The Role of Innate Immunity in Healthy Aging Through Antimicrobial Peptides
Funding: This work was supported by Korea Food Research Institute (E0210101).
ABSTRACT
In a super-aging society, the increase in the elderly population is closely tied to a rise in infectious diseases due to factors such as weakened immune systems and decreased vaccine efficacy in older adults. Various opportunistic pathogens commonly encountered in everyday life can cause infections and diseases when an individual's immune defence is weakened due to aging. These factors underscore the importance of preventive measures against pathogenic infections and the aging of immune systems in the elderly. The immune response acts as the defence mechanism against foreign substances, including pathogens and abnormal cells. Specifically, the innate immune response is the body's first line of defence, offering a rapid and nonspecific response to pathogens. Advances in the study of innate immunity's regulatory functions in both immune and non-immune cells have broadened our understanding of innate immune responses' impact on health. This includes a focus on immune effectors like antimicrobial peptides (AMPs) and their potential implications for health and longevity. This review summarises the common principles and evolutionary adaptations of innate immunity via AMPs, in mammals and invertebrates. Especially, this review discusses the conserved mechanisms regulating AMP production and the role of AMPs in modulating aging and diseases from invertebrate to human. Therefore, it highlights the potential role of innate immunity in addressing aging through AMPs.
1 Introduction
Aging is a complex biological process marked by the gradual decline of physiological functions, which increases susceptibility to disease and mortality. Understanding the underlying mechanisms of aging is essential for developing strategies to promote healthy aging and prevent age-related diseases. Recent studies have identified several key factors in the aging process, including genomic instability, telomere shortening, epigenetic changes, protein homeostasis disruption, altered nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell depletion, and changes in intercellular communication [1]. Among these, chronic low-grade immune activity, often called ‘immunosenesence’ has been recognised as a significant contributor to age-related decline [2]. Immunosenescence is characterised by a progressive deterioration of the immune system with age, leading to increased susceptibility to infections, cancer, and autoimmune diseases [3, 4]. This phenomenon involves complex changes in both innate and adaptive immunity, including decreased production of naive T cells, accumulation of memory T cells, reduced B cell diversity, and altered function of innate immune cells such as neutrophils and macrophages. Therefore, modulating immune activity has emerged as a promising approach for preventing or delaying age-related diseases and promoting healthy aging in elderly. Furthermore, understanding the intricate relationships between immune system aging and other hallmarks of aging is providing valuable insights into the overall aging process.
The immune response is the defence mechanism against foreign substances, pathogens, or abnormal cells. The immune response in higher organisms is regulated by a variety of immune cells that are mostly produced and found in the lymphoid organs, including bone marrow [5]. The immune response by immune cells can be broadly categorised into two types. An adaptive immune response involves the activation of lymphocytes (B cells and T cells) and the production of antibodies [6]. The adaptive immune response has a memory component, allowing the body to recognise and respond more efficiently to previously encountered pathogens. The innate immune response is the body's first line of defence [7]. It provides a rapid, but unlike an adaptive immune response, non-specific response to pathogens. Key components of the innate immune response include physical barriers (e.g., skin and mucous membrane), natural killer cells, phagocytic cells, and various proteins and enzymes (like complement proteins and interferons). Various non-immune cells, including epithelial cells, endothelial cells, and mesenchymal cells also exhibit innate immune responses [8]. Advances in research into the regulatory functions of innate immunity have expanded the understanding of how innate immune responses affects health and longevity [9]. The reduced production of immune cells and diminished response to antigens lead to increased susceptibility to infections in elderly [10, 11]. Thus, the elderly individuals are particularly vulnerable to opportunistic pathogens [12], include a wide range of microorganisms, including bacteria, viruses, and fungi that cause disease primarily when the host's immune defences are compromised.
Antimicrobial peptides (AMPs) represent significant components of innate immune responses, contributing to the defence against pathogens in both innate immune cells and non-immune cells [13]. AMPs are short proteins, typically comprising 12–50 amino acids. They are present in a wide range of organisms, from microorganisms to humans, and are produced by various cells and tissues [14]. AMPs are found on mucosal surfaces (such as in the gut, respiratory tract, and skin), where they form a protective barrier against microbial invasion [15, 16]. AMPs are generally cationic and amphipathic [17]. While animal cell membranes are usually neutral or amphipathic, bacterial cell membranes tend to have a more negative polarity. This difference in polarity allows antimicrobial peptides to bind more strongly to and disrupt bacterial cell membranes. Bacterial cell membranes are composed of phospholipid bilayers with embedded proteins. The cell wall, primarily made of peptidoglycan, differs between Gram-positive and Gram-negative bacteria. Gram-positive bacteria have a thick peptidoglycan layer with teichoic acids, while Gram-negative bacteria have a thin peptidoglycan layer and an outer membrane containing lipopolysaccharides. AMPs exhibit diverse mechanisms to disrupt bacterial cell walls and membranes. For cell walls, AMPs inhibit peptidoglycan synthesis, directly degrade peptidoglycan structures, and in Gram-negative bacteria, bind to and disrupt lipopolysaccharides (LPS) in the outer membrane [18]. Regarding cell membranes, AMPs form ion channels, increase membrane permeability, and neutralise membrane charges [19]. These actions lead to membrane lysis and cellular content leakage. AMPs could penetrate microbial cells and disrupt internal processes, such as inhibiting enzyme activity or interfering with DNA and RNA synthesis [20]. Moreover, AMPs could modulate immune responses. For instance, AMPs can enhance the release of IL-8, also known as CXCL8, which is a pro-inflammatory chemokine [21] that attracts immune cells like neutrophils and macrophages to infection sites, thereby enhancing phagocytosis and other defence mechanisms. They also help limit the extent of inflammation, thereby preventing excessive inflammation and tissue damage [22, 23]. AMPs have recently attracted attention in aging research due to their potential role in immunity. This review highlights the role of AMPs as key effectors of the innate immune response in animal kingdoms. In addition, this review explores the impact of AMPs on longevity regulation, and disease prevention, thereby highlighting the roles of innate immunity in healthy aging.
2 The Innate Immune Responses in Mammals With AMPs
The mammalian innate immune system employs a sophisticated array of defence mechanisms to combat invading pathogens. At the forefront of this defence is phagocytosis, a process by which specialised cells engulf and destroy foreign particles. During this process, phagocytes deploy a variety of antimicrobial agents, including antimicrobial peptides (AMPs), reactive oxygen species (ROS), reactive nitrogen species (RNS), enzymes, and cytokines, all working in concert to eliminate engulfed pathogens. AMPs play a particularly crucial role in pathogen removal. These small, cationic peptides possess broad-spectrum antimicrobial activity and can directly attack and eliminate pathogens by disrupting microbial membranes or interfering with essential cellular processes. Both innate immune cells and non-immune cells contribute to pathogen elimination through the production and release of these antimicrobial agents.
2.1 Immune Cells
Innate immune cells secrete AMPs to eliminate pathogens. Neutrophils produce defensins, cysteine-rich, cationic peptides that kill a broad range of microorganisms including bacteria, yeast, and viruses [24], including α-defensins consisting of human neutrophil peptide (HNP) 1-4 and human defence (HD) 5-6, and β-defensins (HBD1-4). These AMPs are also found in macrophages, lymphocytes, and NK cells [24, 25]. α-defensins insert into the pathogen's cell membrane, forming pores that lead to the pathogen's death. β-defensins have antimicrobial properties as well as immunomodulatory roles, such as recruiting immune cells to the site of infection [26]. Neutrophils and NK cells produce cathelicidins (LL-37 in humans and mCRAMP in mice) [27]. Cathelicidins recruit immune cells to sites of microbial invasion [28] and promote neutrophil extracellular traps (NETs) formation [29], stabilising neutrophil-derived DNA or NETs that resist degradation by bacterial nuclease. C-type lectins function in innate or adaptive antimicrobial immune responses [30]. The binding of c-lectins to pathogens enhances phagocytosis, the process by which macrophages and neutrophils engulf and internalise pathogens [30]. C-lectins can also interact with other components of the immune system, such as complement proteins, to enhance the opsonization and clearance of pathogens [30].
2.2 Non-immune Cells
Epithelial cells across various tissues serve as critical producers of AMPs, each with unique defensive strategies tailored to their specific anatomical environment. In the skin, keratinocytes synthesise β-defensins and cathelicidin (LL-37) [31], which provide a multi-layered defence mechanism against bacterial, fungal, and viral threats by disrupting microbial membranes and modulating local immune responses. The gastrointestinal tract represents a particularly complex ecosystem of AMP production. Paneth cells in the small intestinal crypts of Lieberkühn release α-defensins (cryptdins) [32] that are essential for maintaining gut microbiota balance. Simultaneously, goblet cells produce mucins [33] that physically trap and neutralise potential pathogens, creating an additional protective barrier. Mucosal epithelial cells further contribute to this defence by secreting various defensins and C-type lectins. Specialised AMP production extends to other critical body systems. Urothelial cells in the urinary tract synthesise targeted defensins and cathelicidin to combat uropathogens [34], while respiratory tract epithelial cells generate AMPs that reinforce the mucosal immune barrier. Of particular interest are RegIII proteins, C-type lectins expressed in intestinal epithelial cells [35] that specifically bind to peptidoglycan structures in Gram-positive bacterial cell walls, playing a crucial role in maintaining microbiome homeostasis [35]. This diverse and sophisticated AMP production system demonstrates the intricate mechanisms by which epithelial cells contribute to the body's first line of defence, highlighting the specificity and adaptability of the innate immune response across different tissue environments.
3 The Innate Immune Responses in Invertebrates ( C. elegans and Drosophila) With AMPs
In C. elegans , unlike the adaptive immune system found in mammals, it lacks typical immune cells such as T cells, B cells, and macrophages. Instead, this nematode employs various cell types, including epithelial cells, neurons, and intestinal cells, to mount defences against pathogens [36]. The absence of specialised immune cells in C. elegans highlights the diverse evolutionary strategies that organisms have developed to defend against pathogens. This simplified yet functional immune system makes C. elegans an valuable model for studying fundamental aspects of innate immunity and host-pathogen interactions [37].
In C. elegans , innate immunity primarily focuses on regulating pathogen clearance by the effectors, AMPs, including caenopores (or saposin-like proteins), defensin-like peptides, caenacins (CNCs), C-type lectins, and neuropeptide-like proteins (NLPs) [38]. Caenopores are composed of a secretory signal peptide followed by saposin (lysosomal protein)-like domain [39] and are structurally and functionally similar to pore-forming peptides called ‘amoebapores’ [40]. The most of spp gene (saposin-like protein family) is expressed in the intestine of C. elegans [41]. A direct role of SPP in the innate immune response of C. elegans was first reported when the antimicrobial effect of recombinant SPP-1 was observed in E. coli [42]. In addition, SPP-5 was shown to display pore-forming activity and kill bacteria [39]. The families of CNCs and NLPs are small proteins that consist of 51–82 amino acids with signal peptides at their N-terminus. They are highly induced by fungus infection by Drechmeria coniospora [43-45]. Several nlp and cnc genes are expressed in the epidermis of the worm, where the fungus typically attacks. The chemically synthesised NLP-31 protein inhibited fungal growth in infected worms [43], and the overexpression of the complete nlp-29 or cnc-2 gene clusters renders worms more resistant to Drechmeria coniospora infection [44, 45]. Other AMP, the ASABF (Ascaris suum antibacterial factor)-type antimicrobial peptide, ABF-2, exhibited microbicidal activity against Gram-positive and Gram-negative bacteria [46]. ASABF-type peptides bind to the cell membranes of bacteria and fungi due to their positive charge, which interacts with the negatively charged components of microbial membranes, such as phospholipids and lipopolysaccharides. Once bound, these peptides insert themselves into the lipid bilayer of the microbial membrane and form pores or channels within the membrane, like defensins [47]. Like vertebrate, C-type lectin-like domain (CTLD) proteins, clec-41 and clec-42, in C. elegans induced by pathogen infection and defence against pathogens [48]. The inactivation of clec-41 and clec-42 by RNAi enhanced susceptibility to Gram-positive pathogen Bacillus thuringiensis MYBt18247 (Bt247) [48]. Therefore, in C. elegans , as in many other organisms, AMPs are crucial components of the innate immune system and play an important role that helping the organism defend against a wide range of pathogens, ensuring survival and maintaining homeostasis.
In drosophila, AMPs also play a crucial role in the innate immune system, serving as a primary defence mechanism against various pathogens. The fruit fly's immune system has evolved to distinguish between different classes of microorganisms and mount appropriate responses [49]. Drosophila produces several families of AMPs, including diptericins, cecropins, drosocin, attacins, defensins, metchnikowin, and drosomycin. Each of these AMPs exhibits specific activities against different types of pathogens [50]. For instance, diptericins and attacins are primarily effective against Gram-negative bacteria, while defensins target Gram-positive bacteria. Drosomycin, on the other hand, shows particular efficacy against filamentous fungi [50]. Drosophila AMPs typically function by disrupting microbial membranes, leading to cell lysis. Some AMPs may also have intracellular targets. From an evolutionary perspective, AMP genes in Drosophila show evidence of rapid evolution, likely due to host-pathogen co-evolution. This is particularly evident in the varying number of AMP gene copies among different Drosophila species [51]. Recent research has also suggested roles for AMPs beyond pathogen defence, including potential involvement in aging processes and regulation of the gut microbiome [52].
4 Conserved Synthesis Pathways of AMPs in Animals
In this review, the functions of AMPs as common effector in innate immune responses from invertebrates to human were discussed (Figure 1). Here, the conserved molecular factors that regulate AMP production across the animal kingdom are also discussed.
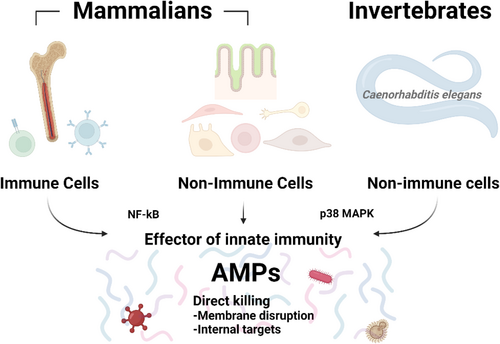
Toll-like Receptors (TLRs) and NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) pathway responds to infection and stress. TLRs are a type of PRR that recognise PAMPs. Activation of TLRs on immune cells initiates signalling cascades that often lead to the activation of NF-κB. Upon activation, NF-κB translocated to the nucleus and induces the expression of various immune-related genes, including those for AMPs. In oral epithelium, NF-κB mediated the induction of human β-defensin 2 (hBD2) expression via TLR2 and NOD1/2 ligands [53]. In primary human airway epithelium, NF-κB induced hBD2 by IL-17 [54]. In Drosophila, NF-κB factors, Dorsal and DIF are mainly associated with the Toll pathway and Relish is associated with the immune deficiency (IMD) pathway [55], play an important role in AMP gene expression [55].
The other molecule, p38 mitogen-activated protein kinases (MAPK), modulates inflammation and immune responses, including the production of AMPs. In humans, the alveolar epithelium serves as an effective barrier against inhaled microorganisms and is involved in initiating the innate host defence. Legionella pneumophila , a causative agent of pneumonia in humans, induces the production of human β-defensin-2 (hBD-2), an antimicrobial peptide. However, L. pneumophila -induced hBD-2 release is significantly reduced by treatment with SB-202190, an inhibitor of p38 MAPK, in A549 cells [56]. In respiratory epithelial cells HBE16 and A549, astragalus polysaccharides (APS), a components in Astragalus membranaceus induced human cathelicidin antimicrobial peptide LL-37 in both mRNA and protein levels [57]. APS increased the level of phosphorylation of p38 MAPK, and APS-induced LL-37 synthesis abolished by p38 MAPK inhibition [57]. Furthermore, in A549 epithelial cells, LL-37 was induced by infection with Mycobacterium bovis bacillus Calmette-Guérin (BCG), and inhibition of the p38 MAPK signalling pathways reduced M. bovis BCG-mediated LL-37 mRNA expression [58]. Thus, these results indicate that p38 MAPK signalling pathways play a critical role in the regulation of inducible AMPs in human cells. In mouse osteoblasts, increased production of β-defensin-14 (MBD-14) by Staphylococcus aureus supernatant treatment was also significantly inhibited by SB203580 (a p38 MAPK inhibitor), which significantly inhibited phosphorylation and mRNA expression of p38 MAPK and simultaneously decreased mouse β-defensin-14 (MBD-14) release [59]. The p38 MAPK pathway plays a crucial role in regulating the production and expression of AMPs in Drosophila, contributing significantly to the innate immune response. This pathway is activated in response to pathogen infection, triggering a cascade of events that ultimately leads to the transcription of AMP genes [60]. Furthermore, the p38 MAPK pathway doesn't operate in isolation but interacts with other immune signalling pathways in Drosophila. Of particular importance is its interaction with the Immune Deficiency (IMD) pathway. This crosstalk between p38 MAPK and IMD pathways leads to a synergistic effect, amplifying the production of AMPs [60]. This cooperative action ensures a robust and efficient immune response against invading pathogens. In C. elegans , PMK-1(p38 MAPK homologue gene) regulates the upregulation of antimicrobial genes, C-type lectins, ShK toxins, and CUB-like genes, upon Pseudomonas aeruginosa infection, and pmk-1 loss-of-function mutants are highly sensitive to the pathogen [61]. The p38 MAPK pathway can modulate the activity of NF-κB that influence NF-κB's transcriptional activity [62, 63]. In addition, both pathways converge on similar sets of genes involved in inflammation and innate immunity, including AMP genes [64-66]. The interplay between NF-κB and p38 MAPK exemplifies the complex network of signalling interactions in the immune response, allowing for the fine-tuning needed to efficiently respond to pathogenic challenges by producing AMPs (Figure 1).
5 Immunity and Aging
Aging profoundly affects the immune system, affecting both innate and adaptive immunity in multifaceted ways. This age-related decline in immune function, known as immunosenescence, is characterised by reduced effectiveness of innate immune cells, thymic involution, and alterations in T and B cell populations [2]. For instance, aging is associated with diminished phagocytic capacity of neutrophils and macrophages, decreased cytotoxicity of natural killer cells, and impaired antigen presentation by dendritic cells [67]. Crucially, this age-related immune dysfunction not only results from the aging process but also contributes to its acceleration. The decline in immune function leads to a state of chronic low-grade inflammation. This inflammatory state, driven by factors such as the accumulation of senescent cells, mitochondrial dysfunction, and persistent viral infections, further exacerbates the aging process across multiple organ systems [68]. Moreover, the compromised immune function in older adults increases susceptibility to infections, cancer, and inflammatory diseases, which in turn can accelerate cellular senescence and tissue damage, further promoting the aging process. Therefore, immune aging and overall organismal aging creates a feedback loop where mutually reinforce each other, and it underscores the critical importance of maintaining immune health as a strategy to promote healthy aging and longevity.
5.1 The Role of AMPs in Longevity
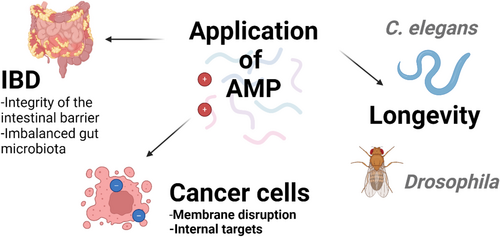
This review highlighting that AMPs are a crucial component of innate immunity that evolved over time. AMPs have recently attracted attention in aging research due to their potential role in immunity. New evidence suggests that AMPs may have broader functions, including roles in inflammation, tissue repair, and possibly in the aging process itself [18]. The connection between AMPs and aging is intricate and not yet fully elucidated. Various small animal models have been utilised in research to understand the mechanisms through which AMPs might influence longevity. Using fruit flies, the role of AMPs on organismal lifespan has been elucidated. In fruit flies, AMPs could be divided into families based on the bacteria they target, defensins (gram-positive bacteria), cecropins, drosocin, attacins, diptericin, and MPAC (gram-negative bacteria) or drosomycin and mechinicowin (fungi) [50]. In flies lacking the seven AMP gene families showed a shortened lifespan [69]. Instead, in germ-free conditions, the lifespan of the mutant flies has increased again [69]. The authors suggested that the reduced lifespan in AMP deletion mutant flies to an imbalance in the microbiota, and AMPs may prevent imbalances in the gut microbiota during aging and affect lifespan [69]. In another study, activation of a single AMP increased lifespan in Drosophila [52]. In flies with AMP activation showed a delayed loss of gut barrier integrity and less intestinal regenerative processes. Furthermore, induction of Drosocin in the intestine protected from infection by Pseudomonas entomophila [52]. In C. elegans , mutations in the daf-2 gene, which encodes an insulin/IGF-1-like receptor, have been widely studied for their effects on longevity and stress resistance [70]. This increased longevity is associated with a variety of physiological changes, including enhanced resistance to pathogens [71]. One notable characteristic of daf-2 mutant strains is the upregulation of genes involved in stress response and immune function, including the increased expression of AMPs. This heightened immune response in daf-2 mutants contributes to their enhanced survival and plays a significant role in extending their lifespan [72]. Overall, the daf-2 mutant strains of C. elegans serve as an important model for studying the genetic and molecular mechanisms of aging, AMPs, immune response, and longevity.
Some research has shown that AMP expression increases with age in various organisms, including fruit flies and humans [73]. This age-related increase in AMP expression has led researchers to consider their potential contribution to chronic inflammation and age-related diseases. However, the precise mechanisms by which AMPs might influence aging, and whether their increased expression is a cause or result of aging, remain to be determined. Given these challenges and the potential importance of AMPs in aging, there is a pressing need for further research in this area.
5.2 The Role of AMPs in Diseases Treatment
AMPs play a crucial role in maintaining gut homeostasis and act as essential regulators in preserving a balanced microbial ecosystem [69], and this impacts on lifespan in animals is through maintaining microbiome balance. By fine-tuning the composition of the gut microbiome, AMPs may enhance immune function, mitigate inflammation, and improve metabolic health in older adults [74]. Furthermore, the ability of AMPs to modulate the immune response in the gut may help counteract the chronic low-grade inflammation often observed in older adults, and promoting healthy aging through their modulation of the gut microbiome. For example, AMPs do have therapeutic potential for diseases, including inflammatory bowel disease (IBD). Inflammatory bowel disease (IBD) encompasses chronic inflammatory conditions of the gastrointestinal tract, primarily Crohn's disease and ulcerative colitis. As individuals age, the diversity of their gut microbiota typically diminishes, potentially leading to various health complications. AMPs help maintain the balance of gut that is crucial in IBD, where dysbiosis (imbalanced gut microbiota) is often observed [75]. In addition, in IBD, the intestinal barrier is often compromised, and AMPs also contribute to the integrity of the intestinal barrier [76]. By strengthening the barrier, AMPs can prevent the translocation of bacteria and other antigens that trigger inflammatory responses. Furthermore, AMPs such as calprotectin [77] and lactoferrin [78] are useful biomarkers for IBD patients. Both calprotectin and lactoferrin have been shown to have an overall diagnostic accuracy of 80%–100% in IBD [79].
In addition, AMP may have anticancer activity. This is a mechanism by which AMP interacts with the negatively charged cell membrane of cancer cells and destroys the membrane. The proportion of phosphatidylserine (negative charge) increases on the surface of cancer cells, which increases the possibility that cationic hydrophilic peptides can selectively act on the surface of cancer cells compared to normal cells [80]. AMP can penetrate cancer cells and induce cell death by interfering with mitochondrial function or modulating intracellular signalling pathways [81].
By manipulating AMP gene expression and observing the effects on health and lifespan, it could illuminate insights into potential therapeutic uses of AMPs for promoting healthy aging and combating age-related diseases (Figure 2). However, it has also been found that over-activation of the AMP gene could shorten the lifespan of fruit flies [82], or antimicrobial peptides and its neuronal receptors signalling pathway could cause the dendritic spine degeneration associated with age or infection [83]. Furthermore, there is still a technical limitation that AMP reacts specifically only to cancer cells. Therefore, further research is essential to understand the role of AMPs and elucidate their application to aging or disease development.
5.3 Molecular Pathways That Regulate Both AMPs Production and Aging Process
The relationship between AMP regulation and aging control is intricate and multifaceted, involving several interconnected signalling pathways. The FOXO transcription factors, which play crucial roles in both AMP regulation and lifespan determination, exemplify the complex interplay between immunity and longevity [84]. FOXO activity is modulated by insulin/IGF-1 signalling, a well-established pathway in aging research in mammals, including human [85]. This same pathway influences AMP production, suggesting a mechanistic link between metabolic regulation, immune function, and aging. Moreover, FOXO factors interact with other longevity-associated pathways, such as the AMPK and mTOR signalling networks [86]. These interactions create a complex regulatory web that balances energy metabolism, stress responses, and immune function—all of which are critical aspects of the aging process. This connection may represent an adaptive strategy to enhance survival in the face of age-related immunosenescence. The relationship between AMPs and aging appears to be a balance between adequate immune defence and the prevention of excessive inflammatory responses. Proper regulation of AMP levels is crucial for maintaining tissue integrity and promoting healthy aging.
6 Conclusion
The mortality rate from infectious diseases in the elderly is increasing due to immunosenescence. However, there are currently no drugs that can prevent or control immune cell senescence, and research in this area is still in its infancy. A holistic approach is needed to more effectively address immune senescence in elderly, and research and strategies aimed at improving overall infection defence in the context of aging are necessary. This review highlights the importance of the innate immune response as a strategy for comprehensive infection defence and the need for intensive research on the role of AMPs in the innate immune response.
In this review, we examined the functions and production mechanisms of AMPs across various species. The roles of AMPs were individually discussed in several model organisms, including humans, mice, C. elegans , and fruit flies. Our analysis confirmed that AMPs serve as crucial components of the innate immune system in diverse species, and the fundamental function of AMPs—microbial defence—is consistently observed across different species. Notably, we discovered that certain elements of the signalling pathways that induce AMP production are similarly conserved among multiple species. It suggests that research on controlling the production mechanism of appropriate AMPs or developing functional synthetic AMPs may open up future possibilities for the decline in immune system function that extends healthy aging. In addition, targeting these conserved pathways for drug development offers the potential for broader applicability across species, thus reducing development costs and timelines. However, it is important to recognise that significant differences exist among species in terms of specific AMP types, production sites, and regulatory mechanisms. Although this review described AMP functions individually for each species, future research should focus on elucidating these commonalities and differences more clearly, and directly comparing AMP functions and production mechanisms across diverse species.
AMPs offer promising clinical applications, particularly for elderly populations facing increased susceptibility to infections and age-related diseases. These naturally occurring molecules demonstrate potent antimicrobial effects against a wide range of pathogens, including those commonly affecting older adults. For instance, the human cathelicidin LL-37 has shown efficacy against respiratory pathogens, suggesting potential therapeutic use in preventing and treating pneumonia in the elderly [87, 88]. Beyond their direct antimicrobial action, AMPs like cathelicidins can modulate immune responses, potentially enhancing vaccine efficacy in older adults whose immune systems often respond poorly to traditional vaccines [89, 90]. Furthermore, recent research has uncovered neuroprotective properties of certain AMPs in models of Alzheimer's disease, opening new avenues for treating age-related neurodegenerative disorders [91]. This knowledge will not only enhance our understanding of AMP biology but also guide the development of more targeted and effective AMP-based therapies. However, research on innate immune regulation by AMPs and the resulting extension of healthy lifespan still requires significantly more time and research effort in many areas. Although hypotheses about potential parallel mechanisms in humans can be generated by examining the shared biological phenomena in animal models, it is acknowledged that further research specific to human systems is necessitated to confirm these possibilities. In addition, despite their potential, translating AMP research into clinical applications faces several challenges. Issues of stability, delivery, and target specificity need to be addressed to maximise therapeutic efficacy while minimising side effects. Furthermore, although their roles in protecting against pathogens, managing chronic inflammation, and maintaining gut health suggest possible indirect effects on overall health and aging processes, but the direct evidence linking AMPs to human longevity is limited. Thus, the more comprehensive human studies needed to establish definitive connections between AMPs and health span.
In conclusion, a holistic approach that considers the interplay between various innate immune effectors, including AMPs, is crucial for comprehensively understanding age-related changes in the immune system. This broad perspective not only provides deeper insights into the innate immune system but also plays a vital role in maintaining immune health during aging and realising healthy aging. By enhancing our understanding of the functions and regulation of AMPs and other innate immune components, it could help to develop effective strategies to prevent or delay immune dysfunction associated with aging. This approach may ultimately contribute to improving individual quality of life and promoting healthy aging.
Author Contributions
Yejin Cho: writing and editing. Jeong-Hoon Hahm: conceptualization, supervision, writing, funding acquisition.
Acknowledgements
The authors declare financial support was received for the research, authorship, and publication of this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




