High Salt Intake and Stroke: Meta-analysis of the Epidemiologic Evidence
Summary
Research on the potential impact of high salt intake on health has grown rapidly over the last decades. Recent studies have suggested that high salt intake could also be associated with adverse effects on cardiovascular system. The review evaluated the current level of epidemiologic evidence on the association between the level of habitual salt intake and stroke outcome. We also suggest further research direction. There were 21 independent samples from 12 studies, with 225,693 participants (follow-up, 3–19 years) and 8135 stroke events. High salt intake was associated with risk of stroke event (pooled odd ratio [OR], 1.34; 95% confidence interval [CI], 1.19–1.51), stroke death (1.40; 1.21–1.63) and stroke onset (1.11; 1.00–1.24), ischemic stroke death (2.15; 1.57–2.95), not associated with risk of ischemic stroke onset (1.07, 0.95–1.2), with no significant evidence of publication bias. High salt intake is associated with significantly increased risk of stroke event. Further research should be directed toward clarifying and quantifying these possible effects and generating testable hypotheses on plausible biologic mechanisms.
Introduction
During the past century, the evidence for the risks imposed on human health by excess salt consumption has become compelling. There is widespread evidence that long-term (years) effects of high salt intake on the risk of cardio-cerebral-vascular disease in the past decades 1-18, and relevant meta-analysis between high salt intake and health effects 19. Most of these studies found evidence of the association between high salt diet and the risk of stroke 4-15, but results are inconsistent. Ikehara et al. 4 and Umesawa et al. 9 studies found that there were significant association between high salt intake and the death as a result of stroke (OR 1.22; 95% CI, 1.00–1.49 for women and 1.55; 1.21–2.00 for men and women). Finding from Kono et al. 5 study shows high salt intake is related to stroke recurrence (1.98, 1.02–4.22), and that from Liang et al. 7 and Ellekjaer et al. 15 studies shows no significant association between high salt intake and ischemic stroke onset.
Past investigations of high salt intake, the stroke event outcome measure primarily focused on overall mortality or morbidity index used such routinely collected data as death counts or death rates, hospitalization or emergency room visit rates, in some investigations, categories of stroke diagnoses were selected as outcome measures. Few studies investigated presented an analysis on the association between high salt diet and stroke events.
Considerable consistency across studies has been observed for many health effects, for example, stoke death, onset or recurrence, particularly for those linked with high salt diet, although the biologic mechanisms of the health effects are unclear.
Methods
Search Strategy for Identification of Study
We identified papers through electronic searches of medical and biologic databases (Chinese Biomedical Database, CBM, 1978–January 2012; Medline 1966–January 2012; Embase 1980–January 2012; CAB Health 1973–January 2012; Science Citation Index, Web of Science 1981–January 2012; Conference Papers Index via Cambridge Scientific Abstracts databases 1982–January 2012, using a comprehensive list of stroke/stroke disease/and salt/salt intake/high salt diet keywords and phrases). We also searched web-based resources (available with the electronic version of these articles at http://www.ncbi.nlm.nih.gov/pubmed/), scanned reference lists (of review articles and published studies), and consulted with key experts in the field. Identified publications were scanned by one of the authors.
Inclusion and Exclusion Criteria
Inclusion criteria were as follows: (1) non-accidental exposure to directly measured sodium; (2) be a prospective population study, assess salt intake as baseline exposure, and determine stroke event prospectively as the outcome; (3) an adult population, and indicate the number of participants exposed and the rate or number of events in the different categories of salt intake; (4) to be original article published from January 1989 to January 2012 in the English language or Chinese language; and (5) follow participants for at least 3 years.
Exclusion criteria were as follows: (1) the outcome related to occupational or accidental exposure; (2) animals served as subjects; (3) the original article did not involve the exposure–response relationship between high salt intake and stroke; (4) repeated report; (5) reviews, letters, and comments; (6) low-quality article; and (7) results presented only as abstracts.
Data Extraction
Papers meeting the inclusion criteria were formally reviewed by pairs of reviewers using a data extraction form based on the previous reviews. This form was piloted before starting the data extraction process. From the identified studies, we recorded publication reference, age (mean, median or ranger), total number of participants, gender, method of assessing salt intake, study design, statistical techniques, country, outcome reported (stroke, ischemic stroke, or hemorrhagic stroke), number of events, method of outcome (death, onset, recurrence, or incidence) assessment, follow-up (years), level of salt intake in different categories, confounding factors, and results.
Table 1 presents the summary of the study design for each study; Table 2 presents the results of the study for each study. There are multiple entries for publications that reported results for more than one outcome. Results were referenced back to the specific publication (rather than to the study) from which they were produced. When possible, effect estimates (odds and risk ratios, mean change) are recalculated as the expected change in outcome for an increase in salt intake levels by 100 mmol/day. This scale is not comparable across studies measuring different salt intake, but its use facilitates comparison among studies using the same salt intake measurements. It was not appropriate to combine the results in a formal meta-analysis because the study methods vary so widely.
| First author | Age (year) | Events | Sex | Sodium intake assessment | Design | Statistical analysis | Country |
|---|---|---|---|---|---|---|---|
| Ikehara et al. 4 | 40–79 | 35515; 49275 | Men and Women | Questionnaire | Prospective cohort study | Cox proportional hazard models | Japan |
| Liang et al. 7 | Mean of 69 | 838 | Men and women | Questionnaire | A case–control study | Logistic regression | China |
| Kono et al. 5 | Mean of 64 | 102 | Men and women | A self-monitoring device | Prospective cohort study | Cox proportional hazard models | Japan |
| Larsson et al. 8 | 50–69 | 26556 | Men | Questionnaire | Prospective ATBC Study | Cox proportional hazard models | Finland |
| Umesawa et al. 9 | 40–79 | 58730 | Men and women | Questionnaire | Prospective cohort study | Cox proportional hazard models | Japan |
| Geleijnse et al. 10 | ≥55 | 1448 | Men and women | Questionnaire and overnight urine sodium | A case–cohort analysis | Cox proportional hazard models | Netherlands |
| Nagata et al. 11 | ≥35 | 13355; 15724 | Men and Women | Questionnaire | Prospective cohort study | Cox proportional hazard models | Japan |
| Tuomilehto et al. 13 | 25–64 | 1173; 1263 | Men and Women | 24- h urinary sodium excretion | Prospective study | Cox proportional hazard models | Finland |
| He et al. 12 | 25–74 | 2688; 6797 | Men and women | 24-h dietary recall | Prospective cohort study | Cox proportional hazard models | US |
| Alderman et al. 14 | 52–54 | 2937 | Men and women | 24 -h urinary sodium excretion | Prospective cohort study | Cox proportional hazard models | US |
| Ellekjaer et al. 15 | Mean 69 | 730 | Men and women | Questionnaire | A case–control study | Logistic regression | Norseland |
| Hu et al. 6 | ≥36 | 8562 | Men and women | Questionnaire | Prospective study | The Mantel-Haenszel procedure | Taiwan |
| First author/year of publication | Sex | Cases | Sodium difference (mmol/day) | Follow-up(years) | Outcome | OR (95% CI) | Controlled factors |
|---|---|---|---|---|---|---|---|
| Ikehara 4 (1)a | Men | 1023 | 12 | 16.4 | Total stroke death | 1.21 (0.99, 1.49) | Age, BMI, diabetes, smoking, education, sport, walking, mental stress, alcohol, and fresh fish intake. |
| Ikehara 4 (2)a | Women | 947 | 13 | 16.4 | Total stroke death | 1.22 (1.00, 1.49) | |
| Liang 7 | Men and women | 374 | 27 | 5 | Ischemic stroke onset | 1.30 (0.73, 2.32) | Weekly energy intake;, sex;, and age. |
| Kono 5 | Men and women | 89 | 183 | 3 | Stroke recurrence | 1.98 (1.02, 4.22) | Sex, disease, age, height, weight, BP, lipid, glucose, HbA1c, smoking, alcohol, exercise, waist, ankle-brachial pressure index, and physical activity. |
| Larsson 8 | Male smokers | 2702 | 84 | 13.6 | Ischemic stroke onset | 1.04 (0.92, 1.18) | Age, supplementation, cigarettes, BMI, BP, cholesterol, diabetes and CHD, physical activity. |
| Umesawa 9 (1)a | Men and women | 986 | 85 | 12.7 | Total stroke death | 1.55 (1.21, 2.00) | BMI, smoking, alcohol, hypertension, diabetes, menopause, hormone, exercise, education, mental stress, calcium and potassium intake |
| Umesawa 9 (2)a | Men and women | 510 | 85 | 12.7 | Ischemic stroke death | 2.04 (1.41, 2.94) | |
| Geleijnse 10 | Men and women | 181 | 69 | 5 | Total stroke death | 1.08 (0.80, 1.46) | Age, sex, 24-h urinary creatinine excretion, BMI, smoking, diabetes, diuretics, education, energy, alcohol, 3calcium, saturated fat intake. |
| Nagata 11 (1)a | Men | 137 | 110 | 7 | Total stroke death | 2.33 (1.23, 4.45) | Age, energy, education, BMI, smoking, exercise, hypertension and intake of protein, potassium, alcohol and vitamin E,diabetes, marital status. |
| Nagata 11 (2)a | Men | 137 | 110 | 7 | Ischemic stroke death | 3.22 (1.22, 8.53) | |
| Nagata 11 (3)a | Men | 137 | 110 | 7 | Hemorrhagic stroke death | 2.27 (0.85, 6.02) | |
| Nagata 11 (4)a | Women | 132 | 92 | 7 | Total stroke death | 1.70 (0.96, 3.02) | |
| Nagata 11 (5)a | Women | 132 | 92 | 7 | Ischemic stroke | 2.10 (0.96, 4.64) | |
| Nagata 11 (6)a | Women | 132 | 92 | 7 | Hemorrhagic stroke death | 1.28 (0.49, 3.37) | |
| Tuomilehto 13 (1)a | Men | 43 | 100 | Total stroke incidence | 1.00 (0.68, 1.47) | Age, education smoking, serum total and HDL cholesterol, systolic blood pressure, and BMI | |
| Tuomilehto 13 (2)a | Women | 41 | 100 | Total stroke incidence | 1.34 (0.87, 2.07) | ||
| He 12 (1)a | Overweight | 430 | 100 | 19 | Total stroke death | 0.99 (0.81, 1.20) | Age, sex, race, SBP,, BMI, diabetes, diuretic use, physical activity,, cholesterol, alcohol consumption, education |
| He 12 (2)a | Normal | 250 | 100 | 19 | Total stroke death | 1.39 (1.10, 1.76) | |
| Alderman 14 | Men and women | 23 | 140 | 3.5 | Total stroke | 1.20 (0.30, 4.00) | Unadjusted |
| Ellekjaer 15 | Men and women | 163 | 3 | Ischemic stroke onset | 0.92 (0.26, 3.31) | Physical activity | |
| Hu 6 | Men and women | 104 | 4 | Total stroke incidence | 1.79 (1.18, 2.71) | Age |
- BMI, body mass index; BP, blood pressure; SBP, systolic blood pressure; CHD, coronary heart disease; HDL, high-density lipoprotein.
- aRank indicates the different findings in the same paper.
Exposure Measurements
Categorization of salt intake differed among studies. Some reported the number of subjects exposed and the number (rate) of events across the distribution of salt intake; others reported differences in the event rate for a 100mmol/day difference in sodium. In the last three cases, we used the relative risk and hazard ratio reported by authors for the analysis. In all the cases in which categorization of the study participants by level of salt intake was available, we calculated the relative risk of higher versus lower salt intake by comparing the event rate in the two categories with a difference in average salt intake closest to 100 mmol of sodium or about 6 g of salt a day.
Stroke Outcomes
Daily counts of stroke event (non-trauma deaths [minus accidents and homicides], attack) were aggregated. Using the International Classification of Disease, tenth Revision (ICD-10) 20, total daily counts of stroke events (ICD-10 codes: I60–I69 or ICD-9: 430–438) were determined.
Study Design
Systematic review and meta-analysis of prospective or case–control study published from 1966 to 2012.
Quality of the Studies
Study quality was described by the extent to which the design, conduct, and analysis minimized selection, measurement, and confounding biases. The following methodological domains have been suggested to describe the quality of observational studies: study design, comparability of subjects, exposure measurement, outcome measurement, statistical analysis, and funding or sponsorship. The main variations among the reviewed studies were in (1) study design (prospective vs. case–control); (2) exposure measurement (follow-up time used; validity and reliability of the measurement method; comparability of measurement across the study groups; and spatial or compositional heterogeneity among the examined districts); (3) adjustment for key confounders (in particular, age, smoking, BMI and so on); and (4) statistical analysis (appropriateness and whether multiple comparisons were taken into consideration in the interpretation of the results). Key points in relation to these criteria are given in Table 1 and taken into account in the narrative summary.
Statistical Analysis
Pooled ORs of high salt intake with 95% confidence intervals (CI) for stroke events in meta-analyses were obtained by using the fixed-or random-effects model, heterogeneity among the risk estimates were tested using Q and I 2 statistics. When the result of the Q-test and I 2 statistics showed evidence for heterogeneity (P ≤ 0.05 and I 2 > 50%), we used a random effect analysis, following the method of DerSimonian and Laird 21. Otherwise, a fixed-effects analysis was conducted using the Mantel-Haenszel method 22. We also produced forest plots to show ORs from each of the individual studies included in the meta-analyses and the estimation of the pooled OR. The sizes of the markers of each OR in the plots represent the relative weight each study contributed to the pooled estimation. Publication bias was assessed by funnel plots or Begger's test and a weighted Egger test 23. We also performed sensitivity analyses, whereby each study was omitted in turn, recalculating the pooled estimates under extreme conditions. All analyses were performed using software STATA version 12.0 (StataCorp LP, College Station, TX, USA).
Results
Characteristics of the Study
The meta-analysis included 12 studies reporting on 21 independent estimates (Table 1). There were 225,693 participants from 6 different countries (4 studies from Japan; 2 each from Finland, United States, and China; and 1 each from Netherlands and Norseland). Eleven studies recruited both male and female participants, while 1 included only men. Follow-up ranged from 3 to 19 years. Three studies reported only total stroke death, three only ischemic stroke onset, two only total stroke incidence, one only stroke recurrence, one only stroke death (total stroke death, ischemic stroke death, and hemorrhagic stroke death). Salt intake was assessed by 24-h dietary recall (n = 1), 24-h urine excretion (n = 2), food frequency questionnaire (n = 7), a self-monitoring device (n = 1), both questionnaire and overnight urine sodium level (n = 1). In total, there were 8135 stroke events reported. Of the 11 studies that included both men and women, 3 reported outcomes separately. He et al.'s study provided separate findings for normal weight and overweight participants. Overall, data on the relationship between salt consumption and stroke were available from 21 estimates.
Salt Intake and Risk of Stroke Event
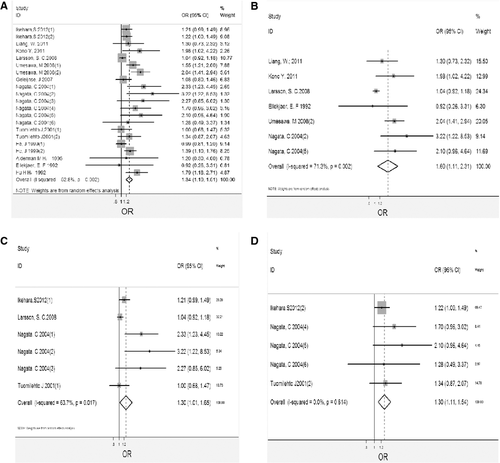
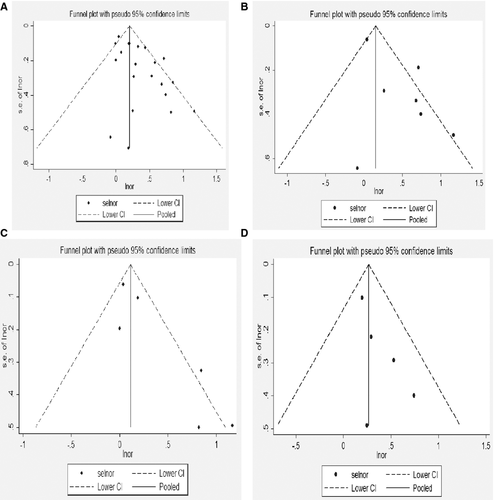
Table 2 provides data on the relation between salt intake and risk of stroke in each of 21 estimates included in the study. Figure 1(A) shows the results of the pooled analysis. In the pooled analysis, high salt intake was associated with risk of stroke (OR, 1.34; 95% CI, 1.19–1.51). There was significant heterogeneity among studies (Q = 42.37, P = 0.002; I 2 = 63.7%). The funnel plot did not show asymmetry, thus excluding publication bias (Begger's test z c = 1.18, P = 0.239; Egger's test t = 0.08, P = 0.094). As shown in Figure 2(A) for the individual estimates included in the analysis, we found a trend toward a direction association between salt intake and risk of stroke in 19 estimates, which was significant in 7. We observed a non-significant inverse trend in 2 estimates. Sensitivity analysis showed the pooled estimate of the effect of salt intake on the risk of stroke did not vary substantially with the exclusion of any one study, as shown in Table 3.
| Model | OR (95% CI) | Q | P | ||
|---|---|---|---|---|---|
| High salt intake and stroke events | Total | Random effects Model | 1.34 (1.19–1.51) | 42.37 | 0.002 |
| Ikehara 4 (1)a excluded | Random effects Model | 1.37 (1.20, 1.56) | 42.36 | 0.002 | |
| Ikehara 4 (2)a excluded | Random effects Model | 1.37 (1.20, 1.56) | 42.37 | 0.002 | |
| Liang 7 excluded | Random effects Model | 1.35 (1.19, 1.52) | 42.32 | 0.002 | |
| Kono 5 excluded | Random effects Model | 1.33 (1.18, 1.50) | 40.57 | 0.003 | |
| Larsson 8 excluded | Random effects Model | 1.38 (1.22, 1.56) | 33.41 | 0.022 | |
| Umesawa 9 (1)a excluded | Random effects Model | 1.32 (1.17, 1.50) | 38.65 | 0.005 | |
| Umesawa 9 (2)a excluded | Random effects Model | 1.29 (1.16, 1.49) | 34.62 | 0.016 | |
| Geleijnse 10 excluded | Random effects Model | 1.37 (1.21, 1.55) | 41.69 | 0.002 | |
| Nagata 11 (1)a excluded | Random effects Model | 1.32 (1.17, 1.48) | 38.45 | 0.005 | |
| Nagata 11 (2)a excluded | Random effects Model | 1.32 (1.18, 1.48) | 38.53 | 0.005 | |
| Nagata 11 (3)a excluded | Random effects Model | 1.33 (1.18, 1.50) | 40.82 | 0.003 | |
| Nagata 11 (4)a excluded | Random effects Model | 1.33 (1.18, 1.50) | 41.07 | 0.002 | |
| Nagata 11 (5)a excluded | Random effects Model | 1.33 (1.18, 1.50) | 40.54 | 0.003 | |
| Nagata 11 (6)a excluded | Random effects Model | 1.35 (1.19, 1.52) | 42.36 | 0.002 | |
| Tuomilehto 13 (1)a excluded | Random effects Model | 1.37 (1.21, 1.55) | 41.30 | 0.002 | |
| Tuomilehto 13 (2)a excluded | Random effects Model | 1.35 (1.19, 1.52) | 41.19 | 0.002 | |
| He 12 (1)a excluded | Random effects Model | 1.38 (1.22, 1.56) | 37.42 | 0.007 | |
| He 12 (2)a excluded | Random effects Model | 1.34 (1.18, 1.52) | 41.10 | 0.002 | |
| Alderman 14 excluded | Random effects Model | 1.35 (1.19, 1.52) | 42.37 | 0.002 | |
| Ellekjaer 15 excluded | Random effects Model | 1.35 (1.20, 1.52) | 42.18 | 0.002 | |
| Hu 6 excluded | Random effects Model | 1.32 (1.17, 1.49) | 39.03 | 0.004 | |
| High salt intake and ischemic stroke event | Total | Random effect Model | 1.60 (1.11, 2.31) | 20.89 | 0.002 |
| Liang 7 excluded | Random effect Model | 1.68 (1.09, 2.60) | 20.75 | 0.001 | |
| Kono 5 excluded | Random effect Model | 1.55 (1.04, 2.32) | 18.70 | 0.002 | |
| Larsson 8 excluded | Fixed effect Model | 1.87 (1.45, 2.41) | 4.24 | 0.516 | |
| Ellekjaer 15 excluded | Random effect Model | 1.67 (1.13, 2.48) | 20.75 | 0.001 | |
| Umesawa 9 (2)a excluded | Random effect Model | 1.48 (1.01, 2.14) | 11.08 | 0.050 | |
| Nagata 11 (2)a excluded | Random effect Model | 1.49 (1.03, 2.14) | 16.64 | 0.005 | |
| Nagata 11 (5)a excluded | Random effect Model | 1.54 (1.04, 2.29) | 18.70 | 0.002 | |
| High salt intake and stroke for male | Total | Random effects Model | 1.30 (1.02–1.65) | 13.76 | 0.017 |
| Ikehara 4 (1)a excluded | Random effects Model | 1.47 (0.99, 2.19) | 13.03 | 0.011 | |
| Larsson 8 excluded | Random effects Model | 1.52 (1.06, 2.19) | 9.97 | 0.041 | |
| Nagata 11 (1)a excluded | Fixed effects Model | 1.10 (0.99, 1.22) | 8.64 | 0.071 | |
| Nagata 11 (2)a excluded | Fixed effects Model | 1.11 (1.10, 1.22) | 9.18 | 0.057 | |
| Nagata 11 (3)a excluded | Random effects Model | 1.25 (0.98, 1.59) | 11.74 | 0.019 | |
| Tuomilehto 13 (1)a excluded | Random effects Model | 1.42 (1.05, 1.91) | 13.41 | 0.009 | |
| High salt intake and stroke for female | Total | Fixed effects Model | 1.31 (1.11–1.54) | 2.61 | 0.614 |
| Ikehara 4 (2)a excluded | Fixed effects Model | 1.52 (1.13, 2.05) | 1.24 | 0.742 | |
| Nagata 11 (4)a excluded | Fixed effects Model | 1.27 (1.07, 1.52) | 1.78 | 0.619 | |
| Nagata 11 (5)a excluded | Fixed effects Model | 1.28 (1.05, 1.51) | 1.21 | 0.751 | |
| Nagata 11 (6)a excluded | Fixed effects Model | 1.31 (1.10, 1.55) | 2.67 | 0.445 | |
| Tuomilehto 13 (2)a excluded | Fixed effects Model | 1.30 (1.09, 1.56) | 2.66 | 0.448 |
- aRank indicates the different findings in the same paper.
Table 2 provides data on the relation between salt intake and risk of ischemic stroke event in each of 7 estimates included in the study. Figure 1(B) shows the results of the pooled analysis. In the pooled analysis, high salt intake was associated with risk of stroke (OR, 1.60; 95% CI, 1.11–2.31). There was significant heterogeneity among studies (Q = 20.89, P = 0.002; I 2 = 71.3%). The funnel plot did not show asymmetry, thus excluding publication bias (Begger's test z c = 0.00, P = 1.000; Egger's test t = −0.38, P = 0.717). As shown in Figure 2(B) for the individual estimates included in the analysis, we found a trend toward a direction of association between salt intake and risk of stroke in 7 estimates, which was significant in 3. We observed a non-significant inverse trend in one estimates. Sensitivity analyses that showed the pooled estimate of the effect of salt intake on the risk of ischemic stroke event were robust, as shown in Table 3.
There were 6 estimates, and 5 estimates reported data for men and women separately for stroke event. The pooled estimates from these estimates were 1.30 (1.02–1.65) and 1.31 (1.11–1.54), as shown in Figure 1(C,D), respectively. Heterogeneity tests showed evidence for heterogeneity and homogeneity among studies for men and women (Q = 13.76, P = 0.017, I 2 = 63.7%; Q = 2.67, P = 0.614, I 2 = 0); the funnel plots were shown in Figure 2(C,D), respectively. Sensitivity analysis showed the pooled estimate of the effect of salt intake on the risk of women stroke event did not vary substantially with the exclusion of any one study, but the results for men were not robust to the inclusion of Ikehara study, and Nagata study, as shown in Table 3.
Salt Intake and Risk of Stroke Death
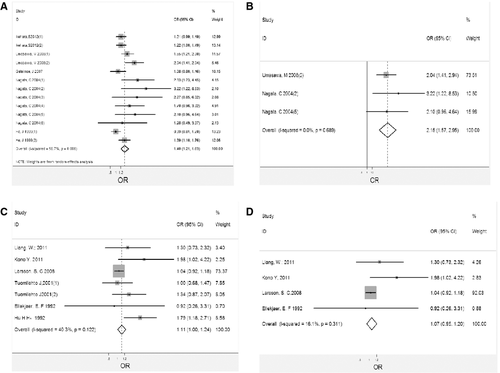
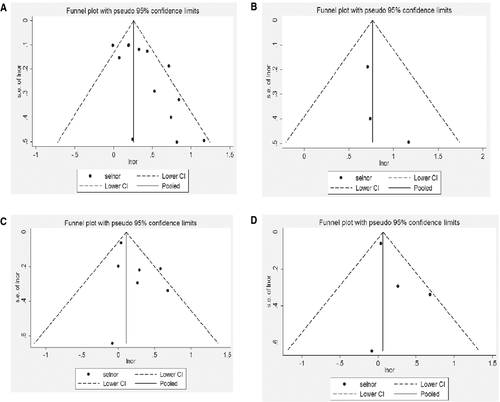
Table 2 provides data on the association between salt intake and the risk of stroke death in 13 cohorts. In the pooled analysis, there was an association between high salt intake and risk of stroke death (OR, 1.40; 95% CI, 1.21–1.63), as shown in Figure 3(A). The heterogeneity among studies was significant (Q = 27.68, P = 0.006; I 2 = 56.7%), but the funnel plot did not show asymmetry, thus excluding publication bias (z c = 1.77, P = 0.077; t = −0.09, P = 0.927), as shown in Figure 4(A).The evaluation of individual estimates showed a trend toward a direct association between salt intake and risk of stroke death in 12 estimates, with significantly higher relative risk in 5. An inverse trend was observed in one estimate but was not significant. Sensitivity analysis showed that the result was robust, as shown in Table 4.
| Model | OR (95% CI) | Q | P | ||
|---|---|---|---|---|---|
| High salt intake and stroke death | Total | Random effects Model | 1.40 (1.21, 1.63) | 27.68 | 0.006 |
| Ikehara 4 (1)a excluded | Random effects Model | 1.45 (1.22, 1.73) | 27.18 | 0.004 | |
| Ikehara 4 (2)a excluded | Random effects Model | 1.45 (1.22, 1.73) | 27.27 | 0.004 | |
| Umesawa 9 (1)a excluded | Random effects Model | 1.39 (1.18, 1.64) | 25.46 | 0.008 | |
| Umesawa 9 (2)a excluded | Random effects Model | 1.34 (1.16, 1.54) | 21.47 | 0.029 | |
| Geleijnse 10 excluded | Random effects Model | 1.45 (1.23, 1.71) | 26.17 | 0.006 | |
| Nagata 11 (1)a excluded | Random effects Model | 1.37 (1.18, 1.58) | 24.42 | 0.011 | |
| Nagata 11 (2)a excluded | Random effects Model | 1.37 (1.19, 1.58) | 24.29 | 0.012 | |
| Nagata 11 (3)a excluded | Random effects Model | 1.39 (1.19, 1.62) | 26.41 | 0.006 | |
| Nagata 11 (4)a excluded | Random effects Model | 1.39 (1.19, 1.63) | 26.80 | 0.005 | |
| Nagata 11 (5)a excluded | Random effects Model | 1.38 (1.19, 1.63) | 26.22 | 0.006 | |
| Nagata 11 (6)a excluded | Random effects Model | 1.41 (1.21, 1.65) | 27.69 | 0.004 | |
| He 12 (1)a excluded | Fixed effects Model | 1.38 (1.25, 1.51) | 18.86 | 0.064 | |
| He 12 (2)a excluded | Random effects Model | 1.42 (1.20, 1.68) | 27.28 | 0.004 | |
| High salt intake and stroke onset | Total | Fixed effects Model | 1.11 (1.00, 1.24) | 10.05 | 0.122 |
| Liang 7 excluded | Fixed effects Model | 1.11 (0.99, 1.24) | 9.77 | 0.082 | |
| Kono 5 excluded | Fixed effects Model | 1.10 (0.99, 1.23) | 7.48 | 0.187 | |
| Larsson 8 excluded | Fixed effects Model | 1.35 (1.10, 1.66) | 5.58 | 0.349 | |
| Tuomilehto 13 (1)a excluded | Fixed effects Model | 1.12 (1.01, 1.26) | 9.72 | 0.083 | |
| Tuomilehto 13 (2)a excluded | Fixed effects Model | 1.10 (0.99, 1.23) | 9.31 | 0.093 | |
| Ellekjaer 15 excluded | Fixed effects Model | 1.12 (1.00, 1.24) | 9.96 | 0.076 | |
| Hu 6 excluded | Fixed effects Model | 1.08 (0.97, 1.20) | 4.71 | 0.452 | |
| High salt intake and ischemic stroke onset | Total | Fixed effects Model | 1.07 (0.95, 1.20) | 3.58 | 0.311 |
| Liang 7 excluded | Fixed effects Model | 1.06 (0.94, 1.20) | 3.11 | 0.211 | |
| Kono 5 excluded | Fixed effects Model | 1.05 (0.93, 1.18) | 0.59 | 0.745 | |
| Larsson 8 excluded | Fixed effects Model | 1.45 (0.95, 2.22) | 1.37 | 0.505 | |
| Ellekjaer 15 excluded | Fixed effects Model | 1.07 (0.95, 1.21) | 3.52 | 0.175 | |
| High salt intake and ischemic stroke death | Total | Fixed effects Model | 2.15 (1.57, 2.95) | 0.74 | 0.689 |
| Umesawa 9 (2)a excluded | Fixed effects Model | 2.49 (1.35, 4.59) | 0.45 | 0.503 | |
| Nagata 11 (2)a excluded | Fixed effects Model | 2.05 (1.47, 2.86) | 0.00 | 0.948 | |
| Nagata 11 (5)a excluded | Fixed effects Model | 2.16 (1.53, 3.05) | 0.74 | 0.389 |
- aRank indicates the different findings in the same paper.
Table 2 provides data on the association between salt intake and the risk of ischemic stroke death in 3 cohorts. In the pooled analysis, there was an association between high salt intake and risk of ischemic stroke death (OR 2.15, 95% CI 1.57–2.95), as shown Figure 3(B). The homogeneity among studies was not significant (Q = 0.74, P = 0.689; I 2 = 0), but the funnel plot did not show asymmetry, thus excluding publication bias (z c = 1.04, P = 0.296; t = 2.42, P = 0.250), as shown in Figure 4(B). The evaluation of individual estimates showed a trend toward a direct association between salt intake and risk of ischemic stroke death in 3 estimates, with significantly higher relative risk in 2. An inverse trend was observed in none. Sensitivity analysis showed that the result was robust, as shown in Table 4.
Salt Intake and Risk of Stroke Attack
Table 2 provides data on the association between salt intake and the risk of stroke attack in 7 estimates. In the pooled analysis, there was an association between high salt intake and risk of stroke attack (OR, 1.11; 95% CI, 1.00–1.24), as shown in Figure 3(C). The homogeneity among studies was not significant (Q = 10.05, P = 0.122; I 2 = 40.3%), but the funnel plot did not show asymmetry, thus excluding publication bias (z c = 0.60, P = 0.548; t = −0.23, P = 0.827), as shown in Figure 4(C).The evaluation of individual estimates showed a trend toward a direct association between salt intake and risk of stroke attack in 6 estimates, with significantly higher relative risk in 2. An inverse trend was observed in one estimate but was not significant. Sensitivity analysis showed that the exclusion of the only estimate showing an inverse trend but not significant, as shown in Table 4.
Table 2 provides data on the association between salt intake and the risk of ischemic stroke attack in 4 cohorts. In the pooled analysis, there was no association between salt intake and risk of ischemic stroke attack (OR, 1.07; 95% CI, 0.95–1.20), as shown in Figure 3(D). The homogeneity among studies was not significant (Q = 3.58, P = 0.311; I 2 = 16.1%), but the funnel plot did not show asymmetry, thus excluding publication bias (z c = 0.34, P = 0.734; t = −0.12, P = 0.914), as shown in Figure 4(D).The evaluation of individual estimates showed a trend toward a direct association between salt intake and risk of ischemic stroke attack in 3 estimates, with significantly higher relative risk in 1. We observed a non-significant inverse trend in 1 estimate. Sensitivity analysis showed that the result was robust, as shown in Table 4.
Discussion
Stroke remains one of the most devastating of all neurological conditions, which caused an estimated 5.7 million deaths in 2005, and 8.7% of these deaths were in low-income and middle-income countries 16. Worldwide it accounts for approximately 5.5 million deaths annually, with 44 million disability-adjusted life-years lost. As a disease of aging, the prevalence of stroke is expected to increase significantly around the world in the years ahead as the global population older than 65 years of age continues to increase by approximately 9 million people per year 17. Stroke is a severely neglected condition in lower-income regions 18. There are many risk factors of stroke, for example, systolic blood pressure, body-mass index, smoking, and environment and so on 20, 24-26; the high salt intake-stroke relationships were also being investigated widely 27-29. However, according to stroke outcome category (death or attack) and diagnosis category (ischemic stroke/hemorrhagic stroke), few studies on the high salt intake-stroke relationships had been investigated systematically.
The systematic review identified 12 relevant and suitable studies published from 1992 to 2012. These studies provided evidence from 225,693 participants contributing overall more than 8000 stroke events.
Stroke events were defined as rapidly developing signs of focal (or global) disturbance of cerebral function lasting >24 h (unless interrupted by surgery or death) with no apparent nonvascular cause. The definition included patients presenting with clinical signs and symptoms suggestive of subarachnoid hemorrhage, intracerebral hemorrhage, or cerebral infarction. Typically, the first 2 types were gone by the name of hemorrhagic stroke, and the last was known as ischemic stroke. On the basis of the status within 28 days of onset, stroke events were subdivided into first or recurrent and into fatal or nonfatal. Stroke attack refers to all strokes (both first and recurrent) within 28 days 30.
According to the World Health Organization, 62% of all strokes are attributable to high blood pressure 31. The direct relation between levels of dietary salt intake and blood pressure has been established through experimental, epidemiological, migration, and intervention studies. However, the relation between salt intake and stroke events was devoid of system study. Up to now, according to our inclusion criteria in the study, there was the sole study involved the association between salt intake and hemorrhagic stroke death, none involved hemorrhagic stroke attack. Many studies focus on the effect of salt intake to total stroke events or ischemic stroke events.
We used subgroup to assess the influence of gender on the association between high salt intake and risk of stroke. For stroke event outcome, separate analyses of the male and female cohorts suggest that the associations are consistent and not significantly different between the sexes. Similar results were obtained with respect to the method of assessment of salt intake used in the various studies.
Meta-analyses suggest unequivocally that high salt intake was related to statistically significant increases in risk of death as a result of stroke events, especially ischemic stroke events, and high salt intake to ischemic stroke death, which was not inconsistent with hemorrhagic stroke that was the finding from sole study by Nagata et al. 11. Strazzullo et al. 19 concluded that high salt intake was associated with risk of stroke (a pooled relative risk of 1.23, 1.06–1.43) based on 14 cohorts, which is slightly lower than our meta-analysis estimates of 1.34 (1.19–1.51) based on 21 estimates of 12 studies. Meta-analyses show that high salt intake statistically significant increases in risk of stroke attack, but found no statistically significant increases in the risk of ischemic stroke attack in relation to high salt intake. We lacked a sufficient number of studies to calculate hemorrhagic stroke estimate for Nana study only. Whether there is any association between high salt intake and hemorrhagic stroke merits to further study. This review and its meta-analysis raise important issues that may guide both the design and presentation of future studies.
Large heterogeneity in estimated high salt intake-stroke event risk estimates was observed across studies. We found some suggestive evidence that type-specific factor (e.g., ischemic stroke and hemorrhagic stroke) can explain some of the heterogeneity. However, the fact that analyses of the same country data (e.g., Japan) by different researchers resulted in markedly different estimates suggests a large influence of model specification on the results. An analysis of multiple cities using several alternative model specifications would provide information on the extent of model uncertainty.
Most adult populations around the world have average daily salt intakes higher than 6 g/day, and for many in Eastern Europe and Asia higher than 12 g/day. International recommendations suggest that average population salt intake should be <5–6 g/day 31.
Six studies provided data adjusted for hypertension status or baseline blood pressure.
Overweight and obesity are often associated with high blood pressure and causally involved in the development of hypertension. Eight of 12 studies included in the meta-analysis provided relative risk estimates adjusted for BMI or body weight at entry into the study. All 12 of these studies lacked adjustment particulate matter; however, we found that particulate matters were related to stroke attack.
Clearly, our meta-analysis has many limitations. Although we attempted to capture the crucial dimensions of methodological heterogeneity, there are many factors either difficult to quantify or unreported by the authors that could influence effect estimates. The studies included in the meta-analysis were heterogeneous regarding sample size, study design, number of events, and duration of follow-up, with a few cohorts having small numbers. In the calculation of the pooled estimate, we weighted the results of the individual studies for sample size but did not account for the duration of follow-up.
Another limitation is the fact that the estimate of the baseline population salt intake in each study was based on a single measurement (whether through 24-h urine collection or dietary assessment). We were therefore unable to correct for the bias. Because of the large day-to-day variability within people in salt consumption and the consequent effect imposed on the average estimate of exposure, our estimates of risk are probably underestimated.
Finally, categorization of salt intake was also heterogeneous: Some studies gave us difference in outcome for a given difference (e.g., 100 mmol/24 h) in sodium intake or excretion; other studies stratified the population by categories of sodium intake and compared stroke outcomes across categories. To standardize our comparison between higher and lower salt consumption, we sought to refer a difference as close as possible to 100 mmol or 6 g a day between high and low salt intakes. Nevertheless, there remained appreciable difference in this respect among studies. How to overcome the problem was the merit to further study.
Despite these limitations, we can draw some conclusions that are useful for public policy. First, there are high salt intake–stroke relationships, especially ischemic stroke. Second, few studies on high salt intake–stroke by diagnosis category have been investigated. Third, the robustness of the high salt intake–stroke relationship, even when controlling for key confounders and effects modifiers, indicates that inclusion of high salt intake–stroke in future regulatory impact analyses may be warranted, although further investigation is need into particulate matter (PM) confounding and the personal exposure–ambient concentration relationship. Future studies should explicate PM or other personal exposure surrogates into the estimation of an appropriate high salt intake–stroke relationship.
Acknowledgments
This research was supported by Zhejiang University Student Research Training Program (SRTP) (2012) and Zhejiang Province Experimental Teaching Demonstration Center Construction Projects (2010).
Conflict of Interest
The authors declare no conflict of interest.




