Cytomegalovirus Replication Within the Lung Allograft Is Associated With Bronchiolitis Obliterans Syndrome
Abstract
Early studies reported cytomegalovirus (CMV) pneumonitis as a risk factor for development of bronchiolitis obliterans syndrome (BOS) following lung transplantation. While improvements in antiviral prophylaxis have resulted in a decreased incidence of CMV pneumonitis, molecular diagnostic techniques allow diagnosis of subclinical CMV replication in the allograft. We hypothesized that this subclinical CMV replication was associated with development of BOS. We retrospectively evaluated 192 lung transplant recipients (LTR) from a single center between 2001 and 2009. Quantitative (PCR) analysis of CMV viral load and histological evidence of CMV pneumonitis and acute cellular rejection was determined on 1749 bronchoalveolar lavage (BAL) specimens and 1536 transbronchial biopsies. CMV was detected in the BAL of 41% of LTR and was significantly associated with the development of BOS (HR 1.8 [1.1–2.8], p = 0.02). This association persisted when CMV was considered more accurately as a time-dependent variable (HR 2.1 [1.3–3.3], p = 0.003) and after adjustment for significant covariates in a multivariate model. CMV replication in the lung allograft is common following lung transplantation and is associated with increased risk of BOS. As antiviral prophylaxis adequately suppresses CMV longer prophylactic strategies may improve long-term outcome in lung transplantation.
Abbreviations:
-
- AR
-
- acute cellular rejection
-
- BAL
-
- bronchoalveolar lavage
-
- BOS
-
- bronchiolitis obliterans syndrome
-
- CMV
-
- cytomegalovirus
-
- LTR
-
- lung transplant recipient
-
- OB
-
- obliterative bronchiolitis
-
- PCR
-
- polymerase chain reaction
Introduction
The major cause of long-term morbidity and mortality after lung transplantation is chronic allograft rejection. This manifests histopathologically as obliterative bronchiolitis (OB) and clinically as bronchiolitis obliterans syndrome (BOS) (1). It develops as a result of an abnormal and dysregulated repair response to a preceding epithelial/subepithelial injury from either alloimmune or nonalloimmune pathways acting in isolation or in combination (2). BOS, the clinical correlate of OB, assumes that all other obvious causes of decreased lung function have been excluded and is the most common cause of death in long-term survivors of lung transplantation (3).
In 2001, a consensus group of the International Society for Heart and Lung Transplantation (ISHLT) determined that acute rejection, human cytomegalovirus (CMV) pneumonitis and lymphocytic bronchitis were the three major risk factors for the development of BOS (1). Since that time antiviral prophylactic strategies have evolved, utilizing more potent regimens for longer durations. As a result, the incidence of CMV pneumonitis defined by the presence of CMV inclusions on transbronchial biopsy has declined (4).
Over the last decade CMV diagnostics have also evolved. While histological detection of CMV inclusions in lung biopsies continues to have a role in defining CMV disease, it is limited by sampling errors and the need for an invasive procedure to obtain specimens. The polymerase chain reaction (PCR) test for CMV DNA has increasingly been recognized as the preferred assay for the diagnosis and therapeutic monitoring of CMV replication within the lung allograft (5–8). These molecular assays offer a number of advantages including improved sensitivity, viral load quantification, ability to standardize results and rapid turnaround. Subclinical viral replication in the lung allograft can also be monitored which hitherto was largely unrecognized with the older assays.
In an era of improved antiviral prophylaxis and CMV diagnostics, we explored whether CMV replication within the lung allograft, was associated with the development of BOS in a large cohort of consecutive lung transplant recipients (LTR) receiving short course (3–5 months) CMV prophylaxis. We demonstrated that despite short course prophylaxis, 41% of LTR developed evidence of CMV replication in the allograft and furthermore, that any detection of CMV replication in BAL was associated with an increased risk of BOS.
Methods
Cohort
All patients undergoing lung or heart–lung transplantation between 2001 and the end of 2008 were considered for inclusion in the study. Of 325 consecutive lung transplant recipients (LTR), 133 patients were excluded either because their follow-up was at a distant center (95 patients), they died within 100 days of transplant (n = 26) or were unable to provide pulmonary function tests to evaluate BOS (n = 12). The final cohort consisted of 192 LTR (demographics shown in Table 1).
| Characteristic | n = 192 LTR |
|---|---|
| Gender (male/female) | 112/80 |
| Age, years (range) | 45.8 (10–69) |
| Native disease, patients | |
| COPD/CF/IPF/PAH/Other | 64/49/28/11/40 |
| Transplant type, patients | |
| Bilateral/single/heart-lung | 135/52/7 |
| CMV serostatus donor (D)/recipient (R), patients (%) | |
| D−/R− (low risk) | 36 (19%) |
| D+/R+ or D−/R+ (medium risk) | 112 (58%) |
| D+/R− (high risk) | 44 (23%) |
- COPD = chronic obstructive pulmonary disease; CF = cystic fibrosis; IPF = idiopathic pulmonary fibrosis; PAH = pulmonary arterial hypertension.
All LTR received standard triple immunosuppression with cyclosporine (n = 175) or tacrolimus (n = 17), azathioprine or mycophenolate mofetil and corticosteroids. Induction therapy with the IL-2 receptor blocker basiliximab (20 mg intravenously on days 0 and 4) was given as a calcineurin inhibitor-sparing agent to 73 patients who were identified pretransplant at high risk of developing renal dysfunction.
Patients underwent routine surveillance bronchoscopy with bronchoalveolar lavage (BAL) and transbronchial biopsy at week 2 and months 1, 2, 3, 6, 9 and 12, with additional procedures when clinically indicated. BAL samples were prospectively collected, pooled and processed as described elsewhere (9). Pulmonary function testing was completed serially on each patient throughout the follow-up. The censor date was June 30, 2009.
Definition of CMV risk groups
CMV risk groups were defined by donor (D) and recipient (R) serology as follows: low-risk: D−/R−, medium-risk: D+/R+ and D−/R+, and high-risk: D+/R− (Table 1). All patients received intravenous ganciclovir (5 mg/kg bd) for 14 days. CMV hyperimmuneglobulin (1.5 million units) was additionally administered at days 1, 2, 3, 7, 14, 21 and 28 to high-risk LTR. Following intravenous ganciclovir, patients received either oral ganciclovir until day 90 (prior to May 2004, n = 71) or oral valganciclovir until day 150 post-transplant (since May 2004, n = 121). Low-risk LTR received oral valaciclovir prophylaxis for 90 days.
Definition of CMV replication and pneumonitis
At the time of bronchoscopy, the presence of CMV DNA in the BAL supernatant was prospectively assessed (COBAS Amplicor CMV monitor test, Roche Diagnostic Systems, NSW, Australia; detectable range of 600–100 000 copies/mL). Only BAL specimens prior to attainment of BOS or censor date were included in the analysis. CMV pneumonitis was defined as histopathological evidence of CMV inclusions on transbronchial biopsy in the presence of new pulmonary disease. Episodes of CMV pneumonitis or significant CMV replication within the lung allograft (CMV load > 10 000 copies/mL BAL supernatant) were treated with intravenous ganciclovir (5 mg/kg bd for 2 weeks). Patients with symptoms suggestive of extra-pulmonary CMV infection had quantitative PCR performed on peripheral blood samples and tissue biopsy, if appropriate. CMV recurrence was defined as two episodes of CMV detection in BAL separated by more than 30 days and a negative BAL or negative CMV PCR from peripheral blood. Episodes that did not fit these criteria were deemed persistent.
Definition of airway infection
Airway infections, other than CMV, were defined by a positive BAL bacterial or fungal isolate or positive PCR for community-acquired respiratory viruses (including influenza, parainfluenza, respiratory syncitial virus, paramyxovirus and adenovirus).
Definition of acute cellular rejection
Acute cellular rejection (AR) was diagnosed on transbronchial biopsy specimens according to the ISHLT pathological scoring system. Acute rejection score was defined as the cumulative AR grading divided by the number of evaluable transbronchial biopsies (10). Only AR events prior to BOS or censor date were evaluated. Significant AR was defined as acute rejection ≥ A2.
Definition of bronchiolitis obliterans syndrome
BOS was defined according to ISHLT guidelines as an irreversible decline in FEV1 of at least 20% of the individual baseline without evidence of restriction (1,11). A diagnosis of BOS was only made retrospectively after other causes of allograft dysfunction were excluded.
Statistical analysis
Descriptive statistics were used to determine baseline and clinical characteristics of study subjects. Univariate analysis was performed using the chi-square test for equal proportion, Student's t-test for normally distributed continuous variables, Mann–Whitney U-test for non-normally distributed variables and the Kruskal–Wallis test for comparison involving more than two groups. Variables in the univariate analyses included recipient age, gender, pretransplant disease, transplant type, donor–recipient HLA match and CMV serostatus; ischemic time; induction/maintenance immunosuppression; acute rejection (defined as episodes ≥ AR 2, episodes ≥ AR 1, AR score, acute rejection peak); CMV in the allograft (defined as CMV in BAL > 600 copies/mL at any point prior to attainment of BOS and in first 12 months following transplantation, peak viral load, peak viral load >46 000 copies/mL, presence of inclusions) and peripheral blood and non-CMV organisms in the BAL. The relationship between CMV detection in BAL and BOS was assessed using Cox-proportional Hazards regression and reported using Hazard Ratios (95% CI) and Kaplan–Meier curves. CMV BAL was considered as a time-independent variable (present or absent in the first 12 months posttransplant) and as a time-dependent variable modeling the change in status once CMV BAL was detected at any point after transplantation but before attainment of BOS. The following variables were additionally modeled as time-dependent variables: time to AR ≥ 1, AR ≥ 2, to first detection of respiratory virus, positive fungal culture in BAL and positive bacterial culture in BAL. Multivariate models were constructed using stepwise selection and backwards elimination procedures with all variables from univariate analysis (p < 0.1) being considered for inclusion. All analysis was performed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA). A two-sided p-value of 0.05 was considered to be statistically significant. While the development of CMV is time dependent, the Proportional Hazards Regression procedure in SAS (PHREG) has the capacity to create and accurately model time-dependent covariates.
Results
The study cohort (192 LTR) provided 1749 BAL specimens for analysis of CMV viral load and 1536 transbronchial biopsies for analysis of CMV pneumonitis and acute cellular rejection. The mean number of biopsies (8 vs. 8) and BAL specimens (9 vs. 9) collected from LTR with and without BOS was not significantly different. Patients diagnosed with BOS underwent significantly fewer pulmonary function tests than those without (18 [IQR: 11–30] vs. 24 [IQR: 15–38], p = 0.003). The median follow-up for the study cohort was 1015 days (IQR: 557–1702).
Incidence and time to CMV replication
During the study, 78/192 LTR (41%) had CMV DNA demonstrated in the BAL in the first 12 months following transplantation, with 44 LTR (23%) developing repeated episodes. CMV pneumonitis, defined histologically by the presence of CMV inclusions was diagnosed in 14 patients (8%). Median time to detection of CMV DNA in BAL was 270 days, with most replication detected (71/78 LTR) following the cessation of antiviral prophylaxis (median 133 days [IQR: 82–218 days]). Median viral load (in those positive) was 9410 copies/mL (IQR: 2700–77 300) (Table 2). Though CMV replication in the BAL was not observed in the low-risk LTR, it was more common in the high-risk LTR compared to the medium-risk LTR (61% vs. 46%, respectively; p = < 0.0001). There was no significant difference in time from cessation of antiviral prophylaxis to CMV detection (median 150 [IQR: 97–233] vs. 115 [IQR: 60–204] days posttransplant; p = 0.2), nor in the magnitude of CMV replication within the lung allograft (median 20 200 [IQR: 2830–73600] copies/mL vs. 6942 [IQR: 2405–77350] copies/mL; p = 0.47) (Table 3).
| Characteristic | n = 192 (%) | |
|---|---|---|
| BOS patients (%) | 73 | (38) |
| Median time to BOS (days) | 574 | |
| Acute rejection ≥ grade 2 | 89 | (46) |
| Cytomegalovirus detected in BAL | ||
| At least one episode | 78 | (41) |
| Recurrent episodes | 44 | (23) |
| Time postcessation of prophylaxis, days | 133 | (IQR: 82–218) |
| Median viral load, copies/mL | 9410 | (IQR: 2700–77 300) |
| CMV pneumonitis, patients (%) | 14 | (8) |
| Death, patients (%) | 70 | (36) |
| Median time to death (days) | 787 | (IQR: 565–1159) |
| Follow-up, days | 1029 | (IQR: 557–1702) |
| Characteristic | Low risk n = 36 | Medium risk n = 112 | High risk n = 44 | p |
|---|---|---|---|---|
| BAL CMV, patients (%) | ||||
| At least one episode | 0 | 51 (46) | 27 (61) | <0.001* |
| Median time postcessation of prophylaxis (days) (IQR) | 0 | 150 | 115 | 0.2* |
| (97–233) | (60–204) | |||
| Median viral load | 0 | 6942 | 20200 | 0.47* |
| (2405–77350) | (2830–73600) | |||
| CMV pneumonitis, patients (%) | 0 | 7 (6) | 7 (16) | 0.05* |
| Acute rejection ≥ A2, patients (%) | 11 (31) | 54 (48) | 24 (55) | <0.001§ |
| Bronchiolitis obliterans syndrome | 7 (20) | 53 (47) | 13 (30) | 0.005* |
- * Comparing only medium and high risk groups.
- §Comparing all risk groups.
There was no difference in the incidence of CMV replication detection between patients who did and did not receive basiliximab induction (39% vs. 41%, p = 0.88).
While CMV was not routinely tested for in the blood, 61% of episodes of CMV detected in BAL had a paired blood sample analyzed for CMV DNA. Of note of those concurrently analyzed for CMV DNA in BAL and blood, in 76% CMV replication was only detected in BAL.
Oral ganciclovir versus valganciclovir prophylaxis
CMV replication was observed in 35/71 (49%) LTR receiving oral ganciclovir compared to 43/121 (36%) receiving valganciclovir (p = 0.06) (Table 4). There were seven episodes of persistent CMV infection of which four patients received valganciclovir and three oral ganciclovir prophylaxis. Recurrent or persistent CMV reactivation was more common in the oral ganciclovir group (38% vs. 22%; p = 0.02). During the ganciclovir era the magnitude of CMV replication detected in BAL was higher (median 38 800 copies/mL [IQR: 3450–100 000] vs. 5925 copies/mL [IQR: 1570–18 050]; p = 0.01) and the detection of CMV pneumonitis was more common (14% vs. 3%; p = 0.01).
| Characteristic | Ganciclovir n = 71 | Valganciclovir n = 121 | p |
|---|---|---|---|
| CMV in BAL in first 12 months, patients (%) | 35 (49) | 43 (36) | 0.06 |
| Recurrent CMV in BAL, patients (%) | 27 (38) | 27 (22) | 0.02 |
| Median time to first CMV in BAL, days (IQR) | 275 | 265 | 0.35 |
| (204–380) | (206–344) | ||
| Median BAL CMV load, copies/mL (IQR) | 38 800 | 5925 | 0.01 |
| (3450–100 000) | (1570–18 050) | ||
| CMV pneumonitis, patients (%) | 10 (14) | 4 (3) | 0.01 |
| Acute rejection≥ A2, patients (%) | 52 (74) | 37 (31) | <0.001 |
| Median acute rejection score | 0.545 | 0.143 | 0.003 |
| BOS ≥ 1, patients (%) | 37 (52) | 36 (30) | 0.003 |
| Follow-up, days (IQR) | 1996 | 809 | 0.005 |
| (872–2489) | (470–1119) |
Acute rejection
At least one episode of significant acute rejection (grade ≥A2) was diagnosed in 89/192 (46%) LTR and 46 (24%) LTR experienced recurrent episodes. The incidence of acute rejection within the first 12 months was significantly less during the valganciclovir era compared to the earlier ganciclovir period (31% versus 74%, p < 0.0001) with a lower median acute rejection score (0.14 [IQR: 0.0–0.3] vs. 0.55 [IQR: 0.3–0.8], p = 0.003). Importantly, there was no change in the immunosuppressive regimens between the two eras. There was no temporal association between treatment of acute rejection and subsequent CMV detection. Only 5/151 episodes of CMV replication occurred within 30 days of a previous episode of acute rejection.
CMV replication within the lung allograft as a predictor of BOS
At the end of the study 73/192 LTR fulfilled the diagnostic criteria for BOS. Median time to BOS was 574 days (IQR: 401–973). Seventy patients were deceased (median time to death: 787 days [IQR: 565–1159]). The incidence of BOS was significantly lower in low-risk (as defined by donor-recipient CMV serology) LTR compared to medium- and high-risk (19% vs. 47% vs. 30%; p = 0.005).
To characterize the relationship between CMV detection in the BAL and development of BOS, CMV detection was considered in two separate models. CMV detection in BAL was initially considered as a time-independent variable occurring at any point in the first 12 months following transplant. This was significantly associated with the development of BOS (HR 1.8 [1.1–2.8], p = 0.02). Secondly, CMV detected in BAL was considered more accurately as a time-dependent variable occurring at any time posttransplantation but prior to the attainment of BOS. This was significantly associated with an increased risk of BOS (HR 2.1 [1.3–3.3], p = 0.003) (Figure 1). Details of the univariate analysis are included as a supplement. After multivariable adjustment for other significant covariates (acute rejection score, CMV serostatus, transplant type and COPD), CMV detection in BAL remained a significant predictor of BOS (HR 1.9 [1.2–3.1], p = 0.007) (Table 5).
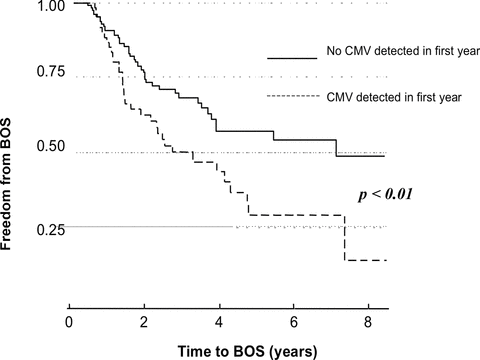
CMV detection in BAL in the first 12 months increases the risk for BOS.
| Time to BOS CMV variable type | Univariate model | Multivariate model1 | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| CMV in first 12 months | 1.8 (1.1–2.8) | 0.02 | 1.5 (1.0–2.4) | 0.08 |
| CMV prior to BOS (time dependent) | 2.1 (1.3–3.3) | 0.003 | 1.9 (1.2–3.1) | 0.007 |
- 1Adjusted for acute rejection score, CMV serostatus, single lung transplant and COPD.
CMV replication within the lung allograft as a predictor of mortality:
CMV detection in BAL was not associated with death or time to death on univariate or multivariate analysis (see Supplement 2). On univariate analysis the development of BOS, acute rejection score, single lung transplant and detection of bacteria in BAL were significantly associated with mortality. These associations persisted in the multivariate analysis (Table 6).
| Death | Univariate model | Multivariate model1 | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| BOS | 4.4 (2.3–8.6) | <0.001 | 3.1 (1.5–6.0) | 0.0013 |
| Acute rejection score | 2.4 (1.2–4.9) | 0.01 | ns | |
| Single lung transplant | 2.0 (1.1–3.6) | 0.03 | 2.4 (1.16–5.2) | 0.02 |
| Detection of bacteria in BAL | 2.0 (1.1–3.6) | 0.03 | 2.7 (1.2–6.1) | 0.02 |
Discussion
In light of the evolution of CMV prophylactic strategies and diagnostics we explored the association between CMV detection in BAL as measured by real-time quantitative PCR and the development of BOS in a large cohort of LTR, all of whom received 3–5 months of antiviral prophylaxis. The analysis showed that the detection of CMV DNA in the BAL was associated with the development of BOS irrespective of the magnitude of viral replication, the presence of tissue invasive disease (CMV-P) or whether viral replication was symptomatic or asymptomatic.
Antiviral prophylaxis is largely effective in controlling CMV replication. CMV detection predominantly (90%) occurred following the cessation of ganciclovir or valganciclovir prophylaxis, a pattern that is well recognized (12,13). Compared to 3 months of oral ganciclovir prophylaxis, the use of oral 5 months of valganciclovir was associated with a reduced peak of CMV replication, as well as a reduced incidence of acute rejection. This association between augmented antiviral prophylaxis and reduced cellular rejection has been previously demonstrated in renal transplant recipients (14) and also identified in lung transplant recipients (15,16).
Despite a number of studies examining this, the link between CMV and BOS has not been uniformly observed (17–19). This inconsistency is potentially attributable to differences in LTR cohort sampling and follow-up, varying definitions of CMV infection and BOS and changes in viral prophylaxis regimens over time. There is however, an emerging consensus that CMV disease is associated with the development of BOS and mortality following lung transplantation (20–23). A recent analysis by Snyder et al. reported that treated CMV-P was a risk factor for the development of BOS and reduced survival following lung transplantation (22). The rate of CMV-P was significantly higher in their cohort compared to ours (19% vs. 8%); an observation that maybe related to the shorter courses of antiviral prophylaxis that the majority of Snyder cohort received (4 weeks of intravenous ganciclovir for medium risk serostatus and 14 weeks for high risk serostatus). In our own center prolonged antiviral prophylaxis has reduced the incidence of CMV-P from over 30% to less than 10% (8). The Snyder analysis adds weight to the studies that have reported a decrease in the incidence of BOS with the use of CMV prophylaxis (12,16,21) and support our findings, suggesting that CMV replication within the allograft may result in subsequent chronic allograft dysfunction.
In contrast to our results, a recent study by Manuel et al. (25) reported no increased risk of BOS in a cohort of patients with beta herpesvirus (CMV, HHV6 and HHV7) replication within the lung allograft. The discordant findings between the two studies may be related to differences in the two study populations. The Manuel study cohort, in contrast to our own, included a smaller number of patients (93 vs. 192), providing fewer BAL samples (581 vs. 1756), with reduced follow-up (777 vs. 1015 days). Additionally, their patients received intensive induction immunosuppression (Campath) and overall had a lower incidence of BOS at study completion.
Though our current study does not explore how CMV may lead to BOS, a recent paper by Weight et al. does provide some mechanistic insights. They demonstrated that episodes of CMV pneumonitis and infection lead to upregulation of the chemokines, CCL2 and CCL5, perpetuating inflammation and potentiating the development of allograft dysfunction (24). The low incidence of acute rejection and BOS seen in the absence of CMV replication in the “low-risk” (D−/R−) group would add weight to this argument and suggests that CMV replication has indirect effects on graft function that extend beyond those associated with direct graft infection.
The strengths of our study include a well-characterized cohort of consecutive LTR that received standardized management, surveillance bronchoscopy and follow-up at a single institution with an extended mean follow-up of 2.9 years, allowing adequate time for BOS development. Each patient had the presence of CMV in BAL prospectively analyzed and this was compared to the development of BOS strictly graded according to the ISHLT guidelines allowing for a precise objective determination of outcome. Our statistical analysis additionally recognized and adjusted for the time-dependent nature of CMV DNA replication in the BAL as a risk factor for the outcome of BOS and when considered and adjusted for other BOS risk factors in our final multivariate model, the effect of CMV persisted.
Limitations of the study include recognition that while the sample is representative of a large cohort of LTR we were not able to consider all the factors that could potentially influence BOS development. We were unable to fully consider the impact of respiratory viral infections and nonpulmonary CMV as no serial testing was conducted but rather the tests were completed as clinically indicated. Additionally, as it has not been our practice to repeat bronchoscopy unless clinically indicated, we are unable to assess viral clearance following treatment and as such cannot categorically rule out persistent infection.
Antiviral drugs adequately suppress CMV replication within the lung allograft during the period of prophylaxis. However, patients remain vulnerable to both clinical and subclinical CMV infection on their cessation. A recent randomized control trial by Palmer et al. showed that extending antiviral prophylaxis with valganciclovir decreased the incidence of CMV disease and infection (15) changing the course of late onset disease. This adds high level evidence in support of groups that have previously suggested that longer courses of antiviral prophylaxis are beneficial (16,26,27). Given that clinically apparent CMV antiviral drug resistance was not a significant issue in our patient group (and was also not evident in the Palmer cohort), we have moved to prolonged antiviral prophylaxis (beyond 5 months); a duration of therapy in keeping with that suggested in a recent position paper on the management of CMV in solid organ transplant recipients (28). Further studies will inform us whether this strategy will limit both the direct and indirect sequelae of CMV replication, namely further reduce the incidence of CMV replication, acute rejection and importantly BOS, the major factor limiting successful clinical outcomes following lung transplantation.
This analysis suggests the direct quantitative assessment of CMV replication by PCR within the allograft offers diagnostic and prognostic information beyond that offered by histological detection of CMV inclusions and provides new and essential information in the management of CMV replication following lung transplantation. Despite the evolution of and improvement in antiviral prophylactic strategies, our study demonstrates that CMV replication remains common following short course antiviral prophylaxis and is associated with BOS. These findings mandate us to further improve how we control CMV, the ubiquitous herpesvirus that, perhaps surprisingly, continues to influence the enduring success of lung transplantation.
Contributors
The work present here was undertaken in collaboration between all authors. MP, GW, GS defined the research idea and designed the study's methodology. MP, AG, BL contributed to research data collection and the study design. MB provided the statistical analysis. MP, GW, GS, TW, TK contributed to the discussion, to reviewing and editing of the report. All authors had access to the data, commented on drafts and approved the final submission.
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation: MP, MB and TW have no conflicts of interest to disclose. BL has received a speaker's fee from Janssen-Cilag and has received travel support from Roche, Janssen-Cilag and Novartis. AG has received travel support from Roche and Novartis. GS has received speaker's fees and travel support from Roche and is an investigator for Alnylam. GW has received speaker's fees and travel support from Roche, has been on an Advisory Board for Pfizer and is an investigator for Alnylam. TK has received speaker's fees from Roche and has been on an Advisory Board for Pfizer.




