Gentiopicroside Attenuates Morphine Rewarding Effect through Downregulation of GluN2B Receptors in Nucleus Accumbens
The first two authors contributed equally to this work.
SUMMARY
Aims: Gentiopicroside (Gent) is one of the secoiridoid compound isolated from Gentiana lutea. This compound exhibits analgesic activities and inhibits the expression of GluN2B-containing N-methyl-D-aspartate (NMDA) receptors in the anterior cingulate cortex in mice. Nucleus accumbens (NAc) is a forebrain structure known for its role in drug addiction. However, little is known about the role of Gent on morphine dependence and synaptic transmission changes in the NAc. Methods: Conditioned place preference (CPP) test and behavioral sensitization of locomotor activity were used to investigate drug-seeking related behaviors. Brain slices containing NAc were prepared, and whole-cell patch-clamp recordings were performed to record the excitatory postsynaptic currents (EPSCs). Expression of proteins was detected by Western blot analysis. Results: Systemic administration of Gent attenuated the CPP effect induced by morphine, but had no effect on morphine-induced behavioral sensitization. Gent significantly reversed overexpression of GluN2B-containing NMDA receptors and dopamine D2 receptors in NAc during the first week of morphine withdrawal. However, the compound did not affect the overexpression of GluN2A-containing NMDA receptors, GluA1, and dopamine D1 receptors. Lastly, Gent significantly reduced NMDA receptors-mediated EPSCs in the NAc. Conclusion: Our study provides strong evidence that Gent inhibits morphine dependence through downregulation of GluN2B-containing NMDA receptors in the NAc.
Introduction
Two major systems are involved in morphine addiction and analgesic tolerance in the forebrain. The first system is that of the N-methyl-D-aspartate (NMDA) receptor. Glutamate is the predominant excitatory neurotransmitter in the mammalian central nervous system (CNS), and its effects are mediated via both ionotropic and metabotropic receptors. The NMDA receptor is the most extensively characterized of the three families of ionotropic glutamate receptor. NMDA receptors, including NR1, GluN2A, and GluN2B subunits, are highly expressed in forebrain areas [1]. A number of studies have suggested that NMDA receptors are involved in the morphine-induced conditioned place preference (CPP), locomotor sensitization, and development of analgesic tolerance [2–5]. For example, the expression levels of NR1 and GluN2A subunit proteins were decreased in frontal cortex and hippocampus but significantly increased in the nucleus accumbens (NAc) in morphine-dependent rats [3]. Second is the dopamine (DA) system. The activation of DA neurotransmission plays a crucial role in behavioral responses to drug abuse [6]. DA receptor antagonists inhibit morphine-induced CPP and locomotor hyperactivity [7, 8]. Furthermore, the subunits of DA receptor play different roles in morphine tolerance or dependence. Several reports have revealed a preferential role of D2 receptors in morphine tolerance and D1 receptors in the development of morphine dependence [9].
Gent is a secoiridoid compound isolated from Gentiana lutea, one of the most common herbal medicines used in China. Animal experiments have revealed its choleretic, antihepatotoxic, adaptogenic, and anti-inflammatory activities [10–12]. Recently, this compound has been investigated for its possible effects on the CNS such as antidepressant, anticonvulsant, and analgesic activities in mice [12]. We have reported in our previous studies that analgesic effects of Gent are relative to the downregulation of GluN2B-containing NMDA receptors in the anterior cingulate cortex (ACC) for persistent inflammatory pain [13]. NAc has been documented as a key brain region related to drug addiction [14, 15]. However, little is known about the role of Gent on morphine dependence as well as on the regulation of GluN2B-containing NMDA receptors and DA receptors in the NAc.
In the present study, we used CPP test and behavioral sensitization of locomotor activity to investigate the drug-seeking related behaviors and found that Gent significantly inhibited the CPP effect induced by morphine and decreased GluN2B receptor expression in the NAc. These results suggest that Gent reverses effect of morphine on CPP by inhibiting the expression of GluN2B-containing NMDA receptors in the NAc.
Materials and Methods
Animals
Male adult C57BL/6 mice (8–10-weeks-old) weighing from 18 to 22 g were obtained from the Laboratory Animal Center of the Fourth Military Medical University. The animals were housed in plastic boxes in groups of four with food and water available ad libitum in a colony room with controlled temperature (24°C ± 2°C), humidity (50–60%), and a 12:12-h light–dark cycle. Mice were allowed to adapt to laboratory conditions for at least 1 week before the procedure. The experiments were performed during the light phase of the cycle. This study was performed in adherence with the National Institutes of Health guidelines for the use of experimental animals. The institutional ethical committee of the Fourth Military Medical University specifically approved this study.
CPP Procedures
CPP was performed in a CPP apparatus with a computer equipped with an automatic monitoring system (Jiliang, Shanghai, China). The apparatus comprised two compartments identical in size (30 × 30 × 40 cm each), separated by a platform with the sides painted white and black, respectively. Place conditioning was conducted as described previously with a minor modification [16]. The whole protocol consisted of the following five different phases: preconditioning test (1 trial), conditioning (2 trials/days for 7 days), Gent administration (i.p., twice daily for 8 days), 1 week no treatment, and morphine challenge on D21 (to test locomotor activity), over a total of 22 days (Figure 1A). The activity of each animal was recorded and measured on each test day to determine the time spent on each side of the experimental box when the guillotine doors were raised.
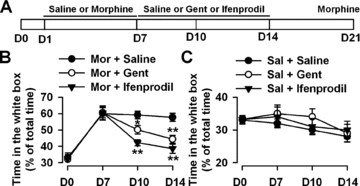
Effects of Gentiopicroside on morphine rewarding. (A) Schedule of the experimental design. The entire experiment was conducted for 21 d, and the mice were tested on D0, D7, D10, D14, and D21. (B) Gent (100 mg/kg) significantly reduced morphine-induced CPP, as shown by the time in the white box, compared with the saline control. n = 6 mice per group. *P < 0.05, **P < 0.01 compared with the saline control. (C) Gent or ifenprodil itself did not show any rewarding behavior. n = 6 mice per group.
Preconditioning
Each mouse was placed in one of the two compartments on day 0 (D0) and allowed access to explore both environments for 15 min. Time spent in each compartment side of the two compartments was determined. Animals that spent more than 10 min in a specific compartment were excluded from the study. All mice showed moderate preference for the black compartment.
Conditioning
During this period (D1–D7), animals received two s.c. injections each day, 10 mg/kg morphine sulfate (Qinghai pharmaceutical Co., Xining, China; morning, started at 8:00 am) and saline (afternoon, started at 2:00 pm), before they were placed in the white or black boxes for 30 min, respectively. The session started immediately afterwards. This dose of morphine was adopted because it is sufficient to produce CPP in mice [16, 17]. The control group was only injected with saline (s.c.). After each trial, the subjects were returned to their own home cages.
Gent Administration and Postconditioning (Test)
From D7 after the last morphine treatment, mice were administrated (i.p.) with Gent (100 mg/kg; The National Institute for the Control of Pharmaceutical and Biological Products, Beijing, China), ifenprodil (5 mg/kg, Sigma), or saline (0.5 mL/kg) twice daily for 8 days (D7–D14). During this period, postconditioning tests were performed on D7, D10, and D14. When the tests began, the guillotine doors were removed to allow animals free access to both compartments for 15 min. Time spent in each compartment was measured. Only animals displaying robust nicotine CPP (i.e., an increase in preference for the drug-paired compartment ≥100 seconds with respect to the preconditioning test) were used in the subsequent phase of the study (extinction).
Washout Time
From D15, the mice were put back to the home cages for 7 days without any drug treatment.
Morphine Challenge and Locomotor Activity (for Behavioral Sensitization Test)
On D21, locomotor activities were recorded after the morphine challenge. Locomotor activity of all the animals was defined as the total distance traveled in the same time and in the same apparatus as the corresponding CPP procedures. Total distance traveled on both sides was analyzed using a PC equipped with an automatic monitoring system.
Slice Preparation and Whole-Cell Patch Clamp Recordings
Coronal brain slices (300 μm), containing the NAc, were prepared as previously described [18]. Slices were transferred to submerged recovery chamber with oxygenated (95% O2 and 5% CO2) artificial cerebrospinal fluid (ACSF) containing (in mM): 124 NaCl, 2.5 KCl, 2 CaCl2, 2 MgSO4, 25 NaHCO3, 1 NaH2PO4, 10 glucose at room temperature for at least 1 h. Experiments were performed in a recording chamber on the stage of an Axioskop 2FS microscope with infrared DIC optics for visualization of whole-cell patch clamp recording. Excitatory postsynaptic currents (EPSCs) were recorded from the shell of NAc neurons with an Axon 200B amplifier (Axon Instruments, Foster City, CA, USA) and the stimulations were delivered by a bipolar tungsten stimulating electrode placed in the shell of NAc ∼100 μm apart from the recording electrode. AMPA receptor mediated EPSCs were induced by repetitive stimulations at 0.02 Hz and neurons were voltage clamped at −70 mV. The recording pipettes (3–5 MΩ) were filled with solution containing (mM) 145 K-gluconate, 5 NaCl, 1 MgCl2, 0.2 EGTA, 10 HEPES, 2 Mg-ATP, and 0.1 Na3-GTP (adjusted to pH 7.2 with KOH). The NMDAR-mediated component of EPSCs was pharmacologically isolated in ACSF containing CNQX (20 μM), glycine (1 μM), and picrotoxin (100 μM). The patch electrodes contained 102 mM cesium gluconate, 5 mM TEA Chloride, 3.7 mM NaCl, 11 mM BAPTA, 0.2 mM EGTA, 20 mM HEPES, 2 mM MgATP, 0.3 mM NaGTP, and 5 mM QX-314 chloride (adjusted to pH 7.2 with CsOH) for the recording NMDAR-mediated EPSCs. Neurons were voltage clamped at −30 mV and NMDAR-mediated EPSCs were evoked at 0.05 Hz. Miniature excitatory synaptic currents (mEPSCs) were recorded in the neurons clamped at −70 mV with 0.5 μM TTX added in the ACSF. Access resistance was 15–30 MΩ and monitored throughout the experiment. Data were discarded if access resistance changed more than 15% during an experiment.
Western Blot
Equal amounts of protein (50 μg) from the NAc were separated and electrotransferred onto PDVF membranes (Invitrogen, San Diego, CA, USA), which were probed with antibodies for GluA1 (dilution ratio 1:300, Abcam), GluN2A (dilution ratio 1:200, Millipore, Billerica, MA, USA), GluN2B (dilution ratio 1:500, Millipore), D1 (dilution ratio 1:1000, millipore), D2 (dilution ratio 1:1000, Millipore), and with β-actin (dilution ratio 1:10000, Sigma) as a loading control. The membranes were incubated with horseradish peroxidase conjugated secondary antibodies (anti-rabbit/anti-mouse IgG for the primary antibodies), and bands were visualized using an ECL system (Perkin Elmer, Waltham, MA, USA).
Data Analysis
Results were expressed as mean ± SEM. Statistical comparisons were performed using a t-test or one-way ANOVA. In all cases, P < 0.05 was considered statistically significant.
Results
Systemic Administration of Gent Attenuated Morphine-Induced Rewarding
The effects of Gent on morphine dependence were tested. Mice were first pretreated with morphine (s.c., 10 mg/kg) with conditioning place training for 7 days (D1–D7), followed by Gent (100 mg/kg, i.p., twice daily) or ifenprodil (5 mg/kg, i.p., twice daily) treatment for 8 days (D7–D14). Mice were then returned to their home cages for another 7 days (D15–D21) and were challenged with morphine (s.c., 10 mg/kg) on D21 (Figure 1A). No mice showed significant place preference for the drug-associated compartment before drug conditioning (D0), indicating that the CPP apparatus used was of a nonbiased design. After 7 days of conditioning with morphine (D1–D7, s.c., 10 mg/kg), mice showed significant place preference for the drug-paired compartment on D7. The mice were then administered with Gent (100 mg/kg) or ifenprodil (5 mg/kg) for 8 days (D7–D14) postmorphine withdrawal. The rewarding behaviors were tested on D7, D10, and D14 to evaluate the drug effects on morphine dependence. The mice still showed significant place preference on D10 and D14 even for 7 days with the administration of same volume of saline (0.5 mL/kg, i.p, twice daily) on D7–D14. However, the drug-seeking effect was attenuated on D10 and D14 (Figure 1B) when Gent (100 mg/kg) or ifenprodil (5 mg/kg) were administered on D7–D14 after morphine conditioning. Gent or ifenprodil itself did not show rewarding, aversive, or drug-seeking effect (Figure 1C).
Gent Reversal of Overexpression of GluN2B and D2 Receptors in the NAc of Morphine-Dependent Mice
Inotropic glutamate receptors, including NMDA and AMPA receptors, play pivotal roles in morphine rewarding [2, 5]. We examined the expression of NMDA and AMPA receptor subunits in total NAc homogenates by Western blot analysis to detect the effects of Gent on these receptors. Results showed that expression levels of AMPA receptor GluA1 subunit, as well as GluN2A- and GluN2B-NMDA receptors, increased after chronic morphine treatment compared with the saline control mice (Figure 2A). The increase in GluA1 and GluN2A subunits remained evident on D21 2 weeks postmorphine withdrawal or 1 week postGent withdrawal. Gent treatment (100 mg/kg) had no effect on the GluA1 and GluN2A subunit expression in the morphine-dependent mice (Figures 2B and C). However, GluN2B subunit expression was significantly reduced in the NAc of Gent-injected morphine-dependent mice (Figure 2D). Thus, the downregulated GluN2B subunit expression by Gent could explain the reduced NMDA receptor-mediated EPSCs in NAc synapses.
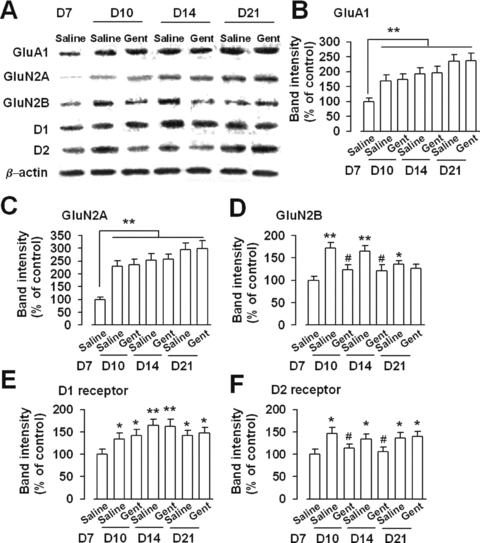
Reduced GluN2B and D2 receptors expression by Gent. (A) Western blot showed the expression levels of GluA1, GluN2A, GluN2B, D1, and D2 receptors. (B) Expression of AMPA receptor GluA1 subunit increased after morphine withdrawal compared with the saline control mice on D7. Gent had no effect on GluA1 expression. (C) Expression of GluN2A receptor increased after morphine withdrawal compared with the saline control mice on D7. Gent had no effect on expression of GluN2A. (D) Expression of GluN2B receptor increased after morphine withdrawal compared with the saline control mice on D7. GluN2B subunit expression was significantly reduced in the NAc of Gent-injected morphine-dependent mice. (E) Expression of D1 receptor increased after morphine withdrawal compared with the saline control mice on D7. Gent had no effect on expression of D1. (F) Expression of D2 receptor increased after morphine withdrawal compared with the saline control mice on D7. D2 receptor expression was significantly reduced in the NAc of Gent-injected morphine-dependent mice. n = 4 mice in each group. *P < 0.05, **P < 0.01 compared with the saline control on D7; #P < 0.05 compared with the saline control on D10 or D14.
Next, we detected the expression of DA receptors, known as the major mediator for the morphine rewarding [7, 8]. The increase in the D1 and D2 receptors remained obvious on D21, 2 weeks postmorphine withdrawal or 1 week postGent withdrawal. Treatment of Gent (100 mg/kg, twice daily) had no effect on D1 receptor expression in the morphine-dependent mice (Figure 2E). However, D2 receptor expression was significantly reduced in the NAc of Gent-injected morphine-dependent mice (Figure 2F).
Downregulation of Excitatory Synaptic Transmission by Gent
The input–output relationships measuring EPSCs amplitude (output) as a function of the afferent stimulus intensity (input) were compared in the shell of the NAc on D14 to detect the effects of Gent on synaptic transmission in morphine-dependent mice (Figure 3A). First, the slope of the AMPA receptor-mediated EPSC (AMPA-EPSC) curves was significantly greater in neurons from morphine-dependent mice compared with saline controls (Figures 2B and C). These data suggest an increase in synaptic efficacy in NAc neurons from morphine-dependent mice. Gent treatment (100 mg/kg) could significantly reverse the enhancement of AMPA receptor-mediated synaptic transmission induced by chronic morphine injection (Figures 3B and C). Then, we recorded the input–output curves of NMDA receptor-mediated EPSC (NMDA-EPSC) and found that Gent also notably reversed the enhancement of NMDA receptor-mediated synaptic transmission in the morphine-dependent mice (Figures 3D and E). These results suggest that Gent could partially downregulate the enhancement of excitatory synaptic transmission in the NAc by chronic morphine administration.
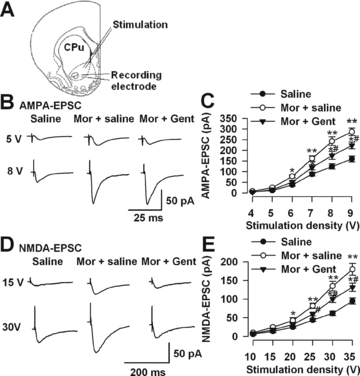
Inhibitory effects of Gent on synaptic transmission. (A) Diagram of a slice showing the placement of a whole-cell patch recording and a stimulation electrode in the shell of NAc. (B) Representative traces show AMPA-EPSC in the saline-, morphine-injected, and Gent-administered mice on D14. (C) Plot of input–output curves illustrates the enhancement of AMPA-EPSC in the shell of NAc of morphine-withdrawn mice. Gent partially inhibited morphine-induced AMPA receptor-mediated synaptic transmission. n = 8 neurons/4 mice in each group. (D) Representative traces show NMDA-EPSC in the saline-, morphine-injected, and Gent-administered mice on D14. (E) Plot of input–output curves shows the enhancement of NMDA-EPSC in the shell of NAc of morphine withdrawal mice. Gent partially inhibited morphine-induced enhancement of NMDA-EPSC. n = 8 neurons/4 mice in each group, *P < 0.05, **P < 0.01 compared with the saline control; #P < 0.05 compared with the morphine-injected mice.
Effects of Gent on Basal Glutamatergic Synaptic Transmission
We recorded AMPA receptor-mediated miniature excitatory postsynaptic currents (mEPSCs) in the shell of NAc on D14 to further determine the presynaptic or postsynaptic component associated with an increase in the synaptic transmission (Figure 4A). A significant increase in mEPSC frequency and amplitude was detected in morphine-dependent mice compared with saline controls (Figures 4B and C). Gent treatment (100 mg/kg, twice daily) could significantly reverse the enhancement of AMPA receptor-mediated mEPSC amplitude induced by chronic morphine injection. However, this compound had no effect on the mEPSC frequency (Figures 4B and C). These results indicate that downregulation of excitatory synaptic transmission by Gent is likely due to postsynaptic AMPA receptor modifications in the NAc synapses of morphine-dependent mice.
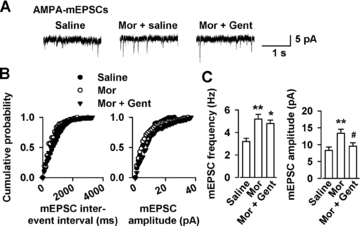
Effects of Gent on basal glutamatergic synaptic transmission. (A) mEPSCs recorded a holding potential of −70 mV in pyramidal neurons from saline, morphine-, and Gent-injected mice on D14. (B) Cumulative frequency (left) and amplitude (right) histogram of the mEPSCs in neurons from saline, morphine-injected, and Gent-injected mice. (C) Summary of mEPSCs frequency (left) and amplitude (right) in neurons from saline (n = 11 neurons/4 mice), morphine- (n = 10 neurons/4 mice), and Gent-(n = 12 neurons /4 mice) treated mice. Gent (100 mg/kg, twice daily) reversed the enhancement of AMPA receptor-mediated mEPSC amplitude induced by chronic morphine injection, but had no effect on the mEPSC frequency. *P < 0.05, **P < 0.01 compared with the saline control; #P < 0.05 compared to the morphine-injected mice.
Noninhibition of Morphine-Induced Behavioral Sensitization by Gent
Repeated administration of morphine results in increased locomotor activity that persisted even after long-term drug abstinence. This mechanism is called “behavioral sensitization.” Locomotor activity was determined on D21 after morphine challenge (10 mg/kg). The 8-day administration (D7–D14) of saline (0.5 mL/kg, i.p., twice daily) or Gent (100 mg/kg, i.p., twice daily) did not induce locomotor sensitization (Figure 5A). The total travel distance significantly increased in the morphine-dependent mice after morphine challenge (10 mg/kg) on D21, indicating that the animals developed behavioral sensitization (Figure 5B). Gent (100 mg/kg) or ifenprodil (5 mg/kg) administered from D7 to D14 after morphine injection did not inhibit total locomotor activity during the morphine challenge on D21 (Figure 5B). These data suggest that Gent cannot inhibit morphine-induced behavioral sensitization, though it can inhibit morphine-rewarding behavior.
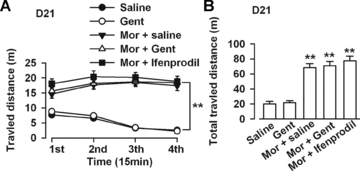
Effects of Gent on morphine-induced behavioral sensitization. (A) Locomotor activity was recorded in the same apparatus as the corresponding CPP procedures for 1 h postmorphine challenge (10 mg/kg) on D21. Travel distance was recorded every 15 min. (B) Saline (0.5 mL/kg) or Gent (100 mg/kg) administration for 8 days (D7-D14) did not induce locomotor sensitization. The total travel distance increased in the morphine-dependent mice after morphine challenge (10 mg/kg) on D21. Gent (100 mg/kg) or ifenprodil (5 mg/kg) treatment during D7–D14 after morphine withdrawal did not inhibit locomotor sensitization after morphine challenge on D21. **P < 0.01 compared with the saline control.
Discussion
This investigation was performed to evaluate the functional activity of Gent, the main secoiridoid compound isolated from G. lutea, on morphine dependence. We demonstrated for the first time that Gent (100 mg/kg) caused a significant reduction of morphine-rewarding behaviors. Furthermore, Gent inhibited GluN2B and D2 receptor expression in the NAc region, and downregulated the enhancement of excitatory synaptic transmission induced by chronic morphine administration through postsynaptic mechanisms.
Expression of NMDA Receptors in Morphine Rewarding
The important roles of NAc in the processing of morphine dependence and behavioral sensitization in animals have been well-documented [14, 15]. In the past decade, accumulated evidence indicate that NMDA receptors play a pivotal role in the development of tolerance and physical dependence to opiates [2]. NAc NMDA receptor subunit expression and function are enhanced in morphine-dependent animals [3]. Our present study shows that the expression of GluN2A was significantly increased in the NAc in morphine-injected mice, consistent with previous reports [3, 19]. Furthermore, we also found that the expression of GluN2B and AMPA receptor GluA1 subunit were significantly increased in the NAc in morphine-dependent mice, despite the discrepancy of GluN2B expression in morphine dependence [3, 20, 21]. The present study reveals that treatment of mice with Gent systematically (i.p.) resulted in a significant inhibition on morphine rewarding and reversed the upregulation of GluN2B receptors in the NAc. Systemic administration of ifenprodil, one specific GluN2B receptors antagonist, significantly reduced rewarding behavior. This result suggests that Gent inhibits morphine rewarding, at least partially by reversing the overexpression of GluN2B receptors. Reports likewise show that Gent treatment significantly suppresses the increase in TNF-α and shows anti-inflammatory activities [22]. We could not exclude the possibility that Gent may take effect by inhibiting the release of other mediators, for example, TNF-α, substance P, bradykinin, or serotonin.
DA Receptors in Morphine Rewarding
DA receptor is known for its crucial role in behavioral responses to morphine [6–8], and the subunits of DA receptor play different roles in morphine tolerance or dependence [9]. D2 receptor deletion significantly alters the reward-enhancing effects of morphine, suggesting that it plays an important role in rewarding effects [23, 24]. In the present study, we found that the expression of D1 and D2 receptors increased the NAc of mice with morphine dependence. Treatment of mice with Gent systematically (i.p.) reversed the upregulation of D2 receptor, but not the D1 receptor in the NAc. However, whether there are functional consequences of such differences of Gent on DA receptor expression remains unclear.
Effects of Gent on the Synaptic Transmission in the NAc of Morphine-Dependent Mice
Previous reports have revealed that the shell and core subdivisions of the NAc play different functional roles in motivated behavior [25–27]. The in vivo neurochemical evidence shows the functional compartmentation within the NAc and the preferential effect of psychostimulants and morphine in the shell of the NAc at doses known to sustain intravenous drug self-administration [25]. In present study, we selected to record in the shell of the NAc because of the more pronounced functional alteration in the shell compared with the core. Numerous studies on morphine dependence on synaptic transmission are focused on the NMDA-EPSCs. However, documents on morphine dependence on NMDA synaptic transmission are not consistent. For example, Martin et al. reported that chronic morphine treatment significantly decrease NMDA receptor-mediated synaptic transmission in the accumbens, and these effects persist for 1 week postwithdrawal [28]. By contrast, recent studies have reported that NMDA receptor subunit expression and function in NAc are enhanced in morphine-dependent rats [3, 20]. In the different brain regions, chronic morphine administration significantly increased protein levels of NR1 and GluN2B subunits in NAc but not in the central medial amygdala. This finding indicates a region-specific response of NMDA receptor subunits to chronic morphine administration at protein levels [29]. In the present study, we found that both AMPA- and NMDA-EPSC were increased in the morphine-dependent mice when recorded in the first week of morphine withdrawal. Gent treatment (100 mg/kg, twice daily) significantly reversed the enhancement of synaptic transmission. Quantal release analysis by AMPA mEPSC recordings revealed that downregulation of excitatory synaptic transmission by Gent was likely due to postsynaptic AMPA receptor modifications in the NAc synapses of morphine-dependent mice. This mechanism was demonstrated by unchanged mEPSCs frequency and decreased mEPSCs amplitude in the recordings and raises the possibility that Gent targets the postsynaptic modulation of GluN2B receptors in the NAc. AMPA receptors are heteromeric complexes composed of four distinct subunits of GluA1–4. AMPA receptor trafficking is perceived to be critical for long-term potentiation and depression [4, 5]. The phosphorylation of the AMPA receptor GluA1 subunit plays a key role in its synaptic expression and synaptic plasticity [6, 7]. In present study, we found that Gent had nonsignificant effect on the increased GluA1 expression caused by morphine as shown by Western Blot analysis. However, whole-cell recording revealed that the enhanced amplitude of AMPA-mEPSCs was significantly reduced by Gent treatment. These results imply that Gent might depress the AMPA receptor-mediated synaptic transmission by attenuating the phosphorylation level of GluA1 or inhibiting the AMPA receptors trafficking to the membrane by morphine.
Effects of Gent on the Morphine Dependence and Locomotor Sensitization
CPP test and behavioral sensitization of locomotor activity were used to investigate drug-seeking related behaviors, which correlate with psychological dependence. Results showed that Gent could completely abolish the CPP effect induced by morphine, but had no effect on morphine-induced behavioral sensitization. These results are consistent with the reports that dextromethorphan (a noncompetitive NMDA antagonist) or siRNA against the GluN2B subunit can completely abolish the CPP effect induced by morphine, but had no effect on morphine-induced behavioral sensitization [30, 31].
Hence, our findings provide evidence that Gent inhibits morphine dependence through the downregulation of GluN2B-containing NMDA receptors in the NAc. Hopefully, this study can help further elucidate the underlying mechanisms of Gent during morphine dependence and aid on the clinical treatment of drug rewarding with traditional herbs.
Acknowledgments
We thank Dr. Tao Li (Xi'an Jiaotong University, China) for valuable discussions and critical reading of this manuscript. This work was supported by National Natural Science Foundation of China No. 30872597-31070923, 2008 ZXJ09004-023, 2009ZX09103-111, and Program for New Century Excellent Talents in University.
Conflict of Interest
The authors declare no conflict of interest.




