Impact of corticosteroid use on outcomes of non–small-cell lung cancer patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis
Abstract
What is known and objective
Administration of corticosteroids to immune checkpoint inhibitors (ICI)–treated patients has raised concerns due to doubts about ICIs' efficacy under those conditions. Hence, we reviewed studies comparing overall and progression-free survival (OS and PFS) outcomes in patients with non–small-cell lung carcinoma (NSCLC) treated with ICI and either with or without corticosteroids for any reason.
Methods
We searched the PubMed Central, Cochrane library, EMBASE and MEDLINE databases from inception until February 2021 for relevant publications. We used the Newcastle-Ottawa scale to assess the quality of the identified studies. We used the published data to carry out a meta-analysis with a random-effects model and report pooled odds ratios (ORs) with 95% confidence intervals (CIs).
Results
We included data from 14 studies with 5461 participants in the meta-analysis. Most studies were retrospective in nature and of low quality, and most of them were conducted in the USA and in European countries. Nivolumab is the most common ICI used in the included studies followed by pembrolizumab. We found that patients using corticosteroids had reduced OSs (pooled HR, 1.82; 95% CI, 1.51–2.18) and PFSs (pooled HR, 1.69; 95% CI, 1.41–2.04) than the patients not using corticosteroids. We identified significant heterogeneity and publication bias for both the outcomes. However, the sensitivity analysis revealed that the estimates were robust to the individual study effects.
What is new and conclusion
Our findings suggest that corticosteroids significantly reduce the OS and PFS of patients with NSCLC under ICI therapy. Hence, clinicians and oncologists should consider this information when prescribing corticosteroids for this target population.
1 INTRODUCTION
Small and non–small-cell lung cancers remain some of the deadliest cancers.1 Amongst these subtypes, non–small-cell cancer (NSCLC) accounts for 85% of all lung cancer cases.2 Monoclonal antibodies (Abs) acting as “immune checkpoint inhibitors (ICIs)” of the “programmed cell protein 1/programmed cell death ligand 1 (PD-1/PD-L1)” pathway have a demonstrated anticancer activity against metastatic and locally advanced NSCLCs.3 This has led to approval of their use as first line or subsequent management medications. They can be used as single agents or combined with chemotherapy.3 ICIs have created a new NSCLC management era with long-lasting remissions for many patients. However, ICIs have also introduced a new spectrum of toxicity and immune-related adverse events (irAEs).4
Exogenous corticosteroid administration has been the cornerstone of treatment for autoimmune disorders for a long time.5 Corticosteroid immunosuppressive mechanisms are multifactorial, and they suppress both adaptive and innate immunity. They act as glucocorticoid receptors’ agonists that activate transcriptional modifiers for many genes involved in priming of the innate immune response.6 As a result of this immunosuppressive property of corticosteroids, patients using more than 10 mg of prednisolone equivalent per day were excluded from the trials leading to the approval of ICIs.7
However, patients with NSCLC require corticosteroids at doses higher than 10 mg prednisolone equivalents for a wide spectrum of underlying aetiologies. The most common causes for corticosteroid administration range widely from cerebral oedema occurring as a result of brain metastases to chronic pulmonary obstructive disease exacerbations.8, 9 However, the administration of corticosteroids to ICI-treated patients has raised concerns due to doubts about ICIs' efficacy under those conditions.
Some studies have reported that administration of high doses of corticosteroids for managing irAEs does not affect ICIs’ efficacy in patients with NSCLC,10 but most studies have found that early administration of prednisone equivalent at doses ≥10 mg daily during ICI initiation is associated with poor outcomes in these patients.11-13 Hence, conclusive evidence about the use of corticosteroids in NSCLC patients receiving ICIs is needed. A meta-analysis done by Petrelli et al (2020)14 assessing the impact of corticosteroids during the management with ICIs included only seven studies on NSCLC and summarized the evidence common for all the cancers. New studies have been published since then; thus, in this updated meta-analysis, we evaluate the impact of corticosteroids on the overall survival, and progression-free survival of patients with NSCLC treated with ICIs.
2 MATERIALS AND METHODS
2.1 Eligibility criteria
2.1.1 Study design
We included relevant randomized controlled trials (RCTs) and observational studies (retrospective/prospective) considering only published full-text studies. We excluded conference abstracts, unpublished data and grey literature.
2.1.2 Participants
We included studies with patients with NSCLC undergoing treatment with ICIs.
2.1.3 Exposure
We included studies reporting relevant outcomes in patients receiving corticosteroids (exposed group) and in those not receiving corticosteroids (non-exposed group).
2.1.4 Outcomes
- Overall survival (OS)
- Progression-free survival (PFS)
2.2 Search strategy
We conducted a comprehensive systematic search in PubMed Central, EMBASE, MEDLINE, and Cochrane library electronic databases. We selected medical subject headings (MeSH) and free-text words for the search during the protocol stage. We used combinations of the following terms in all the search engines and databases: “Corticosteroids,,” “Corticocorticosteroids,,” “Immune checkpoint inhibitors,” “Non-small cell lung cancer,” “Mortality,” “Overall Survival,” “Progression Free Survival,” “Lung Carcinoma.” Our search included appropriate truncations, wildcards and proximity searching of keywords and their synonyms. In addition, we looked for key concepts using the corresponding subject headings in each database. Our final search combined individual search results using appropriate Boolean operators (“OR” and “AND”). The search was narrowed down using the available filters on types of studies. We restricted the search to publications from inception of the databases to February 2021 and in English language only. We also manually searched the bibliographies of the retrieved articles to identify any articles missed during the database search.
2.3 Study selection process
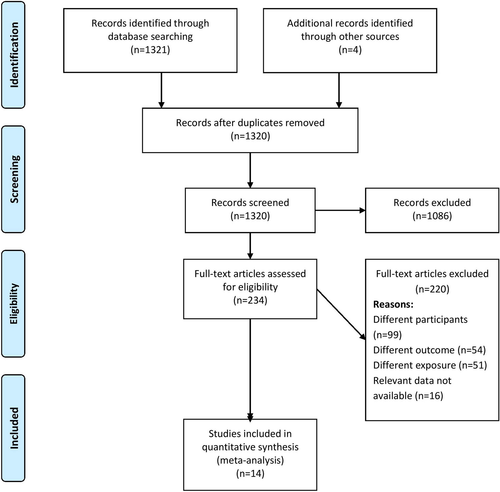
Two independent investigators performed the primary screening of title, abstract and keywords during the literature search. They imported all the citations along with the title and abstract to a specified endnote library and prepared a final list of studies to be screened for inclusion in the study by removing duplicates. Full-text articles were retrieved for these shortlisted studies. Then, the same two authors screened these full texts against our eligibility criteria. They excluded studies not satisfying the eligibility criteria and noted the reasons for exclusion. Any disagreements between the two investigators during the screening process were resolved in consensus with a third investigator for the final study selection. We created a PRISMA flow chart (Figure 1) representing the screening and selection process.
2.4 Data collection process and management
We used a structured data extraction form to manually classify data from the included studies; we obtained general information about each study such as authors, publication year, title, study design, sample size, study participants, inclusion and exclusion criteria, outcome assessment methods, dosage and timing of corticosteroids administration, ICI type, cancer staging, quality-related information and outcome-related information (OS and PFS). Data were entered by the primary investigator collected the data and secondary investigators double-checked the entries for correctness.
2.5 Risk of bias assessment
Two independent investigators (SR and VH) performed the risk of bias assessment using the Newcastle-Ottawa (NO) Quality Assessment Form for observational studies under the three domains: selection, comparability and outcome.15 For each of the above-mentioned domains, we graded the risk of bias as low (adequate information provided), high (inadequate information or “not performed”) or unclear (missing information). We considered all studies having a score of four or more in the NO scale as being of high quality.
2.6 Statistical analysis
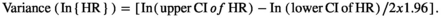
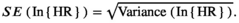
We entered the logarithmic HR and its SE into the STATA software to estimate the pooled effect. We applied a random effects model because of the anticipated heterogeneity, and we reported the final data as the pooled HR with 95% CI for both outcomes (OS and PFS). We represented these pooled estimates visually using a forest plot.
We used the chi-square of heterogeneity and the I2 statistic to assess heterogeneity. We considered chi square test p values ≤0.10 as indicative of significant heterogeneity, and we quantified heterogeneity with the I2 value as mild (<25%), moderate (25%–75%) or substantial (>75%). We performed additional analyses such as a subgroup analysis and a meta-regression to explore reasons for the high heterogeneity we found. We evaluated publication bias and visually represented it through a funnel plot. We also assessed the asymmetry of the plot using Egger's test. We considered a p value <0.10 for publication bias as statistically significant. We performed a sensitivity analysis to identify the individual study effects and the robustness of the obtained results.
3 RESULTS
3.1 Study selection
Figure 1 shows the entire study selection process in the form of a PRISMA flowchart. During the primary screening, we retrieved 234 full-text studies, which become 212 after the removal of duplicates. These studies and four more retrieved from the bibliography of the screened articles underwent a secondary screening. Finally, we included data from 14 studies with 5,461 participants satisfying the inclusion criteria.11-13, 27
3.2 Study characteristics
Most studies 13 out of14 were retrospective, and only the study by Hendriks et al 201922 was prospective in nature. Most studies (4 out of 14) were conducted in United States of America, and others were conducted in European countries (Spain, Italy, France and Sweden). The mean ages of study participants ranged from 63 to 70.5 years. The sample sizes amongst the included studies varied from 67 to 1025. Most studies used corticosteroids as prednisolone equivalents at 10 mg per dose. Indications for corticosteroid use included brain metastasis, cancer-related symptoms, best supportive care and irAEs. Most studies (6 out of 14 studies) used the ICI nivolumab followed by anti-PD-1/PD-L1/anti-PD-L1 and pembrolizumab. Regarding the quality assessment, we found 11 out of 14 poor quality studies, and the rest were of moderate quality (Table 1).
| Study no. | First author and year | Country | Study design | Sample size | Stage | ICIs used | Steroids used and dosage | Reason for steroid use | Type of analysis |
Mean age (in years) |
Quality of evidence |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Adachi et al 201917 | Japan | Retrospective | 296 | Adv. | NIVO | NR | Brain metastasis, fatigue, radiation pneumonitis, autoimmune disease | MVA | 70 | Low |
| 2. | Arbour et al 201818 | USA | Retrospective | 640 | IV | PEMBRO, NIVO, ATEZO or DURVA | NR/10 mg | Brain metastasis, best supportive care | MVA | NR | Low |
| 3. | Drakaki et al 202020 | USA | Retrospective | 862 | IIIB-IV | NIVO, IPI | Dexamethasone, prednisolone | Cancer related symptoms | MVA | 69 | Low |
| 4. | Dumenil et al 201821 | France | Retrospective | 67 | IIIB-IV | NIVO | NR/10-40 mg | Brain metastasis | MVA | 68.5 | Low |
| 5. | Fuca et al 201912 | Italy | Retrospective | 151 | IV | anti-PD−1/PD-L1/ anti-PD-L1 + anti-CTLA−4 | NR | Best supportive care, irAE | MVA | NR | Low |
| 6. | De Giglio et al 202019 | France | Retrospective | 413 | Adv. | anti-PD−1/PD-L1/ anti-PD-L1 | Prednisolone equivalent/10 mg | Cancer related symptoms | MVA | 63 | Low |
| 7. | Hendriks et al 201922 | France | Prospective | 1025 | Adv. | anti-PD−1/PD-L1/ anti-PD-L1 | NR | Brain metastasis | MVA | 64.3 | Moderate |
| 8. | Mountzios et al 202023 | 4 countries (Greece, Italy, Switzerland, Spain) | Retrospective | 265 | IV | PEMBRO | NR | NR | MVA | 67 | Low |
| 9. | Ricciuti et al 201913 | USA | Retrospective | 650 | IV | anti-PD−1/PD-L1/ anti-PD-L1 + anti-CTLA−4 | Prednisolone equivalent/10 mg | Brain metastasis, Best supportive care | MVA | 66 | Low |
| 10. | Riudavets et al 202024 | Spain | Retrospective | 267 | Adv. | anti-PD−1/PD-L1/ anti-PD-L1 | Prednisolone equivalent/10 mg | Cancer related symptoms | MVA | 66.1 | Low |
| 11. | Scott et al 201811 | USA | Retrospective | 210 | IV | NIVO | Prednisolone equivalent/10 mg | Brain metastasis, Best supportive care, irAE | MVA | 67.5 | Moderate |
| 12. | Skribek et al 202025 | Sweden | Retrospective | 196 | Adv. | Mono or combination therapy | Prednisolone equivalent/10 mg | Cancer-related symptoms | MVA | 70.5 | Low |
| 13. | Svaton et al 202026 | Czech Republic | Retrospective | 224 | III/IV | NIVO | Prednisolone equivalent/10 mg | NR | MVA | 67 | Low |
| 14. | Taniguchi et al 201727 | Japan | Retrospective | 201 | IV | NIVO | NR | NR | MVA | 68 | Moderate |
- Abbreviations: Adv, Advanced stage NSCLC; ATEZO, atezolizumab; DURVA, durvalumab; IPI, ipilimumab; irAE, immune-related adverse reaction; MVA, Multivariable analysis; NIVO, nivolumab; NR, Not reported; PEMBRO, pembrolizumab; USA, United States of America.
3.3 Overall survival
In total, 11 studies reported the OSs following corticosteroid administration of patients with NSCLC under ICI therapy.11-13, 18-20, 26 The pooled HR was 1.82 (95% CI, 1.51–2.18), indicating that the patients receiving corticosteroid therapy have significantly worse survivals than those not receiving corticosteroid therapy (Figure 2). However, we found high heterogeneity among the studies (I2=62.6%; chi-square test for heterogeneity, p = 0.003). We explored the sources of heterogeneity using meta-regression with the potential covariates such as study design, country, sample size, mean age and quality of studies, but we found none of these factors had a significant influence on the outcome measures to be the source of heterogeneity.
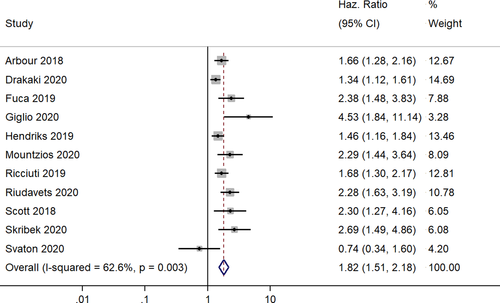
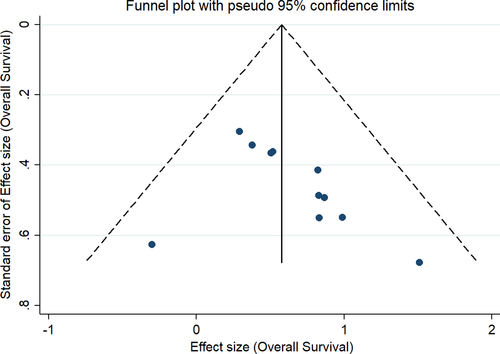
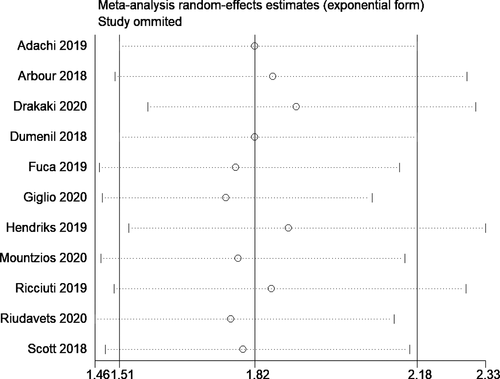
The results of our subgroup analyses for the study designs (prospective/retrospective), sample sizes (< or >300 patients), quality of studies (low or moderate) and study regions (Asia/America/Europe) showed no significant differences in effect estimate in terms of study design (prospective HR, 1.46; 95% CI, 1.16–1.84; n = 1; and retrospective HR, 1.89; 95% CI, 1.54–2.33; n = 10) and study quality (low quality pooled HR, 1.86; 95% CI, 1.50–2.32; n = 9; and moderate quality pooled HR, 1.68; 95% CI, 1.11–2.54; n = 2). However, we found significant variation in effect estimates in sample sizes (<300 sample size pooled HR, 2.12; 95% CI, 1.62–2.77; n=6; and >300 sample size pooled HR, 1.57; 95% CI, 1.32–1.88; n = 5) and study regions (America pooled HR, 1.57; 95% CI, 1.32–1.86; n = 4; and Europe pooled HR, 2.02; 95% CI, 1.49–2.75; n = 7). Subgroup analysis based on the reason for steroid use also showed a difference as the studies reporting the steroid use for supportive had worse OS (pooled HR = 1.94; 95%CI: 1.57–2.20), compared to those using for brain metastasis (pooled HR = 1.62; 95%CI: 1.41–1.86). The funnel plot (Figure 3) showed evidence of significant publication bias (Egger's test p value =0.05). The sensitivity analysis did not reveal a significant variation in the magnitude or direction of the effect estimates due to individual study effects (Figure 4).
3.4 Progression-free survival
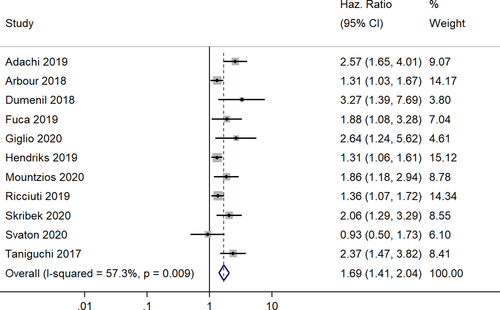
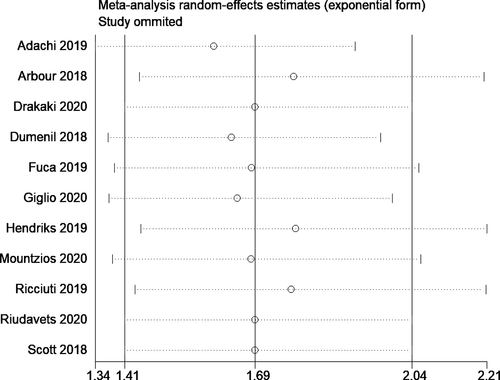
In total, 11 studies reported the progression free survivals following corticosteroid administration of patients with NSCLC under ICI therapy.12, 13, 17-19, 21-23, 25-27 The pooled HR was 1.69 (95% CI, 1.41–2.04), indicating that the patients receiving corticosteroid therapy have significantly shorter progression-free survivals than those not receiving corticosteroid therapy (Figure 5). The heterogeneity between the studies was high (I2 = 57.3%; chi-square test for heterogeneity, p = 0.009). We performed a meta-regression to explore the source of heterogeneity with the same set of covariates. We identified sample size as having a significant influence on the outcome in the univariable meta-regression analysis. Then, we performed a multivariable meta-regression with sample size and mean age (as the P value was lower than 0.20 in the univariable analysis), and the adjusted model explained approximately 64% of the total heterogeneity in the final model.
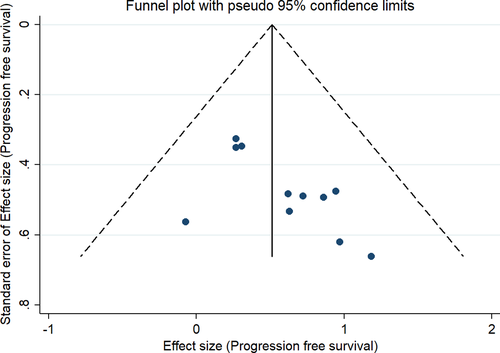
Our subgroup analyses revealed moderate variation in effect estimates in terms of study design (prospective HR, 1.31; 95% CI, 1.06–1.61; n = 1; and retrospective HR, 1.78; 95% CI, 1.44–2.21; n = 10) and study quality (low quality pooled HR, 1.72; 95% CI, 1.38–2.15; n = 9; and moderate quality pooled HR, 1.69; 95% CI, 0.95–3.00; n = 2). However, there was significant variation in effect estimate in terms of sample size (<300 sample size: pooled HR, 2.00; 95% CI, 1.57–2.56; n = 7; >300 sample size: pooled HR, 1.35; 95% CI, 1.18–1.55; n = 4) and study region (Asia: pooled HR, 2.47; 95% CI, 1.79–3.43; n = 2; America: pooled HR, 1.33; 95% CI, 1.13–1.58; n = 2; Europe: pooled HR, 1.72; 95% CI, 1.30–2.25; n = 7). Subgroup analysis based on the reason for steroid use did not show much difference as the studies reporting the steroid use for supportive had similar PFS (pooled HR = 1.55; 95%CI: 1.26–1.92), compared to those using for brain metastasis (pooled HR = 1.56; 95%CI: 1.23–1.97). The funnel plot (Figure 6) showed significant evidence of publication bias (Egger's test p value =0.01). The sensitivity analysis did not reveal significant variations in the magnitude or direction of the effect estimate due to individual study effects (Figure 7).
4 DISCUSSION
ICIs have been approved to manage patients with NSCLC because they provide good clinical activity and durable responses.28 However, ICIs inhibit negative regulators of the adaptive immunity and can lead to toxic effects. Most events are mild in nature and do not require any specific management, apart from basic supportive care. However, when the toxicity becomes serious, moderate to high doses of systemic corticosteroids are needed. Patients with metastasis also require these corticosteroids to manage cancer-related symptoms such as brain oedema, fatigue, pain, dyspnoea and any autoimmune conditions. In spite of their indications, corticosteroids have their own set of harmful side effects leading to progression and worsening of patient outcomes. Therefore, gathering the evidence and assessing the pooled effects on the patient outcomes is important. We conducted this review to assess the impact of corticosteroids on the OS and PFS of patients with NSCLC receiving ICIs.
We included 14 studies with data from 5461 participants in our meta-analysis. Most studies included were retrospective in nature, and most were conducted in the United States (followed by studies conducted in France, Spain and Japan). Most studies were of low quality. Both OS (pooled HR, 1.82; 95% CI, 1.51–2.18) and PFS (pooled HR, 1.69; 95% CI, 1.41–2.04) were poorer for patients with NSCLC receiving corticosteroids than for those patients not receiving corticosteroids. We found this association to be statistically significant as the confidence limit did not cross the null value of 1. Other reviews assessing the impact of corticosteroids on survival outcomes of patients with NSCLC also have revealed similar estimates for OS (pooled HR, 1.62, p < 0.01).14 However, while the previous review analysed data from only 7 studies, our updated review assessed data from 14 studies; moreover, we also calculated PFS outcomes.
Subgroup analysis conducted based on the study design, setting, sample size and study quality revealed significant influences of the sample size and study region on the effect estimates. NSCLC patients in Asia had the poorest PFS outcome (pooled HR, 2.47), while those in Europe had the poorest OS outcome (pooled HR, 2.02). However, we found no studies reporting the OS in Asian patients and this statistic is needed to investigate why this specific population seems to have a higher mortality than other world regions. We found the study results to be robust to the quality of the studies, and the sensitivity analysis revealed no significant influence of individual studies on the overall pooled estimate in terms either magnitude or direction.
Reasons for the high mortality in patients receiving corticosteroids have been explored, and multiple hypotheses have been suggested. One such hypothesis states that corticosteroid intake can significantly reduce the number of circulating CD4 and T-lymphocytes or impair the function of T-lymphocytes by enhancing the PD-1 expression on T cells.29 Another hypothesis states that patients taking corticosteroids at the time of initiation of ICIs can develop strong immune cascade effects leading to poor activation of the anti-tumour immune response.30 Thus, clinicians need to exert caution when prescribing corticosteroids to patients with NSCLC under ICIs as the ultimate goal of treatment is to improve survival and corticosteroids can clash with this goal. Future longitudinal studies should comprehensively assess the factors responsible for such adverse clinical outcomes following corticosteroid intake in these patients and gather evidence on the factors responsible for mortality to be able to take corrective measures and recommend specific interventions.
Our results should be interpreted with caution and inferred accordingly, considering the difference in methods and qualities across the included studies. In our analysis, we found significant between-study variability (significant chi-square test result for heterogeneity and I2 statistic) for both the outcomes. This high heterogeneity can be attributed to methodological differences between the included studies (study design, sample size, mean age and the corticosteroid use patterns).
The strengths of our review include the comprehensive and extensive literature search to gather all the relevant publications to date. We add an updated review to the few available on the impact of corticosteroids on the survival of patients with NSCLC under ICI therapy. Ours is the first meta-analysis on the impact of corticosteroids on survival focussing specifically on patients with NSCLC, and our findings are quite interesting. A similar meta-analysis conducted on this topic analysed data from only 7 studies, but we have doubled the number of studies analysed.14
We are aware of the limitations in our review. We included both prospective and retrospective studies, and we know that inclusion of mixed study designs makes it difficult to infer causal associations between corticosteroid use and mortality. Hence, more prospective studies with larger sample sizes should be done before recommendations can be issued. Most of the studies included in our analysis were of low quality and had high risk of bias. A significant Egger's test means a possibility of publication bias in our review exists. In addition, we tried to overcome the high heterogeneity between the included studies by performing subgroup analyses and meta-regression and identified sample size and mean age of participants as the source of the heterogeneity.
Despite all these limitations, our results suggest that corticosteroid use significantly impacts the survival outcomes of patients with NSCLC under ICI therapy, and we believe our results should be considered by clinicians and oncologists during their routine practice. Our reliable pooled estimates should be made known; however, it will help to overcome this sense of inconsistency and across the findings and provided a for the same. However, more robust prospective studies with larger sample sizes are needed to ensure specific long-term intervention packages will optimize the outcomes of patients with NSCLC receiving ICI therapy.
CONFLICT OF INTERESTS
No conflicts of interest have been declared.
Open Research
DATA AVAILABILITY STATEMENT
All data generated or analysed during this study are included in this published article.




