and loss.
It is mostly unknown whether human β-cell mass accrual is impacted by postnatal overnutrition. Measurement of β-cell mass is currently most accurate by histology at autopsy or pancreatectomy, which are thankfully rare in childhood. In surgical pancreas specimens from adults, a weak positive correlation was observed between self-reported birthweight and both β-cell area and islet size in adults without diabetes.49 Intriguingly, the nondiabetic participants reporting childhood obesity had higher β-cell area, whereas those who developed obesity as adults did not.49 Removing the childhood obesity samples from the analysis further strengthened the relationship between birthweight and β-cell area, suggesting these growth patterns are independently associated with adult β-cell mass. Complicating the extrapolation of these data to other populations, obesity in adolescence did not increase the risk of diabetes in adulthood in a large Japanese cohort.50 The two-stage hyperglycemic clamp with arginine might estimate endogenous β-cell mass51, 52; to our knowledge, glucose-potentiated arginine stimulated insulin secretion has not yet been used to determine whether postnatal growth trajectory influences accrual of β-cell mass.
RODENT MODELS: POSTNATAL OVERNUTRITION LEADS TO OBESITY AND INSULIN RESISTANCE
Mice and rats share many genetic and physiological characteristics with humans, and are used to model metabolic disease to allow genetically controlled studies, genetic or risky interventions and post-mortem experimental analyses.53 In rodents, early postnatal overnutrition increases diabetes susceptibility (Figure 2); like humans, increased adiposity and insulin resistance is evident, whereas a negative impact on β-cell mass or function is less clear.
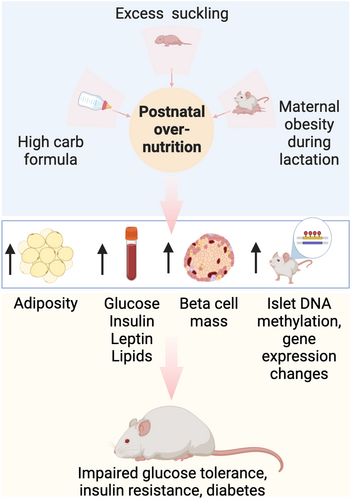
Increased postnatal food consumption leads to increased offspring bodyweight and fat mass
Rodents reared in small litters are prone to early overfeeding, causing them to be heavier and fatter than normal-litter rodents by weaning age, with higher bodyweights as adults.54-58 Intriguingly, pups fed through gastrostomy with a high-carbohydrate formula, but not a high-fat formula, had faster growth and were prone to obesity in later life in comparison with naturally reared pups.59, 60
Maternal obesity impacts offspring health. Pups reared by diet-induced obese dams were heavier than controls at 10 days and 3 months-of-age.61 Male offspring of lean mothers, cross fostered to obese dams, gained 12% more bodyweight from P2 to weaning than those nursed by lean dams, and had increased adiposity as adults.62 Another study, however, found young adult offspring of dams fed an obesogenic diet from pre-pregnancy through lactation to have similar body mass to offspring of mothers fed standard chow.63 As adolescents and adults, offspring that were maintained on fat-rich diets throughout postnatal life had significantly higher daily food intake, increased body mass and adiposity, and reduced energy expenditure compared with offspring nursed by mothers on standard chow.64-69
Postnatal overnutrition increases blood glucose, insulin, leptin, lipids, glucose intolerance and insulin resistance into adulthood
Rodents with early life overfeeding using the small-litter model maintained higher blood glucose and insulin levels during the pre-weaning period, and had elevated fasting glucose, leptin, HOMA-IR, and higher glucose and insulin levels after intravenous glucose challenge as young adults when compared with normal-litter rodents.54, 56, 58 Male offspring seem to be at higher risk for metabolic dysfunction than females. As young adults, postnatally overnourished males had elevated plasma triglyceride levels in addition to diabetes, whereas females remained normal.54 Diabetes incidence was further augmented by consumption of a high-fat diet in adulthood.54
Maternal obesogenic diet during lactation causes impaired glucose tolerance61, 64, 67, 70 and insulin resistance, as measured by an insulin tolerance test.64, 67 Some studies examined prenatal and postnatal exposure separately. When reared by mothers consuming a high-fat diet during lactation only, or throughout gestation and lactation, adult rodents had elevated levels of blood glucose, insulin, leptin and HOMA-IR compared with those reared by mothers on a standard chow diet.65, 68 Adolescent rats nurtured by mothers on a high-fat diet during prenatal or postnatal development were hyperinsulinemic and had higher HOMA-IR than rats fed by mothers on normal chow, but only the rats with postnatal high-fat diet exposure developed hyperglycemia.69 Similarly, adult offspring raised by mothers consuming a diet rich in fat and fructose during lactation developed increased blood glucose and plasma insulin and leptin.67 Adult mice that had been nursed by obese dams, through postnatal fostering, had 51% higher area under the curve for insulin during a glucose tolerance test than those nursed by lean dams, even though weight gain, food intake, glucose and random insulin levels were similar.62
Maternal diabetes can also lead to impaired glucose tolerance in offspring, even when the exposure is only during the lactation period. Neonatal rats born to healthy dams, but nursed postnatally by diabetic mothers, or normal rat embryos transferred to diabetic mothers for both the fetal and postnatal periods, both had poor glucose tolerance as adults.71 The lactation period was dominant; the extent of metabolic dysfunction was similar between offspring exposed to both prenatal and postnatal maternal diabetes and those exposed postnatally only. In another study, small-litter offspring of diabetic mothers had worsened insulin sensitivity index.58
Postnatal diet macronutrient composition impacts risk: early life high-carbohydrate diet leads to hyperinsulinemia and glucose intolerance. Intragastric cannulas in neonatal rats have been used to test the effects of directly feeding a modified high-carbohydrate or high-fat milk formula. Compared with naturally reared pups or pups fed high-fat formula, pups on high-carbohydrate milk were transiently hyperglycemic, but markedly hyperinsulinemic throughout the pre-weaning period,72 and were hyperinsulinemic and had elevated plasma glucose readings after glucose challenge, even much later in life.59, 60 Natural rodent milk is high in fat and low in carbohydrate73; however, maternal diet influences milk composition. Female rats consuming an obesogenic diet during the suckling period produced milk with elevated glucose, cholesterol, triglycerides and total protein.64 In another study, female rats fed an obesogenic diet for 3 months before mating, and throughout pregnancy and lactation produced milk with increased insulin, γ-linolenic 18:3(n-6) and eicosadienoic 20:2(n-6) acid levels, but lower levels of oleic 18:1(n-9) and α-linolenic 18:3(n-3) acids.62
Taken together, many studies confirm that overnutrition in the postnatal lactation period leads to increased bodyweight, adiposity and glucose intolerance associated with hyperinsulinemia and insulin resistance. As type 2 diabetes requires both insulin resistance and relative insulin deficiency, a key question is whether β-cell mass or function are impacted by postnatal overnutrition.
IN RODENTS, POSTNATAL OVERNUTRITION EXPANDS β-CELL MASS, BUT HAS VARIABLE IMPACT ON β-CELL FUNCTION
Because we are currently unable to accurately quantify β-cell mass, islet morphology or islet insulin content over time in human beings, rodent models provide most data on the impact of postnatal overnutrition on these parameters. In addition, rodent studies allow measurement of insulin secretion in vivo and ex vivo, and separation of insulin secretion from insulin production, insulin turnover and β-cell mass. Current knowledge regarding how postnatal overnutrition impacts islet structure and function in rodents is summarized in this section.
Postnatal overnutrition increases pancreatic β-cell mass
Adaptive growth of β-cell mass during lactational overfeeding is reported to occur quite early in postnatal life. At P14, small-litter male mice had markedly increased β-cell mass, even when normalized to body size.54 Increased β-cell mass persisted into adulthood; however, pancreatic insulin content was similar to controls, with an increased number of islets, but reduced insulin content per islet.54 Similarly, evidence of β-cell hyperplasia was present in P12 rat pups fed with direct gastric infusion of high-carbohydrate formula: pancreatic insulin content, percentage of insulin-positive cells and mean islet size were higher than in mother-fed pups.60 The increased β-cell number persisted into adulthood. In a third study, 3-month-old rats experiencing postnatal overnutrition only in the birth-to-weaning period, using the small-litter model, had increased islet number, islet area and insulin immunodensity compared with rats from normal litters.56
Maternal obesity also increases offspring β-cell mass. At P10, pups reared by diet induced obese mothers had increased mean islet diameter, islet mass-to-pancreas mass ratio, β-cell mass-to-pancreas mass and islet proliferating cell nuclear antigen-positive nuclei compared with control offspring.61 Maternal consumption of an obesogenic diet during lactation increased β-cell mass along with a higher proportion of large islets and lower proportion of small islets compared with offspring of mothers fed normal chow.66, 67 Pancreas mass, islet number, islet area and β-cell mass were also increased under these conditions. Islet diameter was enhanced when pups were also weaned to a high-fat diet.64, 66 An obesogenic diet fed only during the lactation period increased islet cell proliferation in 3-month-old rats.69 In contrast, the study on maternal obesogenic diet that did not identify increased bodyweight or adiposity in the offspring also observed no impact on β-cell mass and islet insulin content.63 Maternal diabetes during lactation might negatively affect β-cell mass accrual in the offspring. Neonatal rats born to nondiabetic dams, but nursed by diabetic mothers, had decreased β-cell mass and pancreatic insulin content as adults.71
Postnatal overnutrition might increase β-cell size. Postnatal-only exposure to a high-fat diet increased β-cell size and number, and α-cell number in 3-month-old male rats; in females, α-cell size was the only parameter that was increased.65, 69
Controversy exists regarding whether β-cell compensatory proliferation can occur in the pre-weaning period. A highly cited, carefully performed study in mice concluded that compensatory β-cell proliferation is not enabled until the weaning transition.74 This would seem to contradict the studies cited above that observed increased β-cell mass, islet size and, in some cases, β-cell proliferation, in response to early postnatal overnutrition due to small litter size, maternal obesity or direct feeding of high-carbohydrate formula. The study concluding compensatory replication is absent before weaning examined two models: diphtheria toxin mediated β-cell ablation, and glucokinase activator treatment. The weaning-related switch from higher-fat milk to higher-carbohydrate chow was identified as the trigger that allowed compensatory proliferation to activate. The discrepant conclusions might potentially be related to milk nutrient composition in the early postnatal studies, such as if the intervention reduced milk fat content or increased carbohydrate content. An obesogenic diet that contained sweetened condensed milk and added sucrose was found to increase glucose content of the milk.64 Both this model and the high-carbohydrate formula feeding model could mimic early weaning to a higher-carbohydrate state. We are uncertain whether high-fat feeding of dams or small litter size increases milk carbohydrate content, but lactose content does vary across strains, and duration of lactation75 and litter size impacts mammary gland development.76
Postnatal overnutrition might or might not impair insulin secretory function
In general, studies that report in vivo insulin deficiency also observe ex vivo insulin secretory dysfunction. Islets isolated from small-litter rat pups at P26 were comparable with large-litter islets in size and insulin content, but had impaired glucose-stimulated insulin secretion (GSIS) that persisted into adulthood at P110 and P280.57 This study also observed lower in vivo insulin levels in small-litter rats than controls. Normal neonatal rats cross fostered to diabetic mothers had low in vivo GSIS as adults; effects were more pronounced in males than females.71 In contrast, small-litter rats that were reported to have fasting hyperinsulinemia throughout the suckling phase were observed to have increased GSIS both in vivo and ex vivo at P90.56 Fetal and lactational, or lactational only, exposure to a maternal diet rich in fat and fructose led offspring to have hyperinsulinemia and increased ex vivo insulin release in response to stimulation with 25 mmol/L glucose.67 Adult female offspring with prenatal exposure to maternal obesity showed increased insulin secretion when stimulated by high glucose or leucine/glutamine, whereas there was no change in males.63
Taken together, studies analyzing ex vivo β-cell function after postnatal overnutrition generally show congruent results with insulin levels in vivo, with hyperinsulinemic mice showing increased GSIS ex vivo, and hypoinsulinemic mice showing reduced GSIS ex vivo. However, the literature as a whole does not clearly show how postnatal influences contribute to future β-cell failure. One study captured a dynamic change from hyperinsulinemia early after postnatal overnutrition to later progression to diabetes in some animals in adulthood; this study reported ex vivo GSIS to be unchanged, but GSIS was only measured in islets from nondiabetic animals.54
Molecular mechanisms linked to altered β-cell mass or function after postnatal overnutrition
Small-litter islets with reduced insulin content and GSIS contained lower levels of Ins2 messenger ribonucleic acid (mRNA) and other not-yet-identified genes (in 2002); many mRNA changes persisted into adulthood.57 Expression of Pdx1 and Neurod1, but not Foxo1, Nkx6-1 or Mafa, were decreased in postnatally overnourished males.54 Quantitative bisulfite pyrosequencing showed that islets from small-litter pups were hypermethylated at P21, with accelerated aging-related methylation patterns that resembled control mice at P180.55 Hypermethylation was associated with reduced gene expression of genes relevant to islet function including Ins2, Rab3b, Cacnb3 and Atp2a3.55 Postnatal or prenatal diet rich in fat and fructose led to mRNA and protein reduction of glucokinase and glucose transporter 2, but an increase in insulin, PGC-1α and G6Pase.67 Insulin and leptin signaling pathways were affected in the hypothalamus of offspring reared by obese mothers, characterized by downregulation of leptin receptor, Janus kinase 2, signal transducer and activator of transcription 3, insulin receptor β, phosphoinositide 3-kinases and protein kinase B at the protein level.64 Finally, evidence suggests maternal obesity negatively impacts islet mitochondrial function and stimulus-secretion coupling, particularly in males. Female offspring showed islet hyperresponsiveness at 8 weeks-of-age, with an increased insulin secretion and oxygen consumption rate after glucose challenge, associated with increased mRNA and protein abundance of electron transport chain components and mitochondrial area.63 Males, in contrast, did not show compensatory increase in insulin secretion, respiration or gene expression.63
Postnatal overnutrition is more consequential than prenatal overnutrition for programming future metabolic health in rodents
Two studies comparing the effect of maternal obesogenic diet restricted to either gestation or the lactation period on offspring health found that gestational overnutrition was similar to controls, but postnatal overnutrition led to higher bodyweight, adiposity, leptin, blood glucose and HOMA-IR levels, as well as increased β-cell number and size.65, 68 Bodyweight, leptin, and insulin levels were further elevated when the obesogenic diet included both gestational and lactational periods; however, blood glucose and β-cell number were actually lower when compared with postnatal exposure alone.65 Intriguingly, some of these phenotypes were absent or weaker at younger ages, but seemed to increase as the rats entered full adulthood.69 Similarly, fetal exposure to a high-fat high-fructose diet did not impact offspring health, but lactational or combined fetal-lactational exposure increased bodyweight, adiposity, liver fat content, plasma fatty acids and leptin, insulin resistance, and glucose intolerance.67 In this study, β-cell mass was markedly elevated by postnatal, but not prenatal diet exposure, and combined prenatal and postnatal diet exposure further increased β-cell mass.67
Additional evidence for a dominant role of postnatal nutrition during the lactation period comes from experiments in which offspring of lean or obese female rats were cross-fostered to lean or obese females for the nursing period.62 Offspring from lean mothers cross fostered to obese females gained 12% more bodyweight from P2 to weaning than those fostered to lean females, and had increased adiposity and insulin resistance as adults.62 In contrast, offspring from obese dams fostered to lean females gained 30% less bodyweight, and had improved insulin sensitivity, than those reared by obese females.62
Not all studies found postnatal overnutrition to be dominant; in some cases, results suggest additive or prenatal-dominant effects.77 One study in particular showed opposite results: insulin levels, glucose intolerance, adiposity and β-cell mass were all increased by gestational high-fat diet exposure, but not by lactational exposure.66 Comparing a carbohydrate-rich diet or a saturated fat rich diet across permutations of fetal or lactational exposure, diet condition did not impact offspring bodyweight, glucose, insulin, leptin or glycated hemoglobin percentage.78 Insulin production and insulin secretion by perifusion were also unaffected, but high-fat diet during gestation was negatively correlated, and high-fat diet during lactation was positively correlated, with insulin secretion tested by static incubation under certain conditions.78
Like humans, rodent prenatal undernutrition combined with postnatal overnutrition worsens metabolic dysfunction later in life
The impact of restricted fetal growth on β-cell growth and development has been studied in animal models, such as maternal protein deficiency and uterine artery ligation.79 Offspring with gestational undernutrition are more consistently observed to have increased risk for IGT and type 2 diabetes than offspring exposed to gestational overnutrition.79 β-Cell mass is reported to be lower after fetal undernutrition, even into adult life.80-82 A low-protein diet during the last week of gestation resulted in offspring that were metabolically normal at birth and early adulthood, but predisposed to type 2 diabetes due to insulin resistance and impaired β-cell adaptation.83 A low-protein diet throughout gestation resulted in reduced insulin levels, β-cell mass and β-cell proliferation at birth.84 One study found β-cell mass had recovered by 3 months-of-age,84 but another found β-cell mass recovery after streptozotocin to be impaired.85 The insulin secretion defect was due to reduced insulin production, associated with reduced Pdx1 expression in adulthood. Data also implicate impaired mitochondrial metabolism, oxidative stress, mammalian target of rapamycin signaling, epigenetic deoxyribonucleic acid modifications, and microRNAs in the impaired β-cell adaptability after gestational nutrition restriction.79 Taken together, the combination of prenatal undernutrition with postnatal overnutrition and catch-up growth is detrimental for rodent metabolic risk, as it is for human.
CONCLUSIONS AND GAPS IN KNOWLEDGE
Observations across many populations worldwide show that accelerated childhood growth increases the risk of adult cardiovascular disease and type 2 diabetes, especially when combined with prenatal undernutrition. Clear data link childhood overnutrition with increased total and central adiposity, and insulin resistance. Rodent studies also observe a strong relationship between postnatal overnutrition and adult adiposity and insulin resistance; the effect of overnutrition is stronger for the postnatal period than during gestation. Despite much attention to the hypothesis that pre- and postnatal nutritional insults increase type 2 diabetes risk by impairing β-cell mass and function, evidence in humans supporting this premise remains weak. Many studies document β-cell hyperfunction after postnatal overnutrition, and one study observed increased β-cell mass in adults who reported a history of childhood obesity. How these alterations relate to adult type 2 diabetes risk remains uncertain. Controlled studies show that rodents expand β-cell mass in response to early postnatal overnutrition, especially high carbohydrate exposure, whereas insulin secretory function is reported to be increased, decreased or unchanged. In contrast, fetal undernutrition or growth restriction consistently reduces β-cell mass in rodent models, with some studies indicating residual impaired compensatory adaptation into adulthood. Taken together, the present review of existing data suggests a model in which poor fetal growth impairs β-cell mass accrual, accelerated postnatal growth leads to adiposity and insulin resistance, and the combination leaves individuals at increased risk of type 2 diabetes.
There are many gaps in knowledge. Most prominent is the lack of knowledge around how in utero growth restriction impacts human β-cell mass accrual. When pre- and postnatal growth impacts on the accrual of β-cell mass and function are better understood, more questions will be evident regarding the mechanisms behind the observed biology. As β-cell mass is set in the first 5 years of life,38 and β-cell failure is a key component of type 2 diabetes risk decades later, the pre- and early postnatal period represents a key window to intervene to prevent future metabolic dysfunction. Medicine lacks clinical radiological and biomarker approaches to estimate β-cell mass, proliferation and cell death that could guide postnatal nutrition and growth goals. We also lack tools to correct the root cause of lost β-cell mass or function. Controversy exists regarding whether hyperinsulinemia is cause or consequence of the insulin resistance that leads to type 2 diabetes. Finally, work needs to be done on getting the message out to pediatricians and the public that accelerated childhood growth is potentially harmful, even, and perhaps especially, for those children who were born small.
DISCLOSURE
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
JJNB and LCA jointly conceptualized the review content, identified, read, and interpreted the primary literature, and drafted and revised the manuscript.
ACKNOWLEDGMENTS
Financial support for this work was provided by NIDDK grants R01DK124906, R01DK114686, R01DK113300 and R01DK135304 to LCA.




