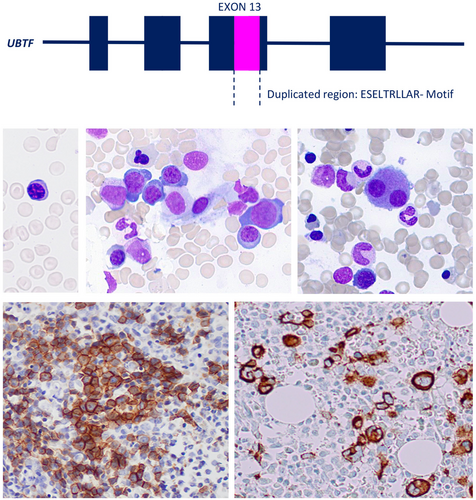
Diagnostic features in paediatric MDS-EB with UBTF-internal tandem duplication: defining a unique subgroup
Abstract
Aim
Tandem-duplications of the UBTF gene (UBTF-TDs) have recently been identified as a new genetic driver in young individuals with acute myeloid leukaemia (AML) and myelodysplastic syndrome (MDS). Disease in these newly defined subgroups is characterized by poor response to standard intensive chemotherapy and inferior survival of the affected patients. However, a thorough analysis of bone marrow histomorphology of UBTF-mutated neoplasia has not been undertaken thus far.
Methods and results
In this retrospective study, we investigated the characteristic histopathological features of a cohort comprising 14 paediatric MDS patients with an excess of blasts (MDS-EB) and UBTF-TD. Bone marrow biopsies from these patients revealed hypercellularity and severe dysplasia across all three haematopoietic lineages. In particular, a marked hyperplastic megakaryopoiesis characterized by the presence of frequent micromegakaryocytes and a high number of monolobulated cells forming small clusters was observed. Additionally, erythropoiesis was left-shifted, with numerous blastoid precursors. The granulopoietic precursors displayed prominent UBTF-positive nucleoli.
Conclusion
The unique combination of these histomorphological features strongly suggests a possible UBTF aberration. It will allow initiating the appropriate genetic testing to confirm the presence of UBTF-TD and identify potential additional genetic alterations. Such molecular profiling will not only contribute to a better understanding of the disease mechanism, but also facilitate more rational treatment approaches for these high-risk paediatric MDS patients.
Graphical Abstract
Distinctive histopathological features of paediatric MDS with UBTF-TD include hyperplastic myelopoiesis with severe atypia, frequent micromegakaryocytes, and immature erythropoiesis with circulating normoblasts. These findings may aid in the early diagnosis and guide targeted molecular testing and treatment strategies, which are critical to improving patient outcomes.
Introduction
The protein of the upstream binding transcription factor (UBTF) plays a key role in ribosomal DNA and RNA transcription. Recent molecular studies on paediatric acute myeloid leukaemia (AML) led to the identification of tandem duplications (TDs) in exon 13 of the UBTF gene in 9% of relapsed AML1 and 1.2%–4% of primary paediatric AML.2, 3 The presence of UBTF-TD was mutually exclusive with other genetically defined AML subgroups and associated with a particular genetic pattern with frequent accompanying FLT3-ITD or WT1 mutations, normal karyotype or trisomy 8. Subsequent studies identified UBTF-TD in 3% of adult AML patients below the age of 65 years, and a strong positive correlation with younger age.4, 5 Like in paediatric AML, the presence of UBTF-TD in young adults was a prognostic factor for inferior outcome, with lower complete remission rates and inferior survival independent of the presence of associated mutations.
Interestingly, the bone marrow morphology of UBTF-mutated AML frequently displayed myelodysplastic changes, and in some cases was compatible with the diagnosis of AML-M6 according to the FAB classification.4 A most recent observation described UBTF-TD as a driver mutation in a substantial portion of children with myelodysplastic syndrome (MDS).6 The recognition of the histopathological pattern of these cases will support the identification of patients in need for UBTF analysis, and thus greatly improve therapeutic decision-making. Therefore, we investigated the histopathology of 14 paediatric patients with de novo MDS with excess blasts (MDS-EB)7, 8 and UBTF-TD.
Materials and Methods
Study design and patient selection
Patients were enrolled in the prospective studies 98 or 2006 (www.clinicaltrials.gov; #NCT00047268, #NCT00662090) of the European Working Group of MDS in Childhood (EWOG-MDS). Here we present 14 patients (Table S1) enrolled in EWOG-MDS and consecutively diagnosed with de novo MDS and UBTF-TD in Germany (n = 13) or Switzerland (n = 1).
Genetic studies
The detection of UBTF-TD was performed through polymerase chain reaction (PCR) followed by fragment length analysis.5 Additional mutations and gene rearrangements were analysed using panel sequencing with an Archer VariantPlex Myeloid panel. Standard methods were employed for cytogenetic studies.
Bone marrow aspirate, biopsy: immunohistochemistry and immunofluorescence
Bone marrow core biopsies and aspirates were evaluated independently by three hematopathologists (MR; SS-F, CG). Haematoxylin and eosin (H&E) staining, reticulin staining, and naphtol AS-D chloroacetate esterase staining were completed on all slides. Immunostaining was performed for CD42b (SP219; Abcam, Cambridge, UK), E-cadherin (EP700y; Zytomed Systems, Berlin, Germany), MPO (Agilent, Santa Clara, CA, USA), TdT (SEN28; Leica, Deer Park, IL, USA), CD20 (L26; Agilent), CD3(SP7; Zytomed Systems), CD123 (AbcamK), CD34 (QBEnd/10; Cell Marque, Rocklin, CA, USA) using an autostainer (Benchmark Ultra; Roche Diagnostics, Germany) following the manufacturer's instructions. For double immunofluorescence, the following antibodies were used: MPO (Agilent) and UBTF (Atlas Antibodies, Bromma, Sweden). An Opal Multiplex 7-colour manual kit (Akoya Bioscience, Boston, MA, USA) was utilized for detection, and the slides were scanned by the Vectra Polaris imaging system (Akoya Bioscience) and analysed using HALO (Indica Labs, Albuquerque, NM, USA).
The histological and cytomorphological evaluation included bone marrow cellularity and the erythroid/myeloid (EP:MP) ratio. Reticulin fibrosis was graded as previously published.9 Erythropoiesis was assessed for abnormal localisation (defined as erythroid cell clusters adjacent to bone trabecular), proerythroblast clusters, and immaturity. Left-shift of granulopoiesis was assessed (defined as >90% precursors in myeloid series).
For quantification, 10 high-power fields (HPFs) were evaluated and the number of specific cell types (as follows) and the total number of cells were counted.
A special focus was the analysis of megakaryopoiesis, which included the count of different cell types according to Cantù Rajnoldi et al.10 (micromegakaryocytes, monolobulated or binuclear megakaryocytes, megakaryocytes with separated nuclei and normal megakaryocytes. CD42b immunohistochemical staining was used for the evaluation of micromegakaryocytes). In addition, abnormal localization of megakaryopoiesis (megakaryopoiesis adjacent to bone trabecular or megakaryocytic clusters) was also noted.
The percentage of CD34, CD20, CD3, TdT, and CD123 positive cells was assessed by counting a minimum of 100 bone marrow cells.
In bone marrow aspirates, myeloblasts were counted in percentage of 100 haematopoietic cells, and blasts with Auer rods were evaluated. In addition, the maturation of granulopoiesis and erythropoiesis (with an increase in proerythroblasts) was recorded and dysplasia of all lineages evaluated.
Results
The median age of the 14 patients with de novo MDS-EB and UBTF-TD was 10.9 years (range 4.7–17.1). There were 11 males and three females (Table S1). Absolute neutrophil count (ANC) was 5.9 g/L (range 2.0–14.5), and all patients presented with anaemia (median haemoglobin concentration 8.2 g/dL, range 6.5–9.4) and thrombocytopenia (median platelet count 60 g/L, range 20–129). The mean corpuscular volume of red cells was elevated for age in five patients (38%). All patients had normoblasts on blood smear (median 3.4/100 WBC, range 1–4), and myeloid precursors were noted in 12 cases (86%).
Cytology of bone marrow aspirate
Bone marrow aspirates were either hypercellular (N = 13) or normocellular (N = 1). The ratio of myeloid to erythroid precursors was decreased in the majority of patients (N = 12; 86%). Dysplastic changes were prominent in all aspirates, affecting erythropoiesis, granulopoiesis, and megakaryopoiesis (>10% dysplastic cells observed in all lineages). Dyserythropoiesis was evident in all patients and included nuclear budding and megaloblastic changes. A significant left-shift of erythropoiesis with numerous proerythroblasts was consistently observed, while ring sideroblasts were not detected. Megakaryocytes displayed atypical features, including numerous mono- or bilobulated megakaryocytes and micromegakaryocytes. Granulopoiesis was left-shifted with hypogranulated cytoplasma and hyposegmented nuclei. Myeloid blasts were increased in all cases (median 11.2%), and only a few Auer rods were detected in three patients (21%).
Histomorphology of bone marrow biopsies
Bone marrow biopsies obtained from UBTF-TD patients exhibited a consistently hypercellular marrow for age in the majority of cases (N = 13; 93%), with a median cellularity of 88% on core biopsies. The distribution pattern of haematopoiesis was found to be diffuse rather than patchy. The architecture of the bone marrow was visibly disturbed, showing atypically located erythropoiesis and a prominent hyperplastic, atypical megakaryopoiesis in all cases. A summary of the morphological features is provided in Table 1 and displayed in Figure 1.
| Cytology (bone marrow aspirate) | ||
| Blast count (%) | Median (range) | 11.2% (5%–19%) |
| EP to MP ratio | Median (range) | 1.8 (0.3–12) |
| Dyserythropoiesis (>10%) | Number (%) | 14 (100) |
| Severe immaturity in erythropoiesis | Number (%) | 14 (100) |
| Dysgranulopoiesis (>10%) | Number (%) | 14 (100) |
| Dysmegakaryopoiesis (>10%) | Number (%) | 14 (100) |
| Histology (core biopsy) | ||
| Hypercellular in BM histology | Number (%) | 13 (97) |
| Severe immaturity in erythropoiesis | Number (%) | 14 (100) |
| Abnormal localisation of erythropoiesis | Number (%) | 14 (100) |
| Megakaryocytes (n/HPF) | Median (range) | 43 (34–51) |
| Micromegakaryocytes (n/HPF) | Median (range) | 17 (12–19) |
| Monolobulated megakaryocytes in % of total megakaryocyte number | Median (range) | 65 (57–69) |
| Bilobulated megakaryocytes in % of total number of megakaryocytes | Median (range) | 12 (11–15) |
| Megakaryocyte with separated nuclei in % of total number megakaryocytes | Median (range) | 2 (1–4) |
- BM, bone marrow; EP, erythropoiesis; MP, myelopoiesis; N, number; HPF, high-power field.
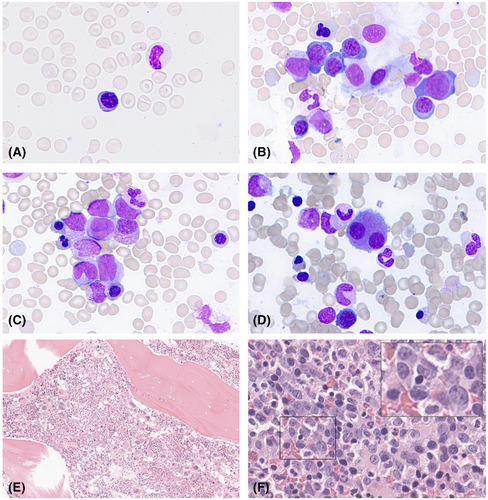
In 12 cases (86%), the ratio of myeloid to erythroid precursors was decreased, with a median ratio of 1.8 (range 0.3–12). Granulopoiesis was left-shifted with immature precursors in the centre of the core biopsy. Notably, 10 patients (71%) displayed myeloid precursors with few but giant nucleoli. Erythropoiesis presented significant dysplastic features, with peritrabecular, atypical localization. Additionally, erythropoiesis was immature, with numerous proerythroblast clusters present in all cases. Megaloblastoid changes were evident in five patients (35%).
Megakaryocytes were markedly increased in number and were observed in small occasional peritrabecular clusters. Prominent atypical features were evident in all cases, with an increase in nuclear bi- or monolobulation and the presence of micromegakaryocytes. Furthermore, blastoid immature megakaryocytes were increased in 10 patients (71%). Reticulin fibrosis was detected in two patients, characterized by the formation of a loose network of reticulin fibres with rare, perivascular intersections, corresponding to a myelofibrosis grade 1. Sinusoids were enlarged in six patients (43%).
Immunohistochemistry and immunofluorescence of bone marrow biopsies
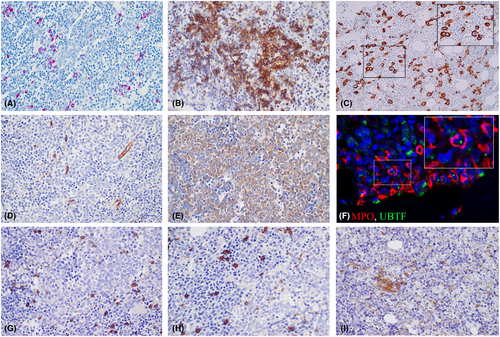
Immunohistochemical analysis revealed scattered CD20-positive B cells, intermingled with CD3-positive T cells, without dense infiltrates or a significant increase in number. The TdT-positive hematogones exhibited a normal distribution and number. Notably, a clear increase in Naphtol–Chloracetate–Esterase-negative blasts was observed in all cases. In all but one case, the blasts were found to be negative for CD34, but positive for CD33. In 36%, blasts were also positive for CD123. CD42b immunohistochemistry highlighted micromegakaryocytes in all cases. Only isolated round-oval mast cells were visible. Additionally, a high number of isolated and small aggregates of CD123 plasmacytoid dendritic cells were observed in close to half of the cases (43%). In all instances, myeloid precursors positive for MPO displayed prominent UBTF-positive nucleoli. Immunohistochemistry and Naphtol–Chloracetat–Esterase stain is shown in Figure 2.
Cytogenetics and molecular findings
Cytogenetic analysis revealed a normal karyotype in 10 patients (71%), with no detection of monosomy 7. Four patients (29%) exhibited a trisomy 8.
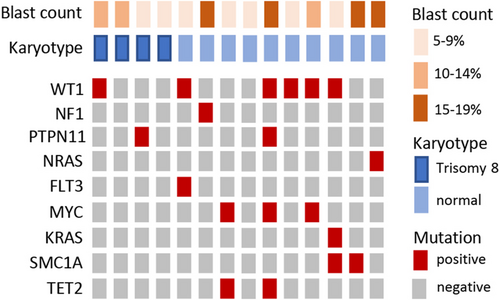
Genetic analysis of UBTF-TD patients did not reveal germline predisposition mutations in GATA2, RUNX1, and SAMD9/9L. Somatic mutations were observed and are summarized in Figure 3. Among the detected mutations, those in WT1 were the most common, being present in six patients (43%). Morphologically, no significant differences in WT1-mutated versus WT1-wildtype could be detected.
Discussion
Since UBTF-TD has emerged as a novel subgroup-defining genetic lesion of AML and MDS in young patients, it is crucial that pathologists recognize the morphological features of these cases. In this study, we aimed to describe the characteristics of cytology and histopathology, which may indicate the presence of UBTF-TD to prompt further molecular profiling. We studied 14 UBTF-TD-positive cases with de novo MDS having excluded disease with prior chemo−/radiation therapy, prior aplastic anaemia, or known inherited bone marrow failure/predisposition syndrome. All cases were classified as MDS-EB according to the International Consensus Classification,7, 8 and qualify as childhood MDS with increased blasts cMDS-IB in the current WHO classification.11
Except for one case with normocellularity, bone marrow biopsies were hypercellular for age. This contrasts with most cases of MDS-EB in GATA2 deficiency,12 SAMD9/9L syndrome,13 or the classical inherited bone marrow failure disorders. Furthermore, it is distinct from MDS presenting as refractory cytopenia of childhood (RCC).8, 14, 15 Next to a hypocellular bone marrow in most instances, RCC displays a typical morphological pattern featuring patchy erythropoiesis and reduced megakaryopoiesis with occasional micromegakaryocytes, which is absent in UBTF-mutated myeloid neoplasia.
Notably, we observed marked dysplasia in all three lineages, particularly in the megakaryopoiesis. While megakaryocytes with dysplastic features are increased in de novo MDS and MDS with germline predisposition, such as GATA2 deficiency,16-18 the predominance of monolobated cells in small clusters and an extremely high rate of atypia with only few normal megakaryocytes (<10%) can serve as a morphologic clue to a diagnosis of UBTF-TD. Another striking feature of UBTF-TD is morphologic dysplasia in the erythropoietic lineage. Erythropoiesis was severely immature, with numerous proerythroblasts and circulating normoblasts. This is in line with the observation that UBTF-TD in adult AML is associated with erythroid leukaemia (M6) and may be attributed to the overexpression of regulators of erythroid differentiation, such as HBB, HBA2, and ABO.4, 5
Molecular analysis of UBTF-TD patients revealed that WT1 mutations were the most common co-occurring mutations, while germline predisposition mutations such as GATA-2 or SAMD9/9L were absent. Additionally, no monosomy 7 was detected. A similar genomic landscape was observed in UBTF-TD-positive adult AML.19 This molecular profile is remarkable, as previously, germline mutations such as GATA-2 deficiency as well as monosomy 7 were the most frequent genetic aberrations in advanced primary MDS of childhood.12
On H&E sections, we noticed prominent nucleoli in UBTF-TD bone marrow biopsies. Double immunofluorescence images confirmed the presence of prominent UBTF-positive nucleoli in myeloid precursors positive for MPO. Interestingly, this finding is consistent with previous reports of gain-of-function mutations in UBTF, such as UBTF E210K, observed in developmental neuroregression and associated with nucleolar dysregulation characterized by few large nucleoli in cultured fibroblasts.18
Nearly half of the patients in our study showed an increase in CD123-positive plasmacytoid dendritic cells. While in adult MDS/myeloproliferative neoplasia, bone marrow dendritic cell aggregates are associated with disease progression and immune dysregulation,17 lymphocyte counts assessed by CD20 and CD3 in UBTF-TD patients were unremarkable.
Immunohistochemical stainings revealed a high number of CD34-negative blasts. The enumeration of myeloid blasts is critical to the classification of MDS, and in this study was completed by quantification of blasts in aspirate smears. It can be assumed that histopathological features in UBTF-mutated disease classified as AML are similar to those outlined in this study for MDS-EB. Biological features rather than an arbitrary cutoff in blast count (of 20% or 30%) are important to differentiate the MDS from AML in young individuals. AML in the young is generally a chemotherapy-sensitive disorder, and most patients are cured by chemotherapy only. In contrast, survival with MDS-EB is extremely poor in the absence of allogeneic stem cell transplantation. Given the severe dysplastic features described above, the poor response to chemotherapy,1-5 and a strikingly lower blast count in UBTF-mutated AML compared to AML with wildtype,4, 5 we propose that myeloid neoplasia with UBTF-TD either classified as MDS or AML may be considered one disease entity requiring a unified MDS-like therapy approach. Current next-generation sequencing (NGS) pipelines for AML and MDS may not include or detect UBTF-TDs, rendering the interpretation of the histopathology by experienced pathologists crucial for expanding standard diagnostic approaches to improve recognition of this distinct subgroup. Lastly, clinical studies providing a therapy algorithm with novel agents, like BCL-2 inhibitors19 or menin inhibitors,20 to patients with UBTF-mutated myeloid neoplasia my pave the way for more rational and disease-specific therapy.
Conclusion
In this study we provide a comprehensive description of the diagnostic features observed in paediatric MDS with UBTF-TD. Our findings reveal distinct histopathologic characteristics that may serve as valuable clues for identifying UBTF-TD in affected patients. Notably, hyperplastic myelopoiesis displaying severe atypia, accompanied by a high frequency of micromegakaryocytes and monolobated cell forms, emerged as prominent histologic features. Furthermore, we observed an immature erythropoiesis with circulating normoblasts, which adds to the diagnostic profile of UBTF-TD. The identification of these specific histopathologic traits is of utmost importance, as it can prompt clinicians to conduct further molecular studies, leading to early and accurate diagnosis. Early detection of UBTF-TD is critical in guiding appropriate therapeutic strategies tailored to the individual patient's needs. Timely intervention may significantly impact patient outcomes, particularly considering the aggressive nature of UBTF-TD-associated MDS.
Acknowledgements
We thank Anja Heier from the Pathological Institute of the LMU for excellent technical assistance and the EWOG-MDS data management team for support.
Author contributions
All authors read and approved the final article. Stephan Schwarz-Furlan, Carole Gengler, Ayami Yoshimi-Nöllke, and Franziska Schreiber analysed the data. Christian Thiede provided molecular data. Martina Rudelius, Miriam Erlacher, and Charlotte Niemeyer designed the study and edited the article. Guido Piontek, Yuki Schneider-Kimoto, and Markus Schmugge performed experiments and interpreted data. Martina Rudelius wrote the article.
Conflicts of interest
The authors declare no conflicts of interest.
Open Research
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.