A case of type 2 diabetes mellitus with weight gain and worsening of glycemic management after tezepelumab administration for severe bronchial asthma
Abstract
Some cases of bronchial asthma are refractory to conventional therapies. As the pathogenesis of bronchial asthma has been clarified, new treatments, such as bronchial thermoplasty and biological drugs, have been developed. Tezepelumab, an anti-thymic stromal lymphopoietin antibody, has been reported to inhibit the exacerbation of severe asthma; however, its adverse effects on glucose metabolism have not yet been reported. We encountered a case of weight gain and worsening glycemic management in a patient with type 2 diabetes and refractory bronchial asthma after the initiation of tezepelumab treatment. It has been reported that the overexpression of thymic stromal lymphopoietin in mice resulted in an enhanced release of free fatty acids from adipose tissues and the liver; thus, the administration of anti-thymic stromal lymphopoietin antibodies in the present case might have caused obesity, fatty liver and lower glucose tolerance.
INTRODUCTION
In Japan, the number of patients with bronchial asthma has been increasing since 2008, and was estimated to be approximately 1.79 million in 2020. Although the mortality rate has been decreasing year over year as a result of the development of various treatments, approximately 1,000 people still die from asthma annually in Japan1. The pathogenesis of bronchial asthma is gradually being clarified, and novel therapies for severe asthma that is refractory to existing treatments are being developed based on this new information.
The latest international guidelines for asthma from the Global Initiative for Asthma 2022 recommend using biologic drugs and bronchial thermoplasty as a step five treatment for patients whose asthma is not adequately controlled by the combination of inhaled corticosteroids, long-acting β2 agonist, long-acting muscarinic antagonist and leukotriene receptor antagonists2. TSLP, interleukin-25 and interleukin-33 are produced by the airway epithelium in response to allergens, infections and irritants. Tezepelumab, an anti-TSLP antibody, has been recently developed as a novel treatment for bronchial asthma3. The NAVIGATOR trial, a phase III clinical trial of tezepelumab, showed that its use significantly reduced the rate of asthma exacerbations compared with the placebo (response rate 0.44; 95% confidence interval 0.37–0.53)4. We report a case of weight gain and worsening of glycemic management in a patient with type 2 diabetes mellitus and refractory bronchial asthma after the initiation of tezepelumab treatment.
CASE REPORT
The patient was a 54-year-old woman. Her medical history included severe asthma and fatty liver disease. Her father also had diabetes mellitus.
She had developed severe asthma 7 years prior, and had been started on inhalers and other medications. Owing to poor control, she had been referred to Center Hospital, National Center for Global Health and Medicine, Tokyo, Japan, 4 years prior. Thermoplasty proved ineffective, so she was started on daily systemic steroids. Nevertheless, the patient experienced shortness of breath at rest and limited her physical activity, including walking. She had developed diabetic ketosis 2 years prior. Multiple daily injections of insulin were started at that time, and glycemic management was monitored using intermittently-scanned continuous glucose monitoring. She had been recently treated with inhaled corticosteroids/long-acting β2 agonist, long-acting muscarinic antagonist, leukotriene receptor antagonists, prednisone 5 mg/day, triamcinolone 40 mg injection monthly and dupilumab injections. However, additional prednisone two or three times per week was required. In October, the patient was started on tezepelumab to replace dupilumab.
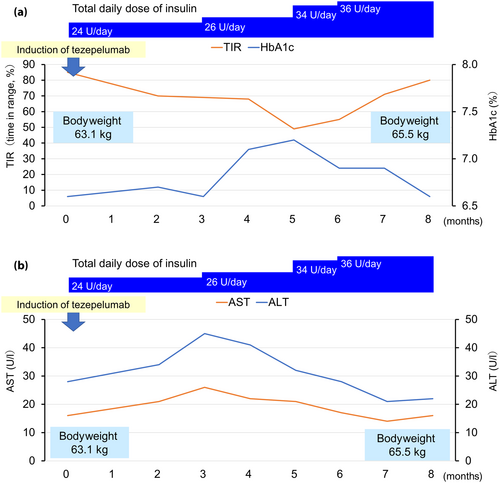
At the time of tezepelumab induction, the patient weighed 63.1 kg and had a body mass index of 26.3 kg/m2. The total daily dose of her insulin treatment was 24 units, and her hemoglobin A1c (HbA1c), time in range, time above range and time below range indicators were 6.6%, 72%, 28% and 0%, respectively. No diabetes-related complications were observed. After starting tezepelumab, her dyspnea improved, walking in daily life became possible without respiratory distress, her need for regular prednisone dropped to 4 mg/day and the need for additional doses fell to just a few times per month in June of the following year.
However, the patient's glycemic management gradually worsened thereafter. Her HbA1c, time in range and time above range were 7.2%, 49% and 51%, respectively, in March of the following year. As a result, her insulin dose was increased by 1.5-fold compared with before the initiation of tezepelumab, after which her glycemic management recovered to the previous level. No medications other than insulin had been prescribed for her diabetes. Her bodyweight increased by 2.4 kg to 65.5 kg. In terms of liver function, the aspartate aminotransferase and alanine aminotransferase levels increased in January of the following year, but recovered to their previous levels after improvement of glycemic management in June (Figure 1). At the start of tezepelumab treatment, her triglyceride, high-density lipoprotein (HDL) cholesterol, and low-density lipoprotein (LDL) cholesterol levels were 139, 80, and 125 mg/dL, respectively. At 8 months, they increased to 237, 65 and 138 mg/dL, respectively.
DISCUSSION
In this patient, anti-TSLP antibody treatment improved bronchial asthma, leading to a decrease in prednisone requirement and an increase in physical activity. However, the patient had increased insulin requirements, worsened glucose tolerance, weight gain and elevated liver enzymes-suggesting exacerbation of fatty liver compared with pre-tezepelumab treatment, although there were no other changes in medications that were likely to have affected weight or calory intake. Hypoglycemia, which can lead to weight gain due to increased carbohydrate intake, was not observed.
TSLP has been reported to promote tumor growth and metastasis, dendric cell fibrosis, and vascular endothelial cell proliferation, among other non-immune effects5. In non-obese diabetic mice, TSLP has been reported to induce regulatory T-cell differentiation, and increase and suppress diabetogenesis6. Additionally, TSLP-overexpressing mice showed decreased triglyceride deposition in the white adipose tissue and liver, improved glucose tolerance through improved insulin resistance, and increased sebum secretion7. The regulation of TSLP expression might represent a new therapeutic target for obesity and type 2 diabetes from both immune and non-immune perspectives. Therefore, in the present case, the inhibition of TSLP by tezepelumab might have induced obesity, fatty liver and worsened glucose intolerance, likely associated with increased insulin resistance.
One of the major strengths of the present case study is that we were able to evaluate glucose metabolism in detail using intermittently-scanned continuous glucose monitoring. Its major limitations, in contrast, include inadequate evaluation of body composition or liver steatosis before and after the start of tezepelumab treatment, inadequate evaluation of endogenous insulin secretory capacity and insulin resistance, and inability to evaluate fat content, such as sebaceous glands and hair tissue. It is also possible that the patient's increased insulin dosage directly caused her weight gain. Regarding change in HbA1c levels, we cannot exclude the influence of seasonal fluctuation in HbA1c, in addition to the adverse effect of tezepelumab8, 9.
The administration of an anti-TSLP antibody might cause obesity, fatty liver disease and glucose intolerance. Therefore, adequate follow up is necessary when administering this treatment, particularly in patients with obesity and diabetes.
Acknowledgments
We thank the staff for their contributions to this study. This manuscript has not been published elsewhere and is not under consideration for publication in any other journal.
Disclosure
Kohjiro Ueki is an Editorial Board member of Journal of Diabetes Investigation and a co-author of this article. To minimize bias, they were excluded from all editorial decision-making related to the acceptance of this article for publication.
Approval of the research protocol: N/A.
Informed consent: Written consent was obtained from the patient to present this case.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.




