Calcium signaling in endocardial and epicardial ventricular myocytes from streptozotocin-induced diabetic rats
Abstract
Aims/Introduction
Abnormalities in Ca2+ signaling have a key role in hemodynamic dysfunction in diabetic heart. The purpose of this study was to explore the effects of streptozotocin (STZ)-induced diabetes on Ca2+ signaling in epicardial (EPI) and endocardial (ENDO) cells of the left ventricle after 5–6 months of STZ injection.
Materials and Methods
Whole-cell patch clamp was used to measure the L-type Ca2+ channel (LTCC) and Na+/Ca2+ exchanger currents. Fluorescence photometry techniques were used to measure intracellular free Ca2+ concentration.
Results
Although the LTCC current was not significantly altered, the amplitude of Ca2+ transients increased significantly in EPI-STZ and ENDO-STZ compared with controls. Time to peak LTCC current, time to peak Ca2+ transient, time to half decay of LTCC current and time to half decay of Ca2+ transients were not significantly changed in EPI-STZ and ENDO-STZ myocytes compared with controls. The Na+/Ca2+ exchanger current was significantly smaller in EPI-STZ and in ENDO-STZ compared with controls.
Conclusions
STZ-induced diabetes resulted in an increase in amplitude of Ca2+ transients in EPI and ENDO myocytes that was independent of the LTCC current. Such an effect can be attributed, at least in part, to the dysfunction of the Na+/Ca2+ exchanger. Additional studies are warranted to improve our understanding of the regional impact of diabetes on Ca2+ signaling, which will facilitate the discovery of new targeted treatments for diabetic cardiomyopathy.
Introduction
Cardiac contractility is controlled by variations in the concentration of intracellular free Ca2+. During the process of excitation–contraction coupling, depolarization of the ventricular myocyte causes the opening of L-type Ca2+ channel (LTCCs) and the influx of small amounts of Ca2+ into the cardiac cells. This influx of Ca2+ stimulates a large release of Ca2+ (Ca2+ transient) from the sarcoplasmic reticulum (SR) through the ryanodine receptors (RyR) resulting in a transient rise of intracellular Ca2+ (Ca2+ transient). Ca2+ attaches to troponin C, which initiates and regulates the process of myocyte contraction. During the process of myocyte relaxation, Ca2+ is removed from the cytoplasm through four main pathways: (i) the uptake of Ca2+ into the SR through SR Ca2+-ATPase (SERCA pump); (ii) efflux of Ca2+ from the cell primarily through the Na+/Ca2+ exchanger (NCX); and (iii) to a lesser extent the Ca2+-ATPase in the plasma membrane1; and (iv) also uptake of Ca2+ by the mitochondria2, 3.
Disturbances in the mechanism(s) of Ca2+ signaling will inevitably have implications for cardiac myocyte contraction. Previous studies of left ventricle cells obtained from the STZ-treated rat have shown alterations in the Ca2+ transient, including prolonged TPK4, 5, prolonged THALF relaxation6 and unaltered7 or reduced8 amplitude of Ca2+ transient. These alterations in Ca2+ transient might be attributed variously to diminished SR Ca2+ content, release and uptake9-11, reduced activity of L-type Ca2+ channel12-14 and NCX7, 13.
Supporting these findings, earlier studies of STZ-treated rats variously reported reduced cardiac output, fractional shortening and ejection fraction4, 14, 15. In cardiac cells isolated from STZ-treated rat, there was a prolonged time course of shortening6, 16, 17, frequently prolonged relaxation4, 16, and slowed velocity of shortening and re-lengthening14. Studies also showed either no significant change6, 12 or reduction14 in the amplitude of shortening. These different results could be partly attributed to the duration of diabetes or the methodology used in the experiments.
It is well known that the electromechanical characteristics of cardiac cells across the ventricular walls are different. This heterogeneity is partly attributed to distinctive expression of different ion channels and associated ionic currents that lead to variation in action potential waveforms seen in epicardial (EPI), midmyocardial and endocardial (ENDO) cells18-20. For example, in vivo echocardiographic studies in rat ventricular myocardium have shown that layers of the epicardium contract less and slower than the layers of the subendocardium21. It is important to highlight that any disruption in the differential distribution of ion channels responsible for Ca2+ transport across the walls of the ventricles is expected to be involved in the dysfunction of cardiac cells in disease7, 15, 22, 23. Earlier work in spontaneously hypertensive rats has shown a transmurally altered time course and amplitude of action potential, variation in Ca2+ transients, and contraction in sub-EPI and sub-ENDO myocytes during compensated hypertrophy24. Transmural alteration in the activity of NCX, Ca2+ content of the SR and amplitude of Ca2+ transient have also been shown in an earlier study in spontaneously hypertensive rat25.
To date, only a few studies have explored the transmural effect of STZ-induced diabetes on Ca2+ signaling in ventricular myocytes26-28. For this reason, the current work was undertaken to investigate the progressive regional effects of diabetes on ventricular myocytes. To the best of our knowledge, this is the first study to investigate the effects of STZ-induced diabetes on LTCC current simultaneously measured with Ca2+ transients. Previous experiments generally measured LTCC current and Ca2+ transient in separate experiments. The influx of Ca2+ through LTCCs is inextricably linked to the release of Ca2+ from the SR (Ca2+-induced Ca2+ release). Therefore, this study has investigated the effects of STZ-induced diabetes on LTCC current simultaneously measured with intracellular Ca2+, and also NCX current in EPI and ENDO ventricular myocytes from rats 5–6 months after the induction of diabetes.
Methods
Experimental model
Experiments were carried out in STZ-induced diabetic rats29, 30. A single intraperitoneal injection of STZ (60 mg/kg bodyweight) in citrate buffer was used to induce diabetes in young adult (220–250 g) male Wistar rats. Age-matched control animals were injected with citrate buffer alone. Rats were kept in a 12/12 h light/dark cycle. Food and water were supplied ad libitum. Both the control and experimental groups received standard pelleted rat chow.
Before each experiment, body and heart weights, in addition to non-fasting blood glucose level (One Touch Ultra, LifeScan Inc., Milpitas, CA, USA) were measured. All experiments were carried out 5–6 months after treatment with STZ in ENDO and EPI myocytes. Ethical approval of the work was received from the UAE University Animal Research Ethics Committee (UAE University, A11-15, 2015). All experiments were carried out according to the institution guidelines.
Isolation of ventricular myocytes
Ventricular myocytes were obtained by enzymatic and mechanical dispersal techniques in accordance with previously described protocols24, 27, 28. Animals were euthanized with a guillotine. Hearts were isolated, mounted on a Langendorff system for retrograde perfusion and perfused at 36–37°C with a cell isolation solution (see Appendix S1)27. The perfusion flow rate was 8 mL/g heart−1·min−1. On stabilization of heart contraction, perfusion was switched to Ca2+-free cell isolation solution containing egtazic acid (0.1 mmol/L) for 4 min, and then to cell isolation solution containing Ca2+ (0.05 mmol/L), type 1 collagenase (0.60 mg/mL) and type XIV protease (0.075 mg/mL) for 6 min. On completion of enzymatic treatment, hearts were detached from the Langendorff perfusion system and the left ventricle was further dissected as described in earlier studies27, 28. Thin sections were carefully dissected by fine scissors from the outermost layer (EPI) and innermost layer (ENDO) of the left ventricle. The sections were gently minced and shaken in an isolation solution containing collagenase and 1% bovine serum albumin. Cells were then filtered at 4-min intervals and re-suspended in cell isolation solution that contained 0.75 mmol/L Ca2+.
Simultaneous recording of L-type Ca2+ current and Ca2+ transient
Simultaneous recording of LTCC current and Ca2+ transients were measured in EPI and ENDO ventricular cells according to modifications of previously described methods27, 28, 31. All experiments were carried out at 34°C. LTCC current was measured with an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA, USA). Analog signal was filtered using a four-pole Bessel filter (bandwidth of 5 kHz), and digitized at a sampling rate of 10 kHz using a Digidata 1550 digitizer (Molecular Devices). The voltage clamp protocols and data acquisition were controlled by WinWCP 4.05 software (Strathclyde Electrophysiology Software, Glasgow, UK). Patch pipettes were made from filamented borosilicate glass. Resistance of electrodes was 2–4 MΩ. Seal resistances were 1–5 GΩ. A current–voltage (I-V) relationship was achieved by the application of 300 ms test pulses (−30 to +50 mV) in 10 mV steps from a holding potential of −40 mV. Intracellular Ca2+ recordings were obtained by loading the cells with 30 μmol/L fura-2-pentapotassium salt through the patch pipette for 5 min, and excited at 360- and 380-nm wavelengths with dual LED light source, controlled by a spectrophotometry unit at 100 Hz. The fluorescence signal at 510 nm was collected through an adjustable diaphragm and a photomultiplier tube. WinWCP 4.05 software was used to sample signals at 10 kHz. All data were stored on a computer for subsequent analysis. After the subtraction of background signal, fluorescent signals were recorded as F360/F380 (ratio of the fluorescent intensity when excited at 360 nm [F360] to that when excited at 380 nm [F380] and expressed as a ratio unit). The whole-cell bath solution and pipette solution used are described in the Appendix S1.
Measurement of Na+/Ca2+ exchange current
The NCX current was recorded according to modifications of previously described techniques31. Experiments were carried out at 34°C. Recording of the NCX current was obtained with an Axopatch 200B amplifier using a voltage ramp protocol. Cells were held at a holding potential of −40 mV. Voltage ramps were deployed at a steady rate of 0.1 V/s from +60 mV to −100 mV. Resistance of electrodes was 3–5 MΩ. Seal resistances were 1–5 GΩ. Oubain (100 μmol/L), nifedipine (10 μmol/L) and niflumic acid (30 μmol/L) were used to block Na+-K+-ATPase, Ca2+ and Cl− currents, respectively. Nickel chloride solution (10 mmol/L) was used to block NCX. The NCX current was measured as the current sensitive to nickel (Ni2+). NCX current was isolated by subtracting Ni2+-insensitive component from the total current31. Whole-cell bath solution and pipette solution used are described in Appendix S1.
Statistical analysis
Results were represented as mean ± standard error of the men of “n” observations. As appropriate, independent samples t-test or one-way anova followed by Bonferroni corrected t-tests were used for statistical comparisons. A P-value <0.05 was considered significant.
Results
General characteristics
Bodyweight and heart weight were significantly reduced (P < 0.05) in STZ-pretreated diabetic rats. In comparison with their respective age-matched controls, heart weight/bodyweight and non-fasting blood glucose were greater in STZ-pretreated diabetic rats (Table 1).
| Control | Streptozotocin | |
|---|---|---|
| Bodyweight (g) | 417.3 ± 9.9 | 274.2 ± 8.6† |
| Heart weight (g) | 1.45 ± 0.03 | 1.28 ± 0.03† |
| Heart weight/bodyweight (mg/g) | 3.50 ± 0.07 | 4.68 ± 0.10† |
| Non-fasting blood glucose (mg/dL) | 83.3 ± 1.31 | 483.9 ± 20.2† |
- Data are the mean ± standard error of the mean, n = 15–17 rats.
- † P < 0.05 for diabetic compared with controls.
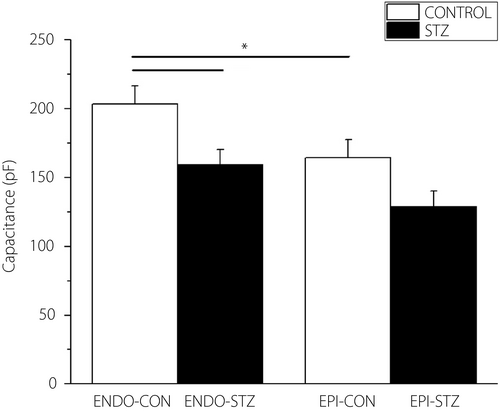
Cell capacitance
Cell capacitance was significantly lower in ENDO-STZ compared with ENDO-CON ventricular cells (P < 0.05; Figure 1). There was a small variation in cell capacitance between EPI-STZ and EPI-CON myocytes; however, the variation was not significant (P > 0.05).
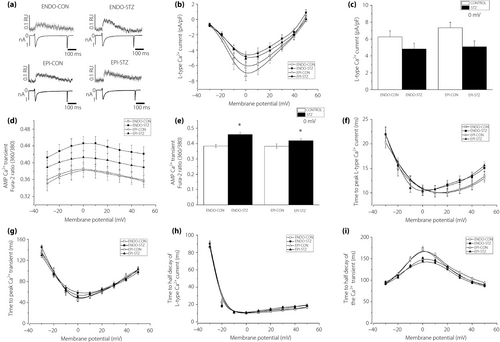
L-type Ca2+ current and Ca2+ transient
Typical tracings of LTCC current and Ca2+ transients measured at 0 mV test potential are shown in Figure 2a. The mean current–voltage (I/V) relationships of the LTCC current for ENDO-STZ, ENDO-CON, EPI-STZ and EPI-CON ventricular cells are shown in Figure 2b, and the currents generated at 0 mV test voltage are represented in Figure 2c. LTCC current amplitudes were not significantly (P > 0.05) different in ENDO-STZ and EPI-STZ compared with their respective controls at 0 mV (Figure 2c). Ca2+ transient fura-2 ratio-voltage relationship for ENDO-STZ, ENDO-CON, EPI-STZ and EPI-CON ventricular myocytes are represented in Figure 2d. Ca2+ transients generated at 0 mV are represented in Figure 2e. The amplitude of Ca2+ transients were significantly (P < 0.05) larger in ENDO-STZ and EPI-STZ compared with their controls (Figure 2e). TPK LTCC current (Figure 2f) and TPK Ca2+ transient (Figure 2g) were not significantly (P > 0.05) different in ENDO-STZ and EPI-STZ compared with their respective controls (P > 0.05). THALF decay of LTCC current (Figure 2h) and THALF decay of the Ca2+ transient (Figure 2i) were not significantly (P < 0.05) different in ENDO-STZ and EPI-STZ compared with their respective controls.
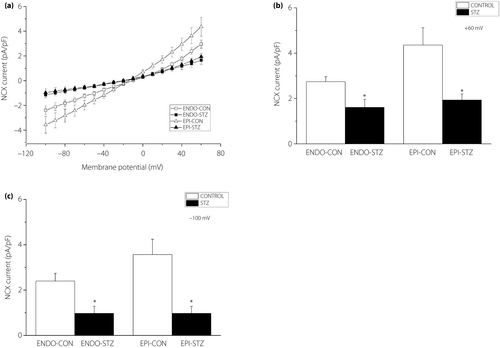
Na+/Ca2+ exchange current
The current of NCX was recorded by applying a voltage ramp between +60 and −100 mV. The NCX current was calculated as the difference between the current generated in the presence of 10 µmol/L nifedipine and the current in the presence of nifedipine and 10 mmol/L Ni2+. Mean recordings of the ramp current in ENDO-CON, ENDO-STZ, EPI-CON and EPI-STZ ventricular cells are represented in Figure 3a. At +60 mV, the amplitude of the NCX current was significantly (P < 0.05) smaller in ENDO-STZ and in EPI-STZ compared with their respective controls (P < 0.05) (Figure 3b). Similarly, at −100 mV, the amplitude of the NCX current was significantly (P < 0.05) smaller in ENDO-STZ and EPI-STZ compared with their respective controls (Figure 3c).
Discussion
The findings of the present study show that STZ-induced diabetes causes significant alteration in Ca2+ transients and NCX current in EPI and ENDO ventricular myocytes. To the best of our knowledge, this is the first study to investigate the regional effects of STZ-induced diabetes on LTCC current simultaneously measured with Ca2+ transients and NCX current in ventricular myocytes from STZ-induced diabetic rat.
Many changes during excitation–contraction coupling might give rise to changes in the Ca2+ transient. In our experiments, the amplitude and the kinetics of the LTCC current were similar in rat ventricular cells from EPI compared with ENDO regions. The amplitude of LTCC currents were not significantly different in ENDO-STZ and EPI-STZ ventricular cells compared with their controls. Although some studies have previously reported decreased amplitude12, 32, many studies have reported no change in LTCC currents in ventricular cells from STZ-treated rat4, 11, 33, 34.
Interestingly, the amplitude of the Ca2+ transients were significantly larger in EPI-STZ and ENDO-STZ myocytes compared with their respective controls. However, no regional difference in Ca2+ transients was observed. Earlier studies reported either reduced4, 15, 35 or unaltered16, 32, 36 Ca2+ transients in ventricular cells, isolated from STZ-induced diabetic animals. In comparison with other studies, in which Ca2+ transients were measured after 3–12 weeks of STZ treatment16, 17, 32, 33, 37, the present experiments were carried out in EPI and ENDO ventricular myocytes 5–6 months after STZ treatment. Thus, the variation in results might be partly related to the degree of progression of diabetes. Furthermore, the discrepancy between the present data and other results, where decreased amplitude38 or no change in amplitude7 of the Ca2+ transients were observed, might be down to the fact that different Ca2+ dyes were used for intracellular Ca2+ recordings. In the present study, a low concentration of fura-2-pentapotassium salt was dialyzed into the cell through the patch pipette. However, other studies7, 38 used ester of Indo-1 to load the indicator. Although both are high affinity indicators that have been used for cytosolic Ca2+ measurements in many studies in cardiac myocytes and other cell types, variation in indicator and/or loading technique might account for different results. For example, the use of ester of Indo-1 dye is sometimes associated with overloading of cytoplasm with Ca2+ indicator leading to increased buffering of Ca2+, slowing down the transients’ decay and reducing their amplitude.
The observed rise in the amplitudes of Ca2+ transients is explained by changes in pathways of Ca2+ extrusion (e.g., NCX) which, along with SERCA, are responsible for decreasing cytosolic Ca2+. In the present study, there was a significant decrease in NCX current in both EPI and ENDO ventricular cells, although no regional difference in the activity of NCX was observed. Earlier studies have also shown that the density of NCX current was reduced7, 13, 39 and current inactivation was prolonged13 in ventricular cells isolated from STZ-treated diabetic rats. These variations in the amplitude and kinetics of current were accompanied with decreased NCX messenger ribonucleic acid (mRNA)7 and reduced or unaltered NCX protein in STZ-induced diabetic rat heart8, 11, 40. It is possible that the decrease in the Ca2+ extrusion rate through NCX leads to a net increase in intracellular Ca2+41, increase in SR Ca2+ load and, as a consequence, increased cytosolic Ca2+ transients11, 14, 17, 33, 42. Alternatively, the increase in the amplitude of Ca2+ transients can be linked to RyR dysfunction. Abnormal function of RyR is associated with various diseases, including cardiac hypertrophy and STZ-induced type 1 diabetes43. It is known that the metabolic changes associated with diabetes stimulate the production of reactive oxygen species. As RyR structure is rich in free thiol groups, it is subject to oxidative stress, changing its tertiary structure and altering its sensitivity to Ca2+43, 44. In an earlier study in 7-week sedentary type 1 diabetic rats, the frequency of Ca2+ spark was threefold higher, and evoked release of Ca2+ was dys-synchronous with the diastolic release. Although the steady-state RyR protein was not altered, its response to Ca2+ was changed. The RyR also showed an approximately 1.5-fold rise in phosphorylation at Ser 2808 and Ser 2814 residues14.
In addition to the effects observed in diabetes, earlier studies have shown that hypertension-induced cardiac hypertrophy causes an alteration in the amplitude and the time course of systolic Ca2+ transients. Fowler et al.25 showed that NCX activity is diminished, whereas the amplitude of Ca2+ transient and SR Ca2+ content are increased in spontaneously hypertensive rats in comparison with their respective controls. It was suggested that alteration in Ca2+ signaling during compensated hypertrophy is attributed to the reduction in ventricular myocyte NCX activity. As a result, SR Ca2+ content increases and thus, the Ca2+ transient amplitude, serving to maintain the force of contraction against the elevated afterload25, 45. This observation is consistent with the increased SR Ca2+ content of spontaneously hypertensive rat ventricular cells reported previously46. Similarly, an increase in SR Ca2+ content, SR Ca2+ release and amplitude of Ca2+ transients were also described in a canine model of compensated hypertrophy that was induced by chronic atrioventricular block47.
Earlier studies have also reported that abnormal intracellular Ca2+ transport is caused by the change in expression of proteins that modulate intracellular Ca2+. For example, Teshima et al.23 showed that expression of SERCA and RyR mRNA were significantly altered in the heart, 3 and 12 weeks post-STZ injection, respectively. Hattori et al.7 showed that the reduction in cardiac NCX activity is due to the reduction in NCX mRNA and protein 6 weeks after the induction of diabetes with STZ. Changes in the expression of mRNA that encodes different proteins in cardiac muscle have also been previously reported in data from our laboratory48.
Transmural gradients in myocyte Ca2+ handling proteins exist across the ventricular wall. For example, it has been shown that Ca2+ handling proteins, such as SERCA, RyR and NCX, are more abundant in the EPI cells than ENDO cells in various species49-51. The results of the present study showed that the amplitude of the NCX current is larger in EPI-CON compared with END-CON. Supporting this finding, Xiong et al.51 reported that the NCX current, protein and mRNA were greater in EPI than ENDO or midmyocardial cells. Wang et al.20 also reported the transmural gradient in NCX with the capacity for Ca2+ extrusion by NCX being ENDO < midmyocardial < EPI. The spatial electrical heterogeneity of ion channels and proteins has a profound effect not only on normal cardiac electrophysiology and contractility, but also on the genesis of cardiac arrhythmias in diseased hearts. For example, the loss of this non-uniformity has been previously reported to be a sensitive index to discriminate physiological from pathological left ventricular remodeling21. The diminished NCX function in diabetic myocytes shown in the present study suggest that NCX function might play an important role when Ca2+ rises to pathological levels, such as in diabetic cardiomyopathy. Furthermore, the disruption of the transmural NCX gradient in diabetic heart highlights a potential substrate for the production of lethal arrhythmias leading to heart failure in diabetes patients.
In conclusion, the present study suggests that STZ-induced diabetes causes a significant rise in Ca2+ transient amplitudes 5–6 weeks after the induction of diabetes with a single intraperitoneal injection of STZ. This change probably stems partly from the dysfunction of NCX. Additional studies would be required to further our understanding of how diabetes alters Ca2+ handling in different regions of the heart. This will contribute to the development of new and regionally targeted treatments for diabetic cardiomyopathy.
Acknowledgments
The work was funded by the College of Medicine and Health Sciences, UAE University in Al Ain; Zayed University in Abu Dhabi; and Al Ain Equestrian, Shooting and Golf Club.
Disclosure
The authors declare no conflict of interest.




