Influence of Recipient Race on the Outcome of Simultaneous Pancreas and Kidney Transplantation
Abstract
Racial differences on the outcome of simultaneous pancreas and kidney (SPK) transplantation have not been well studied. We compared mortality and graft survival of African Americans (AA) recipients to other racial/ethnic groups (non-AA) using the national data. We studied a total of 6585 adult SPK transplants performed in the United States between January 1, 2000 and December 31, 2007. We performed multivariate logistic regression analyses to determine risk factors associated with early graft failure and immune-mediated late graft loss. We used conditional Kaplan–Meier survival and multivariate Cox regression analyses to estimate late death-censored kidney and pancreas graft failure and death between the groups. Although there was no racial disparity in the first 90 days, AA patients had 38% and 47% higher risk for late death-censored kidney and pancreas graft failure, respectively (p = 0.006 and 0.001). AA patients were twice more likely to lose the kidney and pancreas graft due to rejection (OR 2.31 and 1.86, p = 0.002 and 0.008, respectively). Bladder pancreas drainage was associated with inferior patient survival (HR 1.42, 95% CI 1.15, 1.75, p = 0.001). In the era of modern immunosuppresion, AA SPK transplant patients continue to have inferior graft outcome. Additional studies to explore the mechanisms of such racial disparity are warranted.
Introduction
Simultaneous pancreas and kidney (SPK) transplantation is the preferred treatment modality for suitable type 1 diabetic patients with end stage renal disease (ESRD) (1–3). Beside improvement in quality of life, evidence suggests that a successful SPK transplant has the potential to improve cardiovascular risk profile, reduce the progression of macrovascular complication of diabetes and improve patient survival (4–10). With the introduction of more potent immunosuppressive medications as maintenance therapy and widespread use of induction agents, the incidence of acute rejection has decreased and patient and graft survival has improved in SPK transplant patients in the recent era (11,12).
Although the incidence of type 1 diabetes mellitus in African Americans (AA) is lower than that of non-Hispanic white population, the AA diabetic patients have poorer metabolic control and higher incidence of end organ damage including ESRD (13–18). Type 1 diabetic AA ESRD patients have lower rate of access to SPK transplant registration compared to Caucasians (19,20). It is unclear whether such lower access to SPK transplantation in AA is the result of referral bias due to perceived inferiority in the outcome of SPK transplant for AA patients (20). AA kidney transplant patients have higher risk for kidney graft loss and require increased levels of immunosuppression (21–25). However, literature on such racial disparity regarding pancreas transplant outcome in the setting of SPK transplantation remains limited and controversial as most reports were generated from small numbers of AA SPK transplant patients involved in clinical trials or in single-center studies (26–28).
We hypothesized that with the improvement in surgical technique and perioperative care, as well as enhanced immunosuppression, both in term of induction and maintenance regimens, in the recent era, there would be no racial disparity in the outcome in SPK transplantation between AA and non-AA patients. We undertook a retrospective analysis of national registry data to determine race-specific graft survival and mortality risk among SPK transplant patients. Because early pancreas graft failure is not a rare event that may contribute to the difference in overall graft survival, we separately assessed the race-specific risks of early and late graft loss.
Methods
We included all adult primary simultaneous pancreas and kidney (SPK) transplants performed between January 1, 2000 and December 31, 2007 in the United States in the national database provided by the Organ Procurement and Transplantation Network (OPTN)/Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donors, wait-listed candidates and transplant recipients in the US, submitted by the members of the OPTN, and has been described elsewhere (29). The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. The study was approved by the Institutional Review Board (IRB). The follow-up was up to November 30, 2008. We performed separate analyses for early and late graft failure and death. We defined early graft failure as kidney and/or pancreas loss within the first 90 days after transplantation (30,31). The causes of early and late graft failure were separately examined. Baseline recipient and donor-related characteristics were examined and used in multivariate analyses as appropriate. Surgical modalities in exocrine pancreas anastomosis were identified and included as one of the covariates in the analyses. The use of various immunosuppressive agents, induction and maintenance, and their various combinations were obtained from the initial hospital discharge and incorporated in the analyses. Transplant centers were grouped according to the volume of SPK transplants performed at a given year and used as a covariate in the multivariate analyses.
Demographic and baseline characteristics were compared using Student's t-test and χ2-test between AA and non-AA SPK transplant patients. For early graft failure (within 90 days of transplant), a multivariate logistic regression model was used to identify the risk factors associated with graft failure adjusting the volume of SPK transplants at individual centers. For late outcome, conditional Kaplan–Meier survival and multivariate Cox proportional regression analysis with backward selection approach was performed for all SPK patients who had survived up to 90 days after transplantation with both functioning grafts for composite transplant outcome including graft failure (kidney and/or pancreas) and death, and subsequently for death-censored graft survival and patient survival. Finally, the causes of late pancreas and kidney graft loss were identified and a multivariate logistic regression was performed to identify the risk factors associated with acute and chronic rejection related graft loss. Software SAS 9.1 was utilized and statistical significance was set at p ≤ 0.05.
Results
A total of 6742 adult de novo SPK transplant patients were identified in the OPTN/SRTR data set between January 1, 2000 and December 31, 2007. After excluding patients who had incomplete or overlapping information on their initial immunosuppression regimens and final outcome and early loss to follow-up (<90 days), the final cohort included 6585 SPK patients. Among these, 931 (14.1%) reported African American as their racial category. The remainders (5654) were classified as non-AA SPK transplant patients. During the study period, the volume of SPK transplants has remained relatively stable for AA and non-AA patients.
Table 1 shows the demographic and baseline characteristics of study population. Compared to non-AA SPK transplant patients, AA SPK transplant patients were younger (38.5 ± 8.6 years vs. 41.9 ± 8.3 years, p < 0.001) with lower body mass index (BMI) (24.6 ± 4.0 vs. 25.0 ± 4.0, p = 0.01), had shorter duration of diabetes (22.9 ± 7.5 years vs. 27.1 ± 8.1 years, p < 0.001), lower proportion of patients with type 1 diabetes mellitus (90.4% vs. 94.2%, p < 0.001) and longer duration of ESRD (2.3 ± 1.9 years vs. 1.6 ± 1.8 years, p < 0.001). AA SPK transplant patients also had lower rate of preemptive transplant (2.5% vs. 10.0%, p < 0.001), more frequent previous blood transfusion (30.2% vs. 24.5%, p < 0.001), higher levels of panel reactive antibodies at transplant (9.2 ± 20.6 vs. 7.3 ± 18.2, p = 0.008) and received more often organs from younger donors (26.0 ± 10.0 years vs. 26.9 ± 10.8 years, p = 0.007) and AA donors (23.6% vs. 13.4%, p < 0.001). The level of human leukocyte antigen (HLA) mismatch was 4.7 ± 1.0 in AA versus 4.4 ± 1.3 in non-AA SPK transplant patients, respectively (p < 0.001). The delayed kidney graft function was 13.0% in AA compared to 7.2% in non-AA SPK transplant patients (p < 0.001). Fewer AA SPK transplant patients had history of coronary artery disease (4.9% vs. 9.1%, p < 0.001) and peripheral vascular disease (4.8% vs. 7.1%, p = 0.01) prior to transplantation. AA SPK transplant patients also were less likely to have private health insurance (31.0% vs. 51.7%, p < 0.001).
| Non-AA SPK N = 5654 | AA SPK N = 931 | p-Value | |
|---|---|---|---|
| Age (years) (SD) | 41.9(8.3) | 38.5(8.6) | <0.001 |
| Gender, male (%) | 3512(62.1) | 553(59.4) | 0.11 |
| Body mass index (kg/m2)(SD) | 25.0(4.0) | 24.6(4.0) | 0.01 |
| Duration of diabetes (years) (SD) | 27.1(8.1) | 22.9(7.5) | <0.001 |
| Patients with type 1 diabetes mellitus (%) | 5323(94.2) | 842(90.4) | <0.001 |
| Years of dialysis (SD) | 1.6(1.8) | 2.3(1.9) | <0.001 |
| History of dialysis (%) Non dialysis | 563(10.0) | 23(2.5) | <0.001 |
| <1 year | 1935(34.2) | 213(22.9) | |
| 1–3 years | 2319(41.0) | 441(47.4) | |
| >3 years | 837(14.8) | 254(27.3) | |
| Positive Hepatitis C serology (%) | 156(2.8) | 26(2.8) | 0.95 |
| Previous blood transfusion (%) | 1387(24.5) | 281(30.2) | <0.001 |
| Panel reactive antibodies (SD) | 7.3(18.2) | 9.2(20.6) | 0.008 |
| Private health insurance (%) | 2922(51.7) | 289(31.0) | <0.001 |
| Education (%) | |||
| Less than high school | 112(2.4) | 11(1.5) | 0.30 |
| High school | 2089(44.8) | 337(45.5) | |
| College | 2465(52.8) | 392(53.0) | |
| Peripheral vascular disease (%) | 402(7.1) | 45(4.8) | 0.01 |
| Coronary artery disease (%) | 515(9.1) | 46(4.9) | <0.001 |
| Donor age (years) (SD) | 26.9(10.8) | 26.0(10.0) | 0.007 |
| Donor BMI (kg/m2) (SD) | 23.9(4.1) | 24.2(4.2) | 0.10 |
| Donor gender, male (%) | 3827(67.7) | 611(65.6) | 0.22 |
| Donor race, AA (%) | 756(13.4) | 220(23.6) | <0.001 |
| Donor smoking, >20 pack-years (%) | 1352(23.9) | 206(22.1) | 0.24 |
| Donor type, SCD (%) | 5523(97.7) | 917(98.5) | 0.12 |
| Donor hypertension (%) | 378(6.7) | 57(6.1) | 0.52 |
| Donor stroke (%) | 1326(23.5) | 213(22.9) | 0.70 |
| Serum creatinine at donation (mg/dL)(SD) | 1.0(0.8) | 1.0(0.5) | 0.39 |
| Cold ischemia time (h) (SD) | 12.5(5.4) | 12.3(5.7) | 0.41 |
| Delayed graft function (%) | 408(7.2) | 121(13.0) | <0.001 |
| HLA mismatches (SD) | 4.4(1.3) | 4.7(1.0) | <0.001 |
| Surgical technique (%) | 0.87 | ||
| Enteric drainage | 4627(81.8) | 764(82.1) | |
| Bladder drainage | 1027(18.2) | 167(17.9) | |
| Center volume (number/year) (%) | <0.001 | ||
| ≤10 | 2403(42.5) | 414(44.5) | |
| 11–20 | 1534(27.1) | 323(34.7) | |
| >20 | 1717(30.4) | 194(20.8) | |
| Induction agents (%) | <0.001 | ||
| ALG/OKT3 | 2643(46.8) | 427(50.7) | |
| IL-2R blockers | 1361(24.1) | 175(18.8) | |
| Alemtuzumab | 447(7.9) | 47(5.1) | |
| None | 1203(21.3) | 237(25.5) | |
| Calcineurin inhibitors(%) | 0.09 | ||
| Tacrolimus | 4911(86.9) | 833(89.5) | |
| Cyclosporine | 518(9.1) | 68(7.3) | |
| None | 225(4.0) | 30(3.2) | |
| Antiproliferatives(%) | 0.004 | ||
| MMF/MPA | 4681(82.8) | 813(87.3) | |
| mTor inhibitors | 637(11.3) | 82(8.8) | |
| None | 300(5.3) | 34(3.7) | |
| Steroids, yes(%) | 5431(96.1) | 899(96.6) | 0.46 |
| CMV donor/recipient pair(%) | <0.001 | ||
| Negative/negative | 1236(21.9) | 117(12.6) | |
| Negative/positive | 1744(30.9) | 191(20.5) | |
| Positive/positive | 1421(25.1) | 345(37.1) | |
| Positive/negative | 896(15.9) | 207(22.2) | |
| Unknown | 357(6.3) | 71(7.6) |
Induction therapy was used among 78.1% of SPK transplant patients with majority of patients receiving T-cell-depleting antibodies (46.6%). Alemtuzumab was used in 7.5% and interleukin 2 receptor (IL-2R) blockers in 23.3% of patients, respectively. Tacrolimus was the most commonly used calcineurin inhibitor (87.2%) and mycophenolate mofetil/mycophenolic sodium the predominant antiproliferative agent used (83.4%). In addition, 96.1% of SPK patients were receiving steroids at the time of discharge. From the initial transplant hospitalization, the combination of tacrolimus and mycophenolate mofetil/mycophenolic sodium accounted for 75% of maintenance regimen at the time of discharge.
The volume of SPK transplants performed by transplant centers across the United States varies from as few as 1 to as many as 59 per year during the study period (mean 16 and median 13). The enteric exocrine pancreas drainage was used in 82.0% of SPK transplants with no difference between AA and non-AA SPK patients. The use of enteric drainage surgical technique increased over the study period from 71.2% in 2000 to 87.0% in 2007 (p < 0.001).
Early pancreas graft failure (less than 90 days posttransplant) is common affecting approximately 10% of SPK transplant patients (31,32). The leading causes of early failure were thrombosis, leak and infection. A total of 585 pancreas (8.9%) and 149 kidney (2.3%) graft were lost within initial 90 days. The most common reason for graft loss was thrombosis, which accounted for 56.1% of pancreas loss and 35.6% of kidney loss, respectively. Multivariate logistical analyses showed that recipient race had no effect on the risk of early kidney or pancreas graft failure (OR 1.09, 95% CI 0.70, 1.69, p = 0.72 for kidney loss, and OR 0.92, 95% CI 0.71, 1.18, p = 0.44 for pancreas loss). Female recipients (OR 1.62, 95% CI 1.14, 2.31, p = 0.008), delayed kidney graft function (OR 15.75, 95% CI 10.96, 22.62, p < 0.001), older donor age (OR 1.02, 95% CI 1.00, 1.03, p = 0.05), African American donors (OR 1.66, 95% CI 1.08, 2.54, p = 0.02), hypertensive donors (OR 1.86, 95%CI 1.09, 3.15, p = 0.02) and bladder pancreas drainage (OR 1.59, 95% CI, 1.07, 2.37, p = 0.02) were associated with increased risk of early kidney graft failure. Female recipients (OR 1.35, 95% CI 1.13, 1.61, p < 0.001), elevated recipient BMI (OR 1.03, 95% CI 1.01, 1.05, p = 0.005), delayed kidney graft function (OR 1.69, 95% 1.27, 2.26, p < 0.001), older donor age (OR 1.02, 95% CI 1.01, 1.03, p < 0.001), donors with history of hypertension or died of stroke (OR 1.52, 95% CI 1.11, 2.08, p = 0.01, and OR 1.26, 95% CI 1.00, 1.57, p = 0.05, respectively) and lower transplant center SPK volume were each associated with increased risk of early pancreas graft loss (Table 2).
| Variables | Kidney graft loss | Pancreas graft loss | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Recipient race, AA | 1.09 | 0.70, 1.69 | 0.72 | 0.92 | 0.71, 1.18 | 0.44 |
| Recipient gender, male | 0.61 | 0.43, 0.88 | 0.007 | 0.74 | 0.62, 0.88 | <0.001 |
| Recipient BMI (kg/m2) | 1.01 | 0.98, 1.06 | 0.48 | 1.03 | 1.01, 1.05 | 0.005 |
| Cold ischemia time (h) | 0.97 | 0.94, 1.01 | 0.13 | 1.01 | 0.99, 1.03 | 0.07 |
| Delayed graft function | 15.75 | 10.96, 22.62 | <0.001 | 1.69 | 1.27, 2.26 | <0.001 |
| Donor age (years) | 1.02 | 1.00, 1.03 | 0.05 | 1.02 | 1.01, 1.03 | <0.001 |
| Donor race, AA | 1.66 | 1.08, 2.54 | 0.02 | 1.11 | 0.88, 1.42 | 0.38 |
| Donor hypertension | 1.86 | 1.09, 3.15 | 0.02 | 1.52 | 1.11, 2.08 | 0.01 |
| Donor stroke | 1.16 | 0.76, 1.75 | 0.50 | 1.26 | 1.00, 1.57 | 0.05 |
| Bladder pancreas drainage | 1.59 | 1.07, 2.37 | 0.02 | 0.84 | 0.66, 1.06 | 0.14 |
| Center volume (number/year) (%) | 0.40 | <0.001 | ||||
| ≤10 (42.8) | Ref | Ref | Ref | Ref | Ref | Ref |
| 11–20 (28.2) | 0.75 | 0.49, 1.15 | 0.19 | 0.82 | 0.66, 1.06 | 0.06 |
| >20 (29.0) | 0.97 | 0.63, 1.47 | 0.59 | 0.65 | 0.52, 0.80 | <0.001 |
During subsequent follow-up, 544 kidney and 513 pancreas grafts were lost. There was a significant difference in the overall outcome of SPK transplantation between AA and non-AA patients (Figure 1A, log-rank, p < 0.0001). This difference was mainly due to higher death-censored graft failure rate among AA SPK transplant patients for both kidney and pancreas (Figures 1B and C, log-rank, p < 0.0001 for both), but not for death (data not shown). This difference was evident early within the first posttransplant year, with actuarial 1, 3 and 5 years kidney and pancreas graft survivals significantly lower for AA SPK transplant patients compared to non-AA SPK transplant patients (Figure 2A and B). Conditional multivariate Cox regression analyses incorporating various confounding factors demonstrated that AA SPK transplant patients have significantly higher adjusted hazard for death-censored kidney graft failure (HR 1.38, 95% CI, 1.11, 1.73, p = 0.006) and for death-censored pancreas graft failure (HR 1.47, 95% CI, 1.17, 1.85, p = 0.001) (Table 3). When subgroup analyses were performed including only SPK patients with a diagnosis of type 1 diabetes the results remained unchanged (data not shown). Additional risk factors associated with late kidney graft failure were higher degree of HLA mismatch (HR 1.12, 95% CI 1.04, 1.20, p = 0.002), delayed graft function (HR 1.38, 95% CI 1.01, 1.88, p = 0.04), older donor age (HR 1.01, 95% CI 1.01, 1.02, p = 0.01), AA donors (HR 1.73, 95% CI 1.39, 2.16, p < 0.001) and more than 20 pack-years smoking history in donors (HR 1.31, 95% CI 1.07, 1.59, p = 0.008). On the other hand, only donor age (HR 1.01, 95% CI 1.01, 1.02, p = 0.001) and AA donors (HR 1.49, 95% CI 1.17, 1.88, p = 0.001) were associated with increased risk of late pancreas graft failure. Contrary to its negative impact on early pancreas graft survival, transplant center volume did not affect late pancreas graft outcome. Immunological complication, acute and chronic rejection, was the leading cause of late graft failure, accounting for 63.6% of kidney and 53.4% of pancreas graft loss, respectively. In particular, compared to non-AA SPK transplant patients, AA SPK transplant patients had a higher rate of acute and chronic rejection as the reported cause of kidney (80.3% vs. 58.9%, p < 0.001) and pancreas (65.7% vs. 50.1%, p = 0.004) failure and were twice more likely to lose their transplants due to rejection (OR 2.31, 95% CI 1.37, 3.87, p = 0.002 for kidney loss, and OR 1.86, 95% CI 1.18, 2.95, p = 0.008 for pancreas loss).
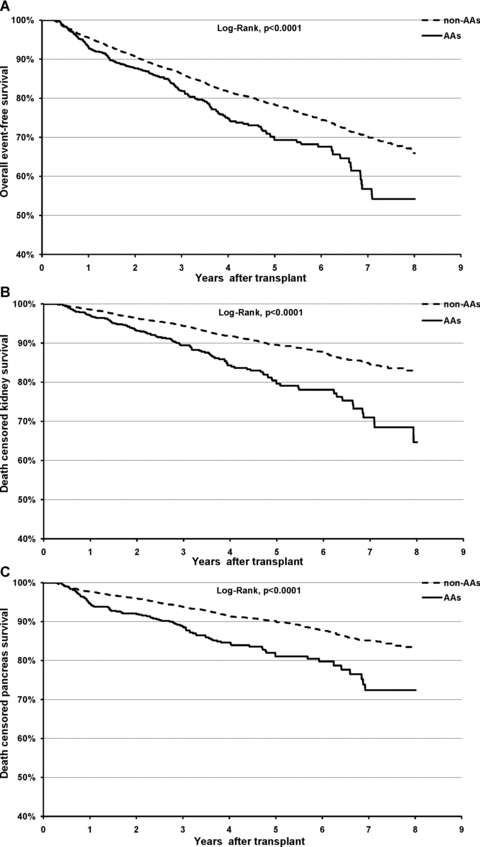
Kaplan–Meier survival analyses between AA and non-AA SPK transplant patients, (A) overall event free survival, (B) death censored kidney graft survival and (C) death censored pancreas graft survival.
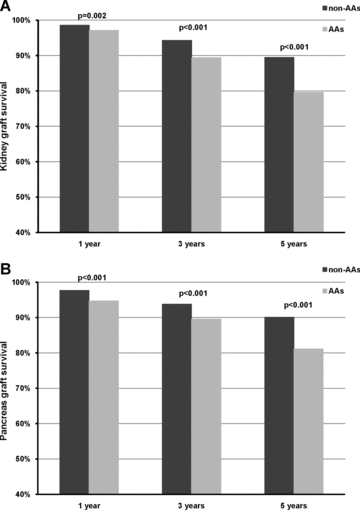
Actuarial graft survival at 1, 3 and 5 years between AA and non-AA SPK transplant patients, (A) kidney and (B) pancreas.
| Variables | Kidney graft loss | Pancreas graft loss | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Recipient race, AA (non-AA) | 1.38 | 1.10, 1.73 | 0.006 | 1.47 | 1.17, 1.85 | 0.001 |
| Recipient age, (years) | 0.97 | 0.96, 0.99 | <0.001 | 0.96 | 0.95, 0.97 | <0.001 |
| Duration of diabetes, (years) | 0.97 | 0.96, 0.98 | < 0.001 | 0.99 | 0.97, 1.01 | 0.10 |
| HLA mismatch, (zero) | 1.12 | 1.04, 1.20 | 0.002 | 0.98 | 0.91, 1.04 | 0.45 |
| Donor age, (years) | 1.01 | 1.01, 1.02 | 0.01 | 1.01 | 1.01, 1.02 | 0.001 |
| Donor race, AA (non-AA) | 1.73 | 1.39, 2.16 | < 0.001 | 1.49 | 1.17, 1.88 | 0.001 |
| Donor smoking, (>20 pack-years) | 1.31 | 1.07, 1.59 | 0.008 | 1.18 | 0.97, 1.45 | 0.10 |
| Delayed graft function, yes (no) | 1.38 | 1.01, 1.88 | 0.04 | 0.88 | 0.61, 1.27 | 0.50 |
The recipient race has no effect on patient survival (HR 1.05, 95% CI 0.80, 1.39, p = 0.72). Older recipient age (HR 1.02, 95% CI 1.01, 1.03, p = 0.001), longer duration of dialysis (HR 1.07, 95% CI 1.03, 1.11, p < 0.001), history of blood transfusion (HR 1.28, 95% CI 1.06, 1.56, p = 0.01), history of coronary artery disease (HR 1.42, 95% CI 1.09, 1.84, p = 0.01), AA donors (HR 1.52, 95% CI 1.19, 1.94, p < 0.001) and stroke as the cause of death in donors (HR 1.31, 95% CI 1.07, 1.61, p = 0.01) were associated with increased risk for death (Table 4). The surgical modality in exocrine pancreas anastomosis did not affect long-term pancreas and kidney graft survival (HR 1.01, 95% CI 0.82, 1.26, p = 0.90 for pancreas, and HR1.17, 95% CI 0.94, 1.45, p = 0.16 for kidney, respectively), but the bladder drainage was associated with increased risk for death (overall death and death with graft function) after adjusting for the effects of transplant era (HR 1.42, 95% 1.15, 1.75, p < 0.001).
| Variables | HR | 95% CI | p |
|---|---|---|---|
| Recipient race, AA (non-AA) | 1.05 | 0.80, 1.39 | 0.72 |
| Recipient age, (years) | 1.02 | 1.01, 1.03 | 0.001 |
| Duration of dialysis, (years) | 1.07 | 1.03, 1.11 | < 0.001 |
| History of blood transfusion, yes (no) | 1.28 | 1.06, 1.56 | 0.01 |
| History of coronary artery disease, yes (no) | 1.42 | 1.09, 1.84 | 0.01 |
| Donor stroke, yes (no) | 1.31 | 1.07, 1.61 | 0.01 |
| Bladder pancreas drainage, (enteric) | 1.42 | 1.15, 1.75 | 0.001 |
| Donor race, AA (non-AA) | 1.52 | 1.19, 1.94 | < 0.001 |
Discussion
This study showed that despite the absence of racial differences in the early outcome of SPK transplants, the long-term outcome of SPK transplants remains significantly inferior for AA SPK transplant patients in the recent era of modern immunosuppressive therapy.
The racial disparity in the outcome of chronic kidney disease in general, and of kidney transplantation in particular has been widely documented over the past 20 years (15,23,33). For example, AA kidney transplant patients have an inferior kidney graft survival compared to non-AA kidney transplant patients and such difference has been attributed to multiple factors including enhanced immune reactivity and lower socioeconomic status (23,33–35). With enhanced immunosuppression, the incidence of acute rejection has been significantly lowered in all categories of transplant patients (34,36,37). However, the reduction in acute rejection has not lead to the elimination of gap in the outcome between AA and non-AA kidney transplant patients (37).
Previous studies of the outcome of simultaneous pancreas and kidney transplantation have included small numbers of AA SPK transplant patients involved in the clinical trials or in single center experiences (26,27,38). Rogers et al. compared 33 AA to 63 non-AA SPK transplant patients and found significantly lower pancreas graft survival at 1, 3 and 5 years after transplantation (26). The authors also reported inferior late graft function and metabolic control in AA SPK transplant patients (28). Zhang et al. reported, on the other hand, comparable outcome in kidney and pancreas graft survival after 5 years follow-up between 36 AA and 55 non-AA SPK transplant patients (27). In studies comparing the outcome of SPK versus kidney transplant alone using large registry data, AA patients had higher risk for kidney graft failure compared to non-AA patients (21,39). This study is the first comprehensive analysis of racial effects on the outcome of simultaneous pancreas and kidney transplantation in the United States. The use of large sample size and the consideration of multiple important demographic and clinical variables allowed us to obtain a reliable assessment of racial disparity among SPK transplant patients. By analyzing separately the early and late outcome, we were able to delineate the predominant nature of graft failure that occurred during each of the two sequential periods. Whereas the improvement in surgical techniques and perioperative management has probably contributed to the equal early outcome between AA and non-AA SPK transplant patients, the use of more modern immunosuppression has failed to eliminate the gap in long-term graft survival, both kidney and pancreas, between AA and non-AA SPK transplant patients. Such difference in the late graft outcome was not related to the inclusion of a small number of type 2 diabetic SPK transplant patients as subgroup analyses including only type 1 diabetic SPK transplant patients yielded identical results. On the other hand, although we did not include early acute rejection episode as one of the covariates in the analyses due to incompleteness of such information in OPTN/SRTR data (more than 40% missing data at 6 and 12 months follow-up), the fact that AA-SPK transplant patients had more immune-mediated kidney and pancreas graft failure, consistent with findings from previous clinical trial (40), suggests that inadequate immunosuppression, either as result of an inherent biological phenomenon or consequence of poor adherence to prescribed immunosuppressive regimens, or other yet identified factors, could be responsible for the observed difference in the graft outcome.
Among many other factors that are involved in determining the graft and patient survival, the effect of donor race appears unique in that the use of organs from AA donors was associated with increased risk for inferior outcome. Several studies of large registry data analyses have previously documented similar findings for kidney graft survival in kidney as well as in SPK transplant patients (21,39,41). The mechanism for such observed difference remains unclear despite adjustment of multiple potential confounding factors. The statistically significant difference conferred by donor race, however, does not appear clinically relevant as the difference in actual kidney, pancreas graft loss and death was about 4.7%, 2.2% and 3.4% after 8 years follow-up, respectively. Most importantly, survival advantage of SPK transplantation over dialysis was clearly demonstrated regardless of donor source (2,42). Nevertheless, it is important to recognize such donor race effect so that future studies can be conducted to improve our understanding of such phenomenon.
A notable finding in this study is the impact of surgical modality in exocrine pancreas anastomosis on the outcome of SPK transplantation. Although bladder drainage did not appear to affect pancreas and kidney graft loss, it was associated with higher risk for patient death, overall and with graft function. Possible explanation for the increased risk for death could be related to patient selection bias, higher rate of infectious and metabolic complications associated with bladder drainage and/or the need for enteric conversion in many instances or other as yet poorly understood mechanisms (43).
There are several limitations related to our study. The possibility of confounding for variables that were not collected by OPTN/SRTR could bias our conclusion in one way or another. In addition, such study also is not able to take into consideration of factors that were important in determining the outcome and that are measured during transplant follow-up, such as the degree of blood pressure and glycemic control, compliance with medical care and follow-up, etc., between AA and non-AA SPK transplant patients. We were not able, for example, to study pancreas endocrine function as data such as fasting glycemia, glycated hemoglobin or use of insulin, etc., was not routinely collected by OPTN/SRTR. As always, association should not be inferred as causality, rather as suggestion that additional studies are needed in the field.
In conclusion, despite the improvement in surgical techniques and perioperative care, and the use of potent immunosuppressive regimens in the most recent era, AA SPK patients continue to have inferior kidney and pancreas graft survival. Additional studies are needed to improve our understanding and to reduce such racial disparity on the outcome.
Acknowledgments
The data reported in this paper have been supplied by the Arbor Research Collaborative for Health (Arbor Research) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
Disclosure
No disclosures.




