B Cells Help Alloreactive T Cells Differentiate Into Memory T Cells
Abstract
B cells are recognized as effector cells in allograft rejection that are dependent upon T cell help to produce alloantibodies causing graft injury. It is not known if B cells can also help T cells differentiate into memory cells in the alloimmune response. We found that in B-cell-deficient hosts, differentiation of alloreactive T cells into effectors was intact whereas their development into memory T cells was impaired. To test if B cell help for T cells was required for their continued differentiation into memory T cells, activated T cells were sorted from alloimmunized mice and transferred either with or without B cells into naïve adoptive hosts. Activated T cells cotransferred with B cells gave rise to more memory T cells than those transferred without B cells and upon recall, mediated accelerated rejection of skin allografts. Cotransfer of B cells led to increased memory T cells by enhancing activated CD4 T-cell proliferation and activated CD8 T-cell survival. These results indicate that B cells help alloreactive T-cell differentiation, proliferation and survival to generate optimal numbers of functional memory T cells.
Abbreviations:
-
- DC
-
- dendritic cell
-
- APC
-
- antigen presenting cell
-
- LN
-
- lymph node
-
- BM
-
- bone marrow
-
- SP
-
- spleen
-
- DST
-
- donor specific transfusion
Introduction
B cells and memory T cells contribute to treatment resistant acute and chronic allograft rejection (1–5). Traditionally, B cells are viewed as antibody producing effector cells that are dependent upon T cell help for their differentiation (6,7). Preformed and de novo alloantibodies in patients often precede not only humoral but also cellular, acute and chronic rejection episodes (8–14). Also, allograft biopsies from patients with acute and chronic rejection show evidence of B and memory T cell infiltrates (2,3,15–17). These observations suggest a link between B-cell and T-cell activation in the alloimmune response.
Long-lived memory T cells have quantitative and qualitative advantages over naïve T cells that are essential for protective immune responses, and jeopardize allograft survival (4, 18–21). Studies on the requirement for B cells in the development of memory responses and protective immunity have yielded conflicting results. B cells were required for CD4 effector and memory T-cell responses in Pneumocystis carinii, acute LCMV and Chlamydia trachomatis lung infections, and protein antigens such as KLH (keyhole lympet hemocyanin), PCC (pigeon cytochrome c) and conalbumin (22–26). In the absence of B cells, CD8 T-cell memory was impaired in chronic LCMV infection (27). Similarly, B cells were required for T-cell activation in rheumatoid arthritis and lupus nephritis (28,29). In contrast to the above studies, B cells were dispensable for CD4 T-cell memory in influenza virus lung and Chlamydia trachomatis genital infections (30,31). Also, CD8 T-cell memory to HY antigen, acute LCMV and Listeria monocytogenes infections was intact in B-cell-deficient mice (32–34). Differences in the type of pathogen studied, route of antigen entry and site of immune response, antigen load, extent of inflammation and requirement for neutralizing antibodies for pathogen clearance could have contributed to the contradictory results observed in these studies. The contribution of B cells to alloimmunity has been largely attributed to alloantibody production. A transplanted organ represents a unique milieu of foreign antigen and it is not known if development of T-cell memory in this setting requires B cells. Understanding the role of B cells in T-cell alloimmunity would assist in the design of therapies to target B cells that inhibit not only antibody production but also T-cell immunity for lasting allograft survival.
Several functions of B cells such as antigen presentation, costimulation and cytokine production can modulate T-cell differentiation and cellular immunity (35–38). It has been shown that in the absence of antigen presentation by B cells, CD4 T-cell activation and alloantibody production were impaired resulting in delayed heart but not skin allograft rejection (39). However, it was not clear whether B cells had any impact on alloreactive T-cell memory. We studied the development of CD4 and CD8 memory T cells in B-cell-deficient and B-cell-competent hosts after allogeneic skin transplantation. We report that in the absence of B cells, CD4 and CD8 T cells differentiate into alloreactive effectors but their development into memory T cells is impaired resulting in decreased numbers of alloreactive memory T cells. We find that B cells promote proliferation of activated CD4 T cells and survival of CD8 T cells leading to increased numbers of memory T cells, and thus, contribute to the quantitative advantage of T-cell memory.
Materials and Methods
Mice
C57BL/6 (CD45.2, H-2b; hereafter wt), B6.SJL-Ptprca Pepcb/BoyJ (CD45.1, H-2b; hereafter CD45.1), B6.129S2-Igh-6tm1Cgn/J (CD45.2, H-2b; hereafter μMT) (40) and BALB/c (H-2d) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All animals were maintained under SPF conditions and procedures were performed as per IACUC guidelines.
Skin transplantation, donor specific transfusion and costimulation blockade
Partial thickness skin transplantation was performed using BALB/c donor abdominal skin (41,42). Skin grafts were monitored daily and rejection was defined as >95% graft necrosis. In some experiments, allograft recipients were treated with donor specific transfusion (DST) (BALB/c splenocytes, 2 × 107, day 0) and anti-CD40L (MR1, 1 mg, days 0, 7, 14) (Bio X Cell, West Lebanon, NH). Log-rank test (Graphpad prism 4.0, Graphpad Software, Inc., La Jolla, CA) was used to assess differences in allograft survival and p-value of <0.05 was considered significant.
Flow cytometry and intracellular cytokine staining
Fluorochrome-tagged antibodies CD8a (53–6.7), CD4 (RM4–5), CD44 (IM7), CD62L (MEL-14), CD45.1 (A20), CD69 (H1.2 F3), CD25 (PC61), CD29 (eBioHMb1–1), CD127 (A7R34), Bcl-2 (3F11) and IFNγ (XMG1.2) for flow cytometry were purchased from BD Pharmingen (San Diego, CA) and eBioscience (San Diego, CA). Intracellular IFNγ in lymphocytes was measured after restimualtion ex vivo with BALB/c splenocytes (H-2d) (1:1) for 6 hours. Flow cytometry acquisition was performed on LSRII analyzers (BD Biosciences) and data analyzed using Flowjo software (Treestar, Ashland, OR). BALB/c-reactive IFNγ+ T cells present within the responder CD4 and CD8 T-cell populations were quantitated after gating on the H-2d negative population.
Cytotoxicity assays
Recipients of BALB/c skin allografts were used as memory mice at 8-weeks after allograft rejection. To assess in vivo cytotoxicity, naïve and memory mice were treated with 200 μg of anti-NK1.1 (PK136) (days -2 and -1) to deplete NK cells and injected with equal numbers of CFSE labeled H-2b (2 × 107, 0.2 μM B6, syngeneic) and H-2d (2 × 107, 2 μM, BALB/c, allogeneic) splenocytes (day 0). 24-hours later, in vivo killing of allogeneic cells was measured as loss of H-2d target cells compared to loss of H-2b syngeneic cells in mice versus naïve control mice using the following formula: 100 –[(%H-2d cells in memory mice/%H-2b cells in memory mice/%H-2d cells in naïve mice/%H-2b cells in naïve mice) × 100] (43). To assess in vitro cytoxicity, spleen (SP) and lymph node (LN) cells from memory mice were purified for T cells by MACS depletion of B220+, NK1.1+, CD11b+ and CD11c+ cells and incubated with calcein labeled BALB/c splenocytes (0.3 mM, 100:1) at 37 °C for 4 hours. BALB/c cell killing was calculated using the formula: (% dead target cells – spontaneous dead target cells/100 – spontaneous dead targets) × 100 (44,45).
Sorting of B cells and activated T cells for adoptive transfer
CD45.1 mice were immunized (3 × 107 BALB/c splenocytes, i.p.) and 8-days later, SP and LN cells were harvested to isolate activated T cells. Harvested cells were labeled with antibodies against CD4, CD8, CD44 and B220, and sorted for CD8+ CD44high, CD4+ CD44high and B220+ (CD4− and CD8−) populations (purity >95%) on BD FACS Aria. 1 × 106 CD4+ CD44high or CD8+ CD44high T cells were transferred with or without 1.5 × 107 B220+ B cells into μMT and wt hosts. In some experiments, B220+ B cells were sorted from unimmunized naïve mice. In experiments testing allograft rejection, 2 × 106 CD8+ CD44high and CD4+ CD44high T cells were transferred into adoptive hosts with or without 1.5 × 107 B220+ B cells. CD8+ CD44high and CD4+ CD44high T cells were labeled with CFSE (2 μM, Molecular Probes, Carlsbad, CA) prior to adoptive transfer in specified experiments (46).
Cell harvest and enumeration after adoptive transfer
Cells were harvested from spleen, LN and bone marrow (BM) of adoptive hosts at indicated times points (1, 2, 3 and 8–12 weeks) after transfer of CD4+ CD44high or CD8+ CD44high T cells with or without B220+ B cells. Bone marrow cells were obtained from femurs and tibia, multiplied by a factor of 5.4 to estimate total body bone-marrow cells (42,47). Harvested live cells from tissues were counted using trypan blue exclusion on a hemacytometer, stained with FACS antibodies and analyzed by flow cytometry after gating on CD4+ or CD8+, CD45.1+ population. Apoptosis was determined after staining cells for Annexin V and 7-AAD (BD Pharmingen). Statistical analyses were performed using unpaired t-test (Graphpad Software, Inc) and differences with p < 0.05 were considered significant.
Results
Alloreactive T-cell memory is impaired in B-cell-deficient hosts
We investigated the role of B cells in alloreactive T cell responses by using B-cell-deficient, μMT (B6, H-2b) mice. Allogeneic BALB/c (H-2d) skin grafts were transplanted onto μMT mice and allograft rejection was compared to that in B-cell-competent wt mice. μMT and wt mice rejected skin allografts comparably (Figure 1A) consistent with previous reports (39,48,49). Spleen (SP), lymph node (LN) and bone marrow (BM) cells were harvested from μMT and wt recipients of BALB/c skin grafts at 14-days after transplantation (effector phase) and at 8 weeks after allograft rejection (memory phase) to measure alloreactive effector and memory T cells, respectively. Alloreactive T cells were identified as IFNγ+ cells present within the responding CD4+ and CD8+ T-cell populations after 6-hour ex vivo rechallenge with BALB/c splenocytes. Since CD4 and CD8 IFNγ+ cells have been reported to mark effector T cells that give rise to memory T cells (50), we utilized ex vivo alloreactive IFNγ production to identify antigen specific cells. CD4+ and CD8+ BALB/c-reactive IFNγ+ T-cell numbers were comparable between wt and μMT mice during the effector phase (Figure 1B). However, the number of CD8 memory T-cell precursors identified as BALB/c-reactive IFNγ+ IL-7Rα+ effector T cells within the CD8+ CD44hi population were decreased in μMT chimeras compared to wt chimeras (45 ± 8% vs. 58 ± 5%, respectively) (Figure 1C). CD4 and CD8 BALB/c-reactive IFNγ+ T cells in the memory phase were significantly fewer in μMT recipients than in wt mice (Figure 1D). Thus, despite comparable skin allograft rejection and differentiation into alloreactive effector T cells in μMT and wt mice, resulting memory T cells were significantly reduced in number in the absence of B cells. Since μMT mice have altered splenic architecture and contain fewer splenic T cells than wt mice (49,51), alloreactive T-cell responses were also tested in chimeras generated in irradiated μMT mice by transplanting syngeneic bone marrow cells from either μMT or wt donors. BALB/c-reactive IFNγ+ CD4 and CD8 memory T cells in μMT chimeras were fourfold and sixfold fewer, respectively, than in wt chimeras (data not shown). To test whether the difference in memory T-cell numbers was significant to affect recall responses, cytotoxic function and allograft rejection were tested in μMT and wt mice at 8-weeks after BALB/c skin allograft rejection. μMT recipients mediated significantly less BALB/c cell lysis than wt mice in the memory phase (Figure 1E and F). To assess allograft rejection mediated by memory T cells, μMT and wt memory mice were rechallenged with BALB/c skin allografts and treated with DST and anti-CD40L to inhibit endogenous naïve T-cell activation (19,52,53). Wt memory recipients rejected BALB/c skin allografts in a significantly accelerated manner compared to μMT memory mice (MST = 20 vs. 30 days, respectively, n = 4/group; p = 0.03) (Figure 1G). Allograft rejection in μMT memory mice was comparable to that of naïve mice (MST = 30 days in both groups, n = 4–9/group) (Figure 1G). Although alloantibodies could contribute to in vivo allogeneic cell lysis in wt memory mice, skin allograft rejection occurs independently of alloantibody mediated graft injury in mice (54–56). Therefore, these data suggest that impaired recall function in μMT memory mice is due to decreased numbers of alloreactive memory T cells in these mice compared to wt memory mice. Thus, in the absence of B cells, although alloreactive effector T-cell differentiation occurs, their subsequent development into memory T cells is significantly impaired resulting in decreased numbers of functional alloreactive memory T cells.
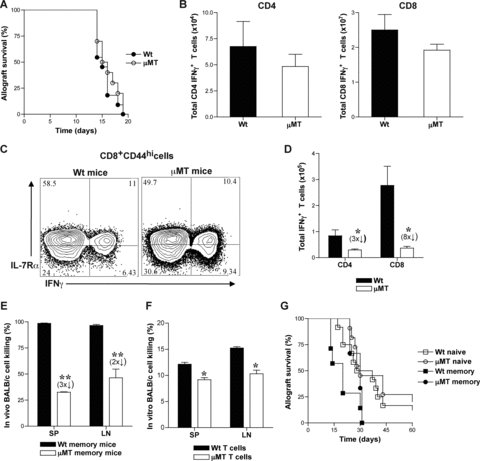
Development of alloreactive memory T cells is impaired in B-cell deficient mice. (A) Rejection of BALB/c (H-2d) skin allografts transplanted to μMT (H-2b) and wt (H-2b) mice (MST = 16 and 15 days, respectively; p > 0.05; n = 10 mice/group). Quantitiation of alloreactive effector (B) and memory (D) T cells in μMT and wt mice. Spleen (SP), lymph node (LN) and bone marrow (BM) cells were harvested from BALB/c skin grafted μMT and wt recipients at 14-days (effector phase) and at 8-weeks after rejection (memory phase) for quantitation of alloreactive T cells. Harvested cells were restimulated ex vivo for 6-hours with BALB/c splenocytes and IFNγ producing CD4 and CD8 T cells were assessed by flow cytometry and enumerated (Mean ± SD; *, p < 0.05; n = 4 mice/group). (C) CD8 memory precursors within alloreactive effector T cells in μMT and wt mice. Representative FACS plots of IL-7Rα expression on BALB/c-reactive IFNγ+ population within CD8+ CD44hi splenic T cells harvested at 14-days after BALB/c skin transplantation are shown. (E and F). Cytotoxic function of μMT and wt memory T cells was assessed by in vivo (E) and in vitro (F) allogeneic cell lysis at 8-weeks after BALB/c skin graft rejection (memory phase).μMT and wt mice were depleted of NK cells and equal numbers of CFSE labeled H-2b (2 × 107, 2 μM B6 wt, syngeneic) and H-2d (2 × 107, 0.2 μM, BALB/c, allogeneic) splenocytes were injected (i.v.). 24-hours later, in vivo killing of BALB/c cells in comparison to B6 cells was measured by flow cytometry (Mean ± SD; **, p < 0.005; n = 3–4 mice/group). Purified T cells from SP and LN cells of μMT and wt memory mice were incubated with calcein labeled BALB/c splenocytes (0.3 mM, 100:1) and 4-hours later, in vitro killing of BALB/c cells was measured by flow cytometry (Mean ± SD; *, p < 0.05; n = 3 -4 mice/group). (G) Allograft rejection in memory μMT and wt mice. At 8-weeks after BALB/c allograft rejection (memory phase), μMT and wt recipients were rechallenged with BALB/c skin grafts and treated with DST (2 × 107) and anti-CD40L (MR1, 1 mg on days 0, 7 and 14 after transplantation). Allograft rejection was assessed in μMT and wt memory mice, and compared to naïve mice (MST = 30, 20 and 30 days, respectively; p < 0.05; n = 4–9 mice/group).
B cells promote differentiation of activated T cells into memory T cells in adoptive hosts
Results in μMT and wt hosts showed that in the absence of B cells, alloreactive T-cell differentiation into effectors was preserved but their development into memory T cells was impaired. These findings raised the question whether B cells are required for the continued differentiation of alloreactive effector T cells into memory T cells. To answer this question, we adoptively transferred activated T cells sorted from alloimmunized mice either with or without B cells into naïve μMT and wt mice and tested their differentiation into functional memory T cells. CD4+ and CD8+ CD44hi activated T cells and B220+ B cells were sorted from SP and LN cells of CD45.1 (B6, H-2b) mice at 8-days after immunization with BALB/c splenocytes (3 × 107, i.p.). Sorted CD4+ CD44hi or CD8+ CD44hi activated T cells (>95% purity) (1 × 106) were transferred either alone or together with B220+ B cells (>97% purity) (1.5 × 107) into congenic naïve μMT (CD45.2) or wt (CD45.2) mice. Sorted T cells were CD44hi, 1B11hi and contained a population of CD69hi, CD25hi and CD62Llo cells consistent with phenotype of activated T cells (data not shown) (42). SP, LN and BM cells were harvested from adoptive hosts, phenotyped and enumerated after gating on CD45.1+ T cells at 1, 2, 3 and 8–12-weeks after transfer. Activated CD45.1+ CD4 and CD8 T cells cotransferred with B cells were found to persist along with CD45.1+ B cells in adoptive hosts better than activated T cells that were transferred alone at 10-weeks after transfer (Figure 2A). Gating on CD8 T cells within the harvested cells, a CD45.1+ population that is CD44hi, CD25lo, CD69lo consistent with memory phenotype and containing CD62Lhi central and CD62Llo effector memory T cells was observed (Figure 2B). CD45.1+ CD4 T cells harvested from μMT hosts were also of similar phenotype and comparable to those harvested from wt adoptive hosts (data not shown). Thus, activated T cells transferred into naïve adoptive hosts had differentiated into memory T cells. Activated T cells that were transferred alone declined more than those cotransferred with B cells over time in adoptive hosts (Figure 2C). The decay of activated T cells in adoptive hosts was most pronounced at 2-weeks with the exception of activated CD4 T cells in μMT hosts that appeared to have declined already at 1-week after transfer (Figure 2C). Although wt hosts had endogenous B cells, cotransfer of B cells from alloimmunized mice was required to attenuate the contraction of activated T cells after transfer suggesting a possible role for cognate interaction between B and T cells (Figure 2C). Upon quantitation of CD45.1+ memory T cells at 8–12-weeks after transfer into μMT recipients, ninefold more CD4 and 13-fold more CD8, CD45.1+ memory T cells were obtained from activated T cells cotransferred with B cells than from those transferred alone (Figure 2D). In wt recipients, activated T cells cotransferred with B cells yielded twofold more CD4 and CD8 CD45.1+ memory T cells than those transferred alone (Figure 2D). Activated T cells cotransferred with B cells from naïve mice also led to formation of sevenfold more CD4 and 17-fold more CD8, CD45.1+ memory T cells in μMT recipients (Figure 2E). These results were comparable to memory T-cell numbers obtained from activated T cells transferred alone into wt recipients containing endogenous B cells (Figure 2D) suggesting a possible noncognate interaction between B and T cells. Thus, in the absence of B cells, activated T cells decayed over time yielding fewer memory T cells. These findings suggest that B cell help for activated T cells promotes their differentiation into memory T cells.
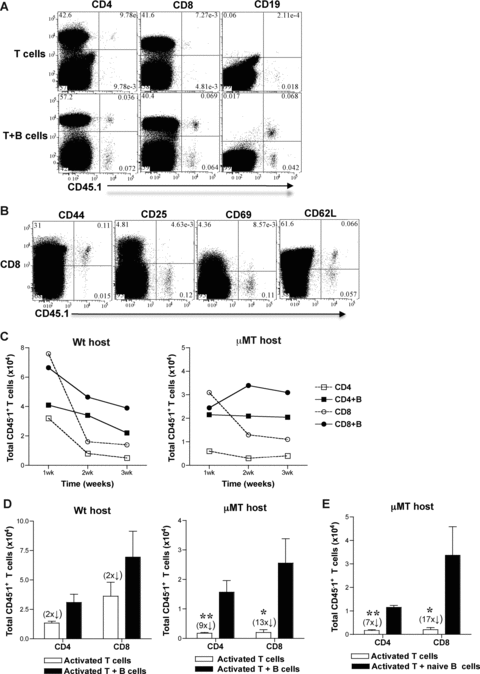
B cells promote differentiation of activated T cells to memory T cells in adoptive hosts. CD45.1 mice were immunized with BALB/c splenocytes (3 × 107) and 8-days later, SP and LN cells were harvested and sorted for CD4+ and CD8+ CD44hi T cells and B220+ B cells. Sorted CD4+ or CD8+ CD44hi activated T cells (1 × 106) were transferred (i.v.) with or without B220+ B cells (1.5 × 107) into μMT and wt mice. SP, LN and BM cells from μMT and wt hosts were harvested at 1, 2, 3 and 8–12 weeks (C and D, respectively) after adoptive transfer and were enumerated after gating on CD4+ or CD8+ CD45.1+ T cells. (A) CD45.1+ T and B cells in μMT adoptive hosts. Representative FACS plots from spleen cells harvested at 10-weeks after transfer from μMT recipients of activated T cells transferred with or without B cells are shown. y-axis legend is depicted on top of the FACS plots. (B) Memory phenotype of CD45.1+ T cells harvested from adoptive hosts at 10-weeks after transfer. Representative FACS plots are shown from spleen cells of μMT adoptive recipient of CD8+ CD44hi activated T cells cotransferred with B220+ B cells. y-axis legend is depicted on top of the FACS plots. (C) Activated T cells transferred without B cells decline over time in adoptive hosts (Mean ± SD, n = 2–3 mice/group). (D and E). Activated T cells cotransferred with B cells develop into more memory T cells in adoptive hosts. B220+ B cells were sorted from either CD45.1 mice immunized with BALB/c splenocytes at 8-days (D) or from unimmunized naïve CD45.1 mice (E) (Mean ± SD; *, p < 0.05, **, p < 0.005; n = 4–6 mice/group).
B cells enhance proliferation and survival of activated T cells to generate more memory T cells
To test how B cells led to development of more memory T cells from activated T cells, proliferation, apoptosis and survival of activated T cells following transfer into adoptive hosts was assessed. CFSE labeled CD45.1+ activated CD4 and CD8 T cells were transferred with or without CD45.1+ B cells into naïve wt and μMT mice. Two weeks later, SP and LN cells from adoptive hosts were harvested, cell proliferation was evaluated by CFSE dilution and cell death was assessed by Annexin V expression on CD4+ and CD8+ CD45.1+ T cells. Activated CD4 and CD8 T cells cotransferred with B cells diluted CFSE more than those transferred alone (Figure 3A). Proliferation index of activated CD4 T cells cotransferred with B cells was 1.5-fold higher than activated CD4 T cells transferred alone whereas activated CD8 T cells transferred with or without B cells showed comparable proliferation index (Figure 3B). There was no difference in Annexin V+ cells detected within the harvested CD45.1+ T cells from adoptive recipients of activated T cells with or without B cells (Figure 3C). Bcl-2 was significantly upregulated in harvested CD8+ CD45.1+ T cells that were cotransferred with B cells compared to those transferred alone and this was not observed in harvested CD4+ CD45.1+ T cells (Figures 3D-E). Despite differences in Bcl-2 expression, apoptosis of transferred CD8 T cells with or without B cells was comparable (Figure 3C) possibly due to a difference that was too small or gradual to be measured, or due to rapid clearance of apoptotic cells in vivo. Taken together, these results suggest that B cells enhance proliferation of activated CD4 T cells and survival of activated CD8 T cells by upregulation of Bcl-2, thus, significantly increasing the number of memory T cells formed.
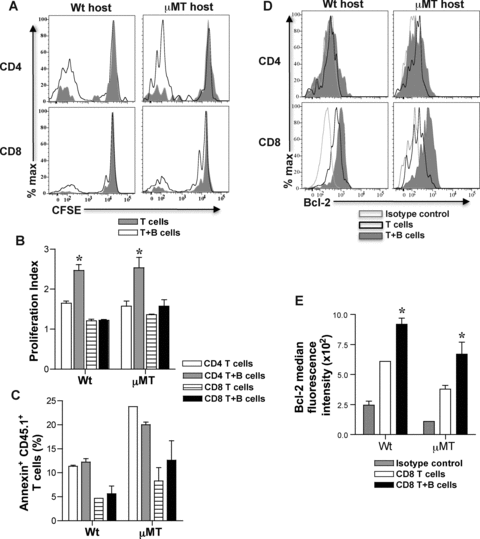
Proliferation and survival of activated T cells in adoptive hosts. Activated CD4+ or CD8+ CD44hi T cells and B220+ B cells were sorted from SP and LN cells of immunized CD45.1 mice (BALB/c splenocytes, 3 × 107) at 8-days. Sorted CD45.1 CD4+ and CD8+ CD44hi T cells (1 × 106) were CFSE labeled and transferred with or without B220+ B cells (1.5 × 107) into μMT and wt adoptive hosts. At 2-weeks after transfer, CFSE dilution was analyzed on harvested SP and LN cells after gating on CD4+ or CD8+ CD45.1+ T cells. Representative histogram overlays of CFSE dilution in CD4+ or CD8+ CD45.1+ T cells from harvested spleens of μMT and wt adoptive hosts are shown (A). (B) B cells help proliferation of activated CD4 T cells in adoptive hosts. Proliferation index is calculated using Flowjo software after gating on CD4+ or CD8+ CD45.1+ T cells (Mean ± SD; *, p < 0.05; n = 3–4 mice/group). (C) Apoptosis of activated T cells in adoptive hosts. Harvested SP and LN cells from μMT and wt adoptive hosts were examined for Annexin V+ staining after gating on CD4+ or CD8+ CD45.1+ T cells (Mean ± SD; n = 3–4 mice/group). (D) Bcl-2 expression in CD4+ and CD8+ CD45.1+ T cells in adoptive hosts. Representative histogram overlays of Bcl-2 expression are shown after intracellular staining and gating on harvested CD4+ or CD8+ CD45.1+ T cells from adoptive hosts. (E) Bcl-2 expression is upregulated in developing CD8 memory T cells in adoptive hosts harboring cotransferred B cells. Median fluorescence intensity of Bcl-2 expression on CD8+ CD45.1+ T cells harvested from adoptive hosts is shown in comparison to isotype control (Mean ± SD; *, p < 0.05, n = 3–4 mice/group).
Memory T cells that develop with B cell help mediate accelerated allograft rejection
We tested the function of memory T cells that developed from transferred activated T cells in adoptive hosts by rechallenging with BALB/c skin transplants. Activated CD4+ or CD8+ CD45.1+ T cells were transferred into μMT and wt adoptive hosts with or without B cells. 12-weeks later, to assess function of memory T cells that developed, mice were treated with DST and anti-CD40L to inhibit endogenous naïve T-cell activation and transplanted with BALB/c skin grafts. BALB/c skin allograft rejection in μMT and wt adoptive hosts of activated CD4 or CD8 T cells cotransferred with B cells was significantly accelerated compared to recipients of activated CD4 or CD8 T cells alone (μMT MST = 20 and 19 days vs. 26 and 28 days, and wt MST = 35 and 32 days vs. 25 and 24 days, respectively, n = 4–6/group; p = 0.003) (Figure 4). BALB/c skin allograft survival in adoptive hosts of activated CD4 or CD8 T cells was comparable to that in naïve recipients (Figure 4). These findings suggest that activated T cells cotransferred with B cells develop into functional memory T cells in adoptive hosts. It's possible that the number of memory T cells in adoptive hosts of activated CD4 or CD8 T cells alone were too few to effect accelerated rejection and may not reflect impaired function of memory T cells on a per cell basis. Since significantly more memory T cells develop from activated T cells with B-cell help (Figure 2D and E), taken together, these data suggest that B cells promote the quantitative advantage of memory T cells essential for recall function.
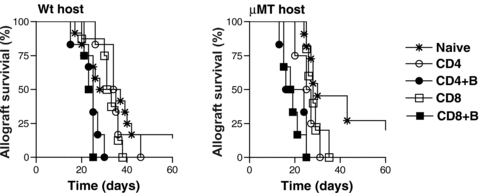
Allograft rejection in adoptive hosts harboring memory T cells. Memory T cells that developed from activated CD4+ or CD8+ CD45.1+ T cells cotransferred with or without B220+ B cells into adoptive hosts were rechallenged with BALB/c skin grafts at 12-weeks after adoptive transfer (memory phase). Skin allograft recipients were treated with DST (2 × 107) and anti-CD40L (MR1, 1 mg on days 0, 7 and 14 after transplantation). Allograft rejection in μMT and wt adoptive hosts harboring memory T cells was compared to naïve mice. MST in wt naïve and adoptive hosts of activated CD4 vs. CD4 + B, CD8 vs. CD8 + B cells were 33, 35 vs. 25 and 32 vs. 24 days, respectively (**, p < 0.005; n = 4–9 mice/group). MST in μMT naïve and adoptive hosts of activated CD4 vs. CD4 + B, CD8 vs. CD8 + B cells were 30, 26 vs. 20 and 28 vs. 19 days, respectively (**, p < 0.005; n = 4–9 mice/group).
Discussion
We investigated in this study whether B cells were required for the development of effector and memory alloreactive T-cell responses. We found that skin allograft rejection and effector T-cell responses were comparable between B-cell deficient and B-cell replete hosts. However, development of alloreactive memory T cells was defective in the absence of B cells resulting in fewer memory T cells and impaired recall function. These findings suggested that although B cells were not required for activation of alloreactive T cells, their subsequent development into memory T cells was dependent upon B cells. Activated T cells were transferred with or without B cells into adoptive hosts to test if B cells were required for their differentiation into memory T cells. Activated T cells cotransferred with B cells gave rise to more memory T cells than activated T cells transferred alone and upon recall, caused accelerated rejection of skin allografts. These results show that B cells help alloreactive T cells differentiate into memory T cells during the alloimmune response yielding increased numbers of memory T cells and contribute to the quantitative advantage of memory responses. It remains to be determined if B cell help also impacts the ‘quality’ of memory T cells generated in the alloimmune response.
Decreased alloreactive T-cell memory in μMT mice despite intact differentiation into effectors (Figure 1B–D) and accelerated decline of activated T cells in μMT adoptive hosts (Figure 2C) is consistent with increased contraction of CD4 and CD8 T cells reported in LCMV and Listeria monocytogenes infections, respectively in the absence of B cells (24,34). Enhanced IL-12 production by DCs in μMT mice due to a relative IL-10 deficient milieu could be contributing to the increased contraction of activated T cells observed in these mice (57,58). Impaired expansion of CD4 T cells (Figure 3B) in adoptive hosts contributed to decreased CD4 memory in the absence of B cells. Attenuated IL-7Rα expression (Figure 1C) and diminished Bcl-2 (Figure 3E) in activated CD8 T cells differentiating without B-cell help suggests that reduced CD8 T-cell survival contributed to decreased CD8 memory (59). It remains to be determined whether impaired CD8 T-cell memory in the absence of B cells is due to lack of B-cell help for CD8 T cells or due to suboptimal CD4 T-cell differentiation resulting in deficient CD4 T-cell help for CD8 T cells.
Activated CD4 and CD8 T cells transferred without B cells declined rapidly in adoptive hosts by 2-weeks suggesting an exaggerated contraction phase in the absence of B cells (Figure 2C) (24,34). It is possible that B cells also play a role in the maintenance of memory T cells since memory CD4 and CD8 T cells derived from activated T cells transferred alone were many fold fewer in μMT than in wt adoptive hosts (Figure 2D) suggesting a continued decline over time in the absence of endogenous B cells. This decline in μMT adoptive hosts is attenuated upon cotransfer of naïve B cells resulting in memory T-cell numbers comparable to wt mice (Figure 2D and E) suggesting a possible noncognate role for B cells in promoting survival of memory T cells. Maintenance of CD8 memory T cells is dependent upon IL-15 while IL-7 plays a role in maintenance of both CD4 and CD8 memory T cells (60–63). It remains to be tested whether endogenous B cells in wt hosts are promoting maintenance of memory T cells directly or indirectly by affecting lymphoid architecture and survival of stromal cells that secrete homeostatic cytokines, IL-7 and IL-15 (64).
Despite a modest difference in memory T-cell numbers, wt recipients of activated T cells cotransferred with B cells mediate accelerated graft rejection compared to wt recipients of activated T cells alone (Figure 4) suggesting that cognate B-cell help for T cells promoted their differentiation into functional memory T cells. Further elucidation of cognate versus noncognate roles of B cells in the development of alloreactive T-cell memory is required and needs models in which antigen-specific B and T cells can be identified and tracked.
Early costimulatory interactions via CD28/B7 and CD40/CD40L and IL-2 regulate clonal expansion of CD4 and CD8 T cells following antigen exposure (65). The role of B cells in this phase could be an indirect one in maintaining lymphoid architecture, shuttling antigen and formation of immune complexes that amplifies the interactions between DCs and T cells (64,66–68). CD27/CD70, 4–1BB/4–1BBL, OX40/OX40L and ICOS/ICOSL costimulatory interactions that occur in the later phase of activation determine the number and quality of CD4 and CD8 memory T cells that are generated (69–72). Activated B cells can entrap antigen and function as potent APCs driving CD4 T-cell expansion and differentiation (23,26,35,73). B cells upregulate CD40, ICOSL, CD70, OX40L costimulatory ligands and secrete cytokines upon activation that can modulate T-cell differentiation (36–38). B cells can thus provide help for T cells via multiple mechanisms promoting T-cell proliferation, differentiation and survival to generate memory T cells (Figure 5). Therefore, B-cell depletion/inhibition early in the alloimmune response could possibly prevent not only alloantibody formation but also generation of long-lived memory T cells improving graft survival.
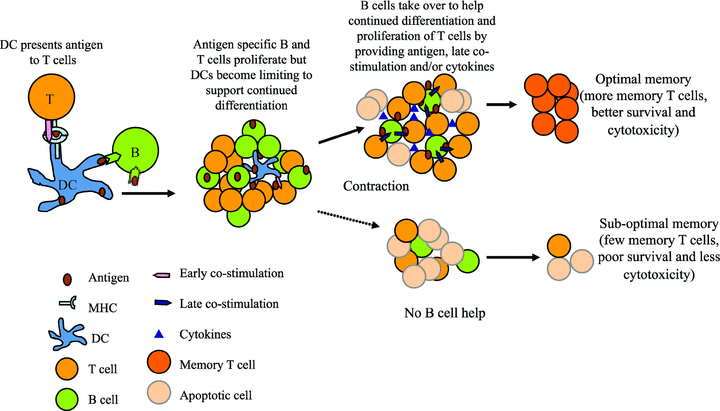
Model of B-cell help for T-cell differentiation to memory. Early in the immune response professional APCs such as dendritic cells (DCs) present antigen to T cells and provide early costimulatory signals. As antigen specific B and T cells proliferate, the nondividing DCs become limiting to support further differentiation of T cells. B cells then take over as ‘helper’ cells to promote continued differentiation and survival of T cells. B cells present entrapped antigen, provide cytokines and/or costimulation to T cells to support their differentiation into long-lived memory T cells.
Acknowledgments
We thank Ms. Autumn Marlowe for technical assistance; Dr. Hongmei Shen for FACS sorting; and Dr. Fadi Lakkis for critical reading of the manuscript.
This work is supported by NIH grants AI059137, AI073578 and AI079177 (GC) and John Merrill Transplant Research Scholar Award (GC) from the American Society of Nephrology and the American Society of Transplantation. Y-HN is a recipient of American Heart Association Physician Scientist Fellowship Award. MHO is a recipient of the American Society of Transplantation International Fellowship Award and the Thomas E. Starzl Fellowship in Transplantation Biology.




