Decreased microcirculatory function measured by perfusion index is a novel indicator of diabetic kidney disease in patients with type 2 diabetes
Abstract
Aims/Introduction
Diabetic kidney disease has been considered as an important risk factor of cardiovascular disease. Chronic hypoxia is considered to be the main cause of renal injury. Diminished microcirculatory blood flow could be associated with hypoxia in the kidney. Whether diminished microcirculation is associated with diabetic kidney disease has not yet been reported. Here, we investigated the correlation between microcirculatory function and diabetic kidney disease in patients with type 2 diabetes.
Materials and Methods
Our cross-sectional study included 574 patients who were admitted to Matsushita Memorial Hospital in Moriguchi, Japan, for type 2 diabetes. Microcirculatory function was assessed using the perfusion index (PI), which represents the level of circulation through peripheral tissues. We measured the PI for all patients.
Results
The median age and PI values were 70 years (range 60–77 years) and 2.8% (range 1.6–4.8%). Multiple regression analyses showed that the PI independently correlated with the logarithm of urinary albumin excretion (P = 0.009) and estimated glomerular filtration rate (P = 0.005), respectively. Multiple logistic regression analyses showed that patients with systolic blood pressure (SBP) greater than the median and PI less than or equal to the median (high-low group) had a significantly increased odds of albuminuria compared with those with SBP less than or equal to the median and PI greater than the median (low-high group), and patients with SBP greater than the median and PI less than or equal to the median (high-low group) had a significantly increased odds of estimated glomerular filtration rate <60 mL/min per 1.73 m2 compared with those with SBP less than or equal to the median and PI greater than the median (low-high group) or SBP greater than the median and PI greater than the median (high-high group).
Conclusions
PI could be a novel indicator of diabetic kidney disease in patients with type 2 diabetes.
Introduction
The prevalence of diabetic kidney disease (DKD) is increasing worldwide in patients with diabetes. DKD develops in approximately 40% of patients with type 2 diabetes and is the leading cause of end-stage renal disease1-3. An elevated albumin excretion rate and declining estimated glomerular filtration rate (eGFR) have been reported to be associated with an increased risk of cardiovascular disease4-6.
In the kidney, microcirculation, such as glomerular and peritubular capillaries, offer oxygen and nutrients to the corresponding region. Chronic hypoxia is considered as one of the main mechanisms of renal disease7. It has been reported that impairment of capillary blood flow results in a decrease in oxygen supply to the kidney8, suggesting that diminished microcirculation could result in renal disease. However, there have been no studies on the association between microcirculation and DKD. In contrast, some studies have reported the significance of microcirculation in the early phase of critical illness9-12. They assessed the validity of sublingual tissue measurements for the evaluation of tissue perfusion. The use of sublingual tissue in some patients is often problematic, and thus microcirculation remains difficult to investigate. With recent development of techniques, the quantitative assessment of peripheral perfusion has been popular in clinical practice. The peripheral perfusion index (PI) is the ratio of non-pulsatile blood flow to pulsatile blood flow in the tissue, and has been suggested to reflect changes in peripheral perfusion13, 14. A low PI value indicates poor microcirculation in critically ill patients13.
Whether diminished microcirculation is associated with DKD has not yet been reported. Therefore, we evaluated the association between PI and albuminuria or eGFR in patients with type 2 diabetes.
Methods
Ethics
The present study was carried out in accordance with the ethical principles of the Declaration of Helsinki. The local research ethics committee approved this study. We obtained informed consent from all patients.
Data collection and patients
A cross-sectional study was carried out with 574 patients who were admitted to Matsushita Memorial Hospital in Moriguchi, Japan, for type 2 diabetes between August 2015 and May 2018. All data were retrieved from the database.
Fasting blood samples were collected in the early morning. Hypertension was defined when systolic blood pressure (SBP) was ≥140 mmHg, their diastolic blood pressure was ≥90 mmHg and/or the patient was prescribed any antihypertensive medications. Diabetes was diagnosed based on a previous guideline15. Urinary albumin excretion (UAE) and urinary creatinine (Cr) concentrations were calculated in the early morning spot urine sample. Albuminuria was defined as UAE ≥30 mg/gCr. We classified patients as never smokers, past smokers or current smokers by a self-administered questionnaire. Patients for whom PI measurements could not be obtained were excluded from this study; those with implanted cardiac pacemakers; arrhythmia, such as paroxysmal atrial fibrillation; or amputations of the lower extremities were also excluded from the study. The eGFR was calculated using the following equation16: eGFR (mL/min per 1.73 m2) = 194 × Cr−1.094 × age−0.287 (×0.739 for women).
Technique for determining PI
The PI value was obtained using a Masimo SET Radical-7 (Masimo Corporation, Irvine, CA, USA) instrument. The patients were placed in the face-up position. A probe was positioned on each toe of the patients. A probe was connected to the Masimo SET Radical-7 machine. The PI was measured three times at every 20 s after the 5-min rest period. We calculated the average of the three values. PI was calculated as the ratio between the non-pulsatile and pulsatile components of the light reaching a light-sensitive cell of the pulse oximetry probe. The reliability and reproducibility of PI have been reported elsewhere17, 18. After PI was determined bilaterally, the lower value was considered as a representative value for each participant.
Statistical analysis
The medians and frequencies of potential confounding variables were calculated. The patients were categorized according to their level of SBP and PI value to examine the association between SBP or PI and UAE or eGFR: the low-high group, SBP less than or equal to the median and PI greater than the median; the low-low group, SBP less than or equal to the median and PI less than or equal to the median; the high-high group, SBP greater than the median and PI greater than the median; and the high-low group, SBP greater than the median and PI less than or equal to the median. The means or percentages were calculated for each group, and associations were assessed by analysis of variance or the 2-test, respectively. The relationships between UAE or eGFR and age, body mass index (BMI), and other variables were examined using Spearman’s rank correlation analyses. We implemented log transformation before a correlation analysis, because triglyceride levels and UAE have skewed distributions. To examine the effects of various factors on the logarithm of UAE, the following factors were considered as independent variables for multiple regression analyses: age, duration of diabetes, BMI, average SBP, PI, hemoglobin A1c, uric acid and creatinine (model 1). Model 2 was adjusted for all variables in model 1 plus sex, total cholesterol, logarithm of triglycerides, smoking status, the use of renin–angiotensin system (RAS) inhibitor, the use of incretin-related therapies, such as dipeptidyl peptidase-4 inhibitors or glucagon-like peptide-1 receptor agonists, the use of sodium–glucose cotransporter 2 (SGLT-2) inhibitor and the use of statin. To examine the effects of various factors on eGFR, the following factors were considered as independent variables for multiple regression analyses; duration of diabetes, BMI, PI, hemoglobin A1c, total cholesterol and uric acid (model 1). Model 2 was adjusted for all variables in model 1 plus sex, average SBP, logarithm of triglycerides, smoking status, the use of RAS inhibitor, the use of incretin-related therapies, the use of SGLT-2 inhibitor and the use of statin. To examine the effects of SBP and PI on albuminuria or eGFR <60 mL/min per 1.73 m2, logistic regression analyses were carried out, and the following factors were considered as independent variables: sex, age, duration of diabetes, BMI, hemoglobin A1c, total cholesterol, triglycerides, uric acid, creatinine and smoking status (model 1); or sex, duration of diabetes, BMI, hemoglobin A1c, total cholesterol, triglycerides, uric acid and smoking status (model 1). Model 2 was adjusted for all variables in model 1 plus the use of a RAS inhibitor, the use of incretin-related therapies, the use of SGLT-2 inhibitor and the use of statin. When comparing SBP-PI categories, the patients with SBP less than or equal to median and PI greater than median (low-high) were referred to as the reference group. Statistically significant was defined as a P-value <0.05.
Results
Table 1 shows the characteristics of all 574 participants in our study. The median age and PI values were 70 years (range 60–77 years) and 2.8% (range 1.6–4.8 %), respectively.
| n (male/female) | 574 (336/238) |
| Age (years) | 70 (60–77) |
| Duration of diabetes (years) | 7.0 (4.0–14.0) |
| Body mass index (kg/m2) | 24.3 (21.4–27.8) |
| Systolic blood pressure (mmHg) | 128 (115–144) |
| Diastolic blood pressure (mmHg) | 75 (65–82) |
| Heart rate (b.p.m.) | 79 (71–90) |
| Perfusion index (%) | 2.8 (1.6–4.8) |
| Hemoglobin A1c (%) | 8.4 (7.3–9.9) |
| Total cholesterol (mg/dL) | 177 (155–210) |
| Triglycerides (mg/dL) | 126 (87.8–190.3) |
| Uric acid (mg/dL) | 5.0 (4.0–6.2) |
| Creatinine (mg/dL) | 0.81 (0.6–61.05) |
| Urinary albumin excretion (mg/gCr) | 37.7 (9.6–147.7) |
| Hypertension (−/+) | 254/320 |
| Smoking status (never/past/recent) | 256/116/202 |
| Renin-angiotensin system inhibitor (−/+) | 316/258 |
| Incretin-related therapies (−/+) | 176/398 |
| Sodium–glucose cotransporter 2 inhibitor (−/+) | 530/44 |
| Statin (−/+) | 336/238 |
- Data are expressed as the median (interquartile range) or absolute number.
Table 2 shows the characteristics of the study participants according to their SBP and PI value. Sex, age, BMI, SBP, PI, hemoglobin A1c, total cholesterol, creatinine, UAE, the use of RAS inhibitor, the use of SGLT-2 inhibitor and the use of statin differed among each group. Patients with SBP greater then the median and PI less than or equal to the median (high-low group) were older, and had lower hemoglobin A1c, higher creatinine and higher UAE than those with SBP less than or equal to the median and PI greater than the median (low-high group). Furthermore, patients with SBP greater than the median and PI less than or equal to the median (high-low group) more frequently used a RAS inhibitor and statin than those with SBP less than or equal to the median and PI greater than the median (low-high group).
| SBP ≤median PI >median (low-high) | SBP ≤median PI ≤median (low-low) | SBP >median PI >median (high-high) | SBP >median PI ≤median (high-low) | P | |
|---|---|---|---|---|---|
| n (male/female) | 140 (88/52) | 148 (76/72) | 146 (100/46) | 140 (72/68) | 0.004 |
| Age (years) | 68 (57–77) | 70 (61–78) | 66 (54.75–75) | 73.5 (66–79) | <0.0001 |
| Duration of diabetes (years) | 5 (3–14) | 7.5 (4–14) | 6 (4–14) | 10 (4–14) | 0.403 |
| Body mass index (kg/m2) | 24.7 (22.4–28.6) | 23.4 (21.2–27.0) | 24.8 (21.8–28.5) | 24.2 (20.7–26.7) | 0.0002 |
| SBP (mmHg) | 115 (104–122) | 116 (108–120) | 139 (134–151) | 145.5 (138–158) | <0.0001 |
| Perfusion index (%) | 4.5 (3.6–6.3) | 1.7 (0.7–2.3) | 5 (3.8–6.9) | 1.8 (0.9–2.3) | <0.0001 |
| Hemoglobin A1c (%) | 8.9 (7.7–10.7) | 8.2 (6.7–10.2) | 8.4 (7.1–10.2) | 8.2 (7.3–9.2) | 0.0008 |
| Total cholesterol (mg/dL) | 182 (157–216) | 169 (145–198) | 188 (155–221) | 184 (158–215) | 0.0003 |
| Triglycerides (mg/dL) | 126 (84–210) | 129 (91–168) | 125 (90–192) | 121 (88–190) | 0.140 |
| Uric acid (mg/dL) | 5.0 (3.8–6.3) | 5.1 (3.8–6.6) | 5 (4.2–6.0) | 5.1 (4–6.1) | 0.812 |
| Creatinine (mg/dL) | 0.81 (0.66–0.98) | 0.8 (0.64–1.11) | 0.76 (0.64–0.98) | 0.89 (0.68–1.16) | 0.002 |
| UAE (mg/gCr) | 22.5 (6.7–74) | 24.1 (8.4–84.4) | 33.2 (12.7–182) | 93.9 (19.3–515.6) | 0.004 |
| Smoking status (never/past/recent) | 58/34/48 | 64/32/52 | 60/26/60 | 74/24/42 | 0.250 |
| RAS inhibitor (−/+) | 92/48 | 68/80 | 90/56 | 66/74 | 0.0006 |
| Incretin-related therapies (−/+) | 44/96 | 42/106 | 59/90 | 34/106 | 0.067 |
| SGLT-2 inhibitor (−/+) | 122/18 | 136/12 | 138/8 | 134/6 | 0.035 |
| Statin (−/+) | 92/48 | 78/70 | 94/52 | 72/68 | 0.018 |
- Data are expressed as the median (interquartile range) or absolute number. PI, perfusion index; RAS, renin–angiotensin system inhibitor; SBP, systolic blood pressure; SGLT-2, sodium–glucose cotransporter 2; UAE, urinary albumin excretion.
The number of patients with albuminuria or eGFR 60 mL/min per 1.73 m2 was 304 or 216 patients, respectively. The number of patients with albuminuria or eGFR <60 mL/min per 1.73 m2 was 62 or 46 patients, 70 or 62 patients, 76 or 42 patients, or 96 or 66 patients in SBP less than or equal to the median and PI greater than the median (low-high group), SBP less than or equal to the median and PI less than or equal to the median (low-low group), SBP greater than the median and PI greater than the median (high-high group), and SBP greater than the median and PI less than or equal to the median (high-low group), respectively.
The simple correlation and multiple regression analyses on the logarithm of UAE and eGFR are shown in Table 3. Multiple regression analyses showed that age, BMI, SBP, PI, total cholesterol, uric acid, creatinine, smoking status, the use of RAS inhibitor or the use of statin independently correlated with the logarithm of UAE (model 2). In addition, multiple regression analyses showed that BMI, PI, hemoglobin A1c, uric acid, the use of SGLT-2 inhibitor or the use of statin independently correlated with eGFR (model 2).
| r | P | Model 1 | Model 2 | |||
|---|---|---|---|---|---|---|
| ß | P | ß | P | |||
| Urinary albumin excretion | ||||||
| Sex | — | — | — | — | 0.054 | 0.236 |
| Age | 0.153 | 0.0002 | 0.125 | 0.004 | 0.135 | 0.003 |
| Duration of diabetes | 0.107 | 0.011 | 0.002 | 0.961 | −0.036 | 0.415 |
| Body mass index | 0.143 | 0.0006 | 0.148 | 0.0003 | 0.128 | 0.002 |
| Average SBP | 0.222 | <0.0001 | 0.191 | <0.0001 | 0.179 | <0.0001 |
| Perfusion index | −0.136 | 0.001 | −0.109 | 0.006 | −0.108 | 0.009 |
| Hemoglobin A1c | −0.093 | 0.025 | 0.027 | 0.517 | 0.001 | 0.982 |
| Total cholesterol | 0.066 | 0.119 | — | — | 0.143 | 0.003 |
| Logarithm of triglycerides | 0.077 | 0.067 | — | — | 0.011 | 0.817 |
| Uric acid | 0.223 | <0.0001 | 0.107 | 0.012 | 0.110 | 0.014 |
| Creatinine | 0.330 | <0.0001 | 0.251 | <0.0001 | 0.213 | <0.0001 |
| Smoking status | — | — | — | — | 0.100 | 0.023 |
| RAS inhibitor | — | — | — | — | 0.087 | 0.041 |
| Incretin-related therapies | — | — | — | — | 0.031 | 0.469 |
| SGLT-2 inhibitor | — | — | — | — | −0.051 | 0.200 |
| Statin | — | — | — | — | 0.104 | 0.013 |
| Estimated glomerular filtration rate | ||||||
| Sex | — | — | — | — | −0.017 | 0.699 |
| Duration of diabetes | −0.240 | <0.0001 | –0.112 | 0.004 | −0.053 | 0.191 |
| Body mass index | −0.144 | 0.0006 | –0.079 | 0.037 | −0.081 | 0.041 |
| Average SBP | −0.074 | 0.075 | –0.051 | 0.170 | −0.032 | 0.390 |
| Perfusion index | 0.160 | 0.0001 | 0.137 | 0.0003 | 0.108 | 0.005 |
| Hemoglobin A1c | 0.120 | 0.001 | 0.157 | 0.0001 | 0.151 | 0.0003 |
| Total cholesterol | 0.103 | 0.014 | 0.0001 | 0.998 | −0.054 | 0.233 |
| Logarithm of triglycerides | 0.038 | 0.374 | — | — | 0.057 | 0.186 |
| Uric acid | −0.447 | <0.0001 | −0.385 | <0.0001 | –0.100 | <0.0001 |
| Smoking status | — | — | — | — | 0.003 | 0.949 |
| RAS inhibitor | — | — | — | — | –0.053 | 0.179 |
| Incretin-related therapies | — | — | — | — | –0.051 | 0.205 |
| SGLT-2 inhibitor | — | — | — | — | 0.114 | 0.002 |
| Statin | — | — | — | — | –0.135 | 0.0006 |
- Sex was defined as female (=0) or male (=1), smoking status was defined as non-smoker (=0), past smoker or current smoker (=1), and medication for hypertension, diabetes or dyslipidemia was defined as without (=0) or with (=1). RAS, renin–angiotensin system inhibitor; SBP, systolic blood pressure; SGLT-2, sodium–glucose cotransporter 2.
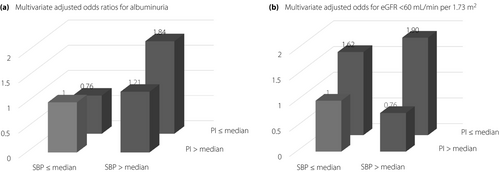
Table 4 and Figure 1 show the unadjusted and multivariate-adjusted odds ratios for albuminuria and eGFR <60 mL/min per 1.73 m2. Multiple logistic regression analyses showed that patients with SBP greater than the median and PI less than or equal to the median (high-low group) had a significantly increased odds of albuminuria compared with those with SBP less than or equal to the median and PI greater than the median (low-high group) and patients with SBP greater than the median and PI less than or equal to the median (high-low group) had a significantly increased odds of eGFR <60 mL/min per 1.73 m2 compared with those with SBP less than or equal to the median and PI greater than the median (low-high group) or SBP greater than the median PI greater than the median (high-high group).
| SBP ≤median PI >median (low-high) | SBP ≤median PI ≤median (low-low) | SBP >median PI >median (high-high) | SBP >median PI ≤median (high-low) | |
|---|---|---|---|---|
| Albuminuria | ||||
| Crude | 1 | 1.13 (0.71–1.80) | 1.37 (0.86–2.18) | 2.74 (1.69–4.50) |
| Multiple (model 1) | 1 | 0.92 (0.54–1.58) | 1.17 (0.69–1.98) | 2.01*,** (1.16–3.49) |
| Multiple (model 2) | 1 | 0.76 (0.44–1.37) | 1.21 (0.70–2.28) | 1.84* (1.05–3.23) |
| Estimated glomerular filtration rate | ||||
| Crude | 1 | 1.47 (0.91–2.39) | 0.77 (0.46–1.28) | 1.92* (1.19–3.14) |
| Multiple (model 1) | 1 | 1.85* (1.04–3.31) | 0.73 (0.40–1.33) | 2.28*,** (1.29–4.09) |
| Multiple (model 2) | 1 | 1.62 (0.88–3.00) | 0.76 (0.40–1.42) | 1.90*,** (1.05–3.46) |
- Independent variables, model 1: sex, duration of diabetes, body mass index, hemoglobin A1c, total cholesterol, triglycerides, uric acid and smoking status. Model 2: model 1 + the use of a renin–angiotensin system inhibitor, the use of incretin-related therapies, the use of sodium–glucose cotransporter 2 inhibitor and the use of statin. PI, perfusion index; SBP, systolic blood pressure.
- * P < 0.05, versus reference.
- ** P < 0.05, versus high-high group.
Discussion
The major finding of the present study was that PI, which represents microcirculatory function, was associated with UAE and eGFR after adjusting for systolic blood pressure. In particular, patients with SBP greater than the median and PI less than or equal to the median (high-low group) had a significantly increased odds of eGFR <60 mL/min per 1.73 m2 compared with those with SBP greater than the median and PI greater than the median (high-high group). PI could be a novel indicator of DKD in patients with type 2 diabetes.
In the kidney, the afferent arteriole gives rise to capillaries of glomerulus, which merge together again to form the efferent arterioles. Efferent arterioles enter the peritubular capillary plexus, which offers oxygen and nutrients to tubular and interstitial cells7. Chronic hypoxia in the tubulointerstitium was considered as a main pathway to renal dysfunction7. Some mechanisms that induce chronic hypoxia in the tubulointerstitium have been identified. First, histological studies have shown that tubulointerstitial injury is associated with diminished microcirculation, such as distortion of peritubular capillaries8, 19-22. Advanced renal disease is decreased in microcirculatory oxygenation and blood supply to the corresponding region. Second, chronic hypoxia could also change the extracellular matrix metabolism of renal cells and activate fibroblasts23, 24. This response leads to the dysfunction of microcirculation. Furthermore, renal tubular cells, which are subjected to chronic hypoxia, result in the mitochondria functional deficit, in turn causing apoptosis25. Taken together, chronic hypoxia could cause transdifferentiation or tubular cell apoptosis, activation of fibroblasts and further diminished microcirculation with progression of fibrosis. Third, microcirculation in the tubulointerstitium occurs downstream of the glomerular efferent arterioles. Damage of the glomerular capillary bed results in a decrease in peritubular perfusion and oxygen supply26. Fourth, imbalances in associated intrarenal vasoconstriction and vasoactive substances can induce hypoxia in the kidney. Elevated efferent arteriolar resistance and decreased microcirculation in the tubulointerstitium have been associated with reversible renal dysfunction27. Fifth, angiotensin II has been reported to cause the loss of microcirculation in the tubulointerstitium28, 29. Angiotensin II induces hypoxia in the tubulointerstitium through both hemodynamic mechanisms, such as the loss of microcirculation, and non-hemodynamic mechanisms, such as oxidative stress. Norman et al.30 showed that microcirculation and tissue oxygenation was preserved in an animal model by blockade of RAS. Finally, there are other mechanisms of chronic hypoxia, such as increased metabolic demands of tubular cells, inappropriate energy use due to the uncoupling of mitochondrial respiration caused by oxidative stress and decreased oxygen supply due to anemia. When these mechanisms are taken together, diminished microcirculation is associated with chronic hypoxia in the kidney.
In the present study, SBP showed a different effect on UAE and eGFR. SBP was higher in patients with albuminuria than in those without albuminuria (132.5 mmHg vs 127.0 mmHg; P = 0.0013). However, SBP was similar in patients with eGFR <60 mL/min per 1.73 m2 and those with eGFR ≥60 mL/min per 1.73 m2 (129.5 mmHg vs 129.0 mmHg; P = 0.728). We hypothesized that patients with decreased eGFR were more frequently prescribed antihypertensive drugs. We found that patients with eGFR <60 mL/min per 1.73 m2 were more frequently prescribed antihypertensive drugs than those with eGFR ≥60 mL/min per 1.73 m2 (65.1% vs 43.6%; P < 0.0001). Then, multiple regression analyses might show that SBP was not independently correlated with eGFR.
Previous studies used the sublingual tissue bed as an indicator of microcirculatory function. Using sublingual tissues in some patients can often be difficult in any clinical care setting. However, the technique described here for the measurement of PI takes approximately 5 min and is simple.
However, the present study had several limitations. Decreased peripheral microcirculation might cause DKD. Simultaneously, DKD could decrease peripheral microcirculation. Indeed, a previous study showed that albuminuria or decreased eGFR were risk factors for atherosclerotic cardiovascular disease31, which decrease peripheral microcirculation. Because the present study was cross-sectional, the causal relationship between peripheral microcirculation and DKD is unclear. Therefore, prospective trials are required to better assess the relationship between peripheral microcirculation and DKD. Because we only targeted Japanese patients, it is unclear whether the results of the present study are applicable to other ethnicities. Furthermore, the volume of information gathered might be insufficient for stringent analyses, because the number of patients was small. Although this investigation suggested that PI was a useful indicator of DKD, further investigations using a larger population with patients of various ethnicities would be crucial to provide better care.
Despite recent therapies, there is a residual risk of DKD development and progression. Therefore, a widespread tool is required to prevent complications for patients with DKD. An improvement of microcirculation could be a target goal in cases of DKD. In conclusion, impaired microcirculation, determined by a simple, non-invasive PI measurement, might be a valuable indicator of DKD in patients with diabetes. Microcirculation could be a target of prevention for DKD.
Disclosure
Michiaki Fukui received grants from the Japan Society for the Promotion of Science, AstraZeneca Plc, Astellas Pharma Inc., Nippon Boehringer Ingelheim Co., Ltd., Daiichi Sankyo Co., Ltd., Eli Lilly Japan K.K., Kyowa Hakko Kirin Company Ltd., Kissei Pharmaceutical Co., Ltd., MSD K.K., Mitsubishi Tanabe Pharma Corporation, Novo Nordisk Pharma Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., Sanofi K.K., Ono Pharmaceutical Co., Ltd. and Takeda Pharmaceutical Co., Ltd., outside the submitted work. The aforementioned sponsors were not involved in the study design; collection, analysis and interpretation of data; writing of this manuscript or decision to submit the article for publication. The authors, their immediate families and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the content of this article. The authors declare that although they are affiliated with a department that is supported financially by a pharmaceutical company, they received no current funding for this study, and this does not alter their adherence to all the journal policies on sharing data and materials. The other authors declare no conflict of interest.




