Efficacy of steroid therapy for improving native liver survival after pediatric acute liver failure with immune activation
Abstract
Aim
Recent evidence suggests that acute liver failure (ALF) in some patients may reflect a dysregulated immune response, and that corticosteroids improve survival of the native liver in ALF patients with high serum alanine aminotransferase levels, which are an indication of liver inflammation. However, it is unclear whether steroids are effective for pediatric acute liver failure (PALF). The aim of this retrospective case–control study is to examine whether steroid therapy for PALF accompanied by immune activation improves the survival of native liver and to identify factors that predict responses to steroid treatment.
Methods
Of 38 patients with PALF treated at Kyoto University Hospital from February 2006 to August 2022, 19 receiving steroids who met the specific criteria for identifying the pathophysiology of immune activity in the liver (the “Steroid group”), and seven steroid-free patients who also met the criteria (“Nonsteroid group”) were enrolled. Patients in the “Steroid group” were categorized as “responders” or “nonresponders” according to treatment outcome. Clinical and histological data were analyzed.
Results
Survival of the native liver in the Steroid group was significantly higher than that in the Nonsteroid group (68% vs. 0%, respectively; p = 0.0052). Nonresponders were significantly younger, with higher Model for End-stage Liver Disease and pediatric end-stage liver disease scores, higher prothrombin time – international normalized ratio, and higher serum ferritin levels than responders. Massive hepatic necrosis was more common in nonresponders.
Conclusion
Steroid therapy is effective for PALF patients with liver inflammation; however, liver transplantation should be prioritized for young children with ALF accompanied by severe coagulopathy or massive hepatic necrosis.
Graphical Abstract
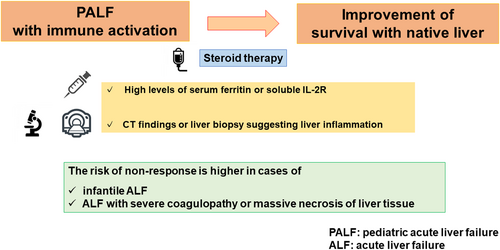
This retrospective study found that steroid therapy improves native liver survival in pediatric acute liver failure (ALF) with immune activation, defined by high serum ferritin or soluble interleukin-2R levels and liver inflammation on computed tomography or biopsy. High risks of nonresponse include infantile ALF, severe coagulopathy, or massive hepatic necrosis.
Abbreviations
-
- AIH
-
- autoimmune hepatitis
-
- ALF
-
- acute liver failure
-
- ALT
-
- alanine aminotransferase
-
- AST
-
- aspartate aminotransferase
-
- CHDF
-
- continuous hemodiafiltration
-
- CT
-
- computed tomography
-
- D/T
-
- direct/total bilirubin
-
- HLH
-
- hemophagocytic lymphohistiocytosis
-
- HSV
-
- herpes simplex virus
-
- MELD
-
- Model for End-stage Liver Disease
-
- mPSL
-
- methylprednisolone
-
- PALF
-
- pediatric acute liver failure
-
- PELD
-
- pediatric end-stage liver disease
-
- POD
-
- postoperative day
-
- POM
-
- postoperative month
-
- PT-INR
-
- prothrombin time – international normalized ratio
-
- sIL-2R
-
- soluble interleukin-2 receptor
INTRODUCTION
Acute liver failure is a heterogeneous and devastating liver disease in patients with previously normal liver function. Acute liver failure is characterized by acute onset liver injury, hepatocellular damage, hepatic encephalopathy, and coagulopathy and carries a high rate of mortality in the absence of liver transplantation. The standard treatment is plasma exchange and/or hemodialysis, but there is no proven effective therapy for ALF, including liver transplantation.1, 2
Even though the use of corticosteroids as a treatment for ALF is controversial,3-10 recent studies suggest that many adult cases of ALF of unknown etiology are actually immune-mediated; indeed, there are features suggestive of autoimmune pathogenesis.11 Therefore, steroids may improve the survival of adult ALF patients with high serum ALT levels (which are indicative of inflammation) and a low MELD score.4, 6, 9 Chapin et al. reported that pediatric patients with indeterminate ALF, defined as ALF of unknown etiology, improved after treatment with corticosteroids.5 However, few studies show that corticosteroids improve survival in such cases in the absence of liver transplantation5, 12; to date, steroid therapy for PALF remains controversial.
Increasing evidence shows that indeterminate PALF is often associated with immune activation and dysregulation, characterized by increased levels of serum sIL-2R or dense hepatic CD8 T cell infiltration.13-17 By contrast, one of the most important etiologies of PALF is a congenital metabolic disease accompanied by minimal hepatic cytotoxic T cell infiltration17; this makes it more difficult to select patients who may benefit from steroid therapy during the early stages of PALF.
At our institution, corticosteroid treatment for PALF is indicated mainly for patients with suspected autoimmune hepatitis, or if the presentation is strongly suggestive of immune activation in the liver (i.e., elevated serum ferritin or sIL-2R, combined with positive pathological findings and/or CT images).
This retrospective case–control study aimed to clarify whether steroid therapy for PALF accompanied by immune activation improves survival of the native liver and to identify predictors of steroid efficacy. To achieve this goal, we compared medical survival rates or clinical characteristics (based on clinical findings suggestive of inflammation of the liver) in patients treated with/without steroids. The results demonstrate the efficacy of steroids for PALF associated with immune-mediated responses.
METHODS
Thirty-eight consecutive patients under 18 years of age, all diagnosed with ALF at Kyoto University Hospital from February 2006 to August 2022, were enrolled.
The eligibility criteria for ALF were as follows18, 19: (1) no medical history of chronic liver disease, (2) biochemical markers compatible with acute liver injury, (3) coagulopathy caused by factors other than vitamin K deficiency, and (4) PT/INR >2.0 or >1.5, with hepatic encephalopathy. These PALF patients were assessed retrospectively to determine whether they received steroid treatment (or not), and for clinical findings suggestive of immune activation in the liver. Twenty-one of the 38 patients received steroid therapy, while 17 did not. As shown in Figure 1, 19 of the steroid-treated patients met the following criteria detecting inflammation in the liver: (1) serum ferritin or sIL-2R more than twice the upper limit of normal, and (2) liver biopsy showing mononuclear cell infiltration, or imaging result suggestive of liver inflammation (including CT findings of periportal edema and gallbladder wall thickening). These patients were classified into the “Steroid group”. The seven patients who did not receive steroid therapy, but who also met the above-mentioned criteria for identifying inflammation in the liver, were classified into the “Nonsteroid group”. Twelve patients were excluded because they did not meet the criteria defining liver inflammation. Among these 12 patients, seven had CT imaging or pathological findings that did not show signs of liver inflammation, and four were either not evaluated for serum ferritin or sIL-2R or did not meet the inclusion criteria levels. Most patients in the Nonsteroid group did not fulfill the criteria for liver inflammation because they were transferred to a transplant surgeon for the purpose of liver transplantation and underwent transplant procedures without evaluation of immune activation in the liver.
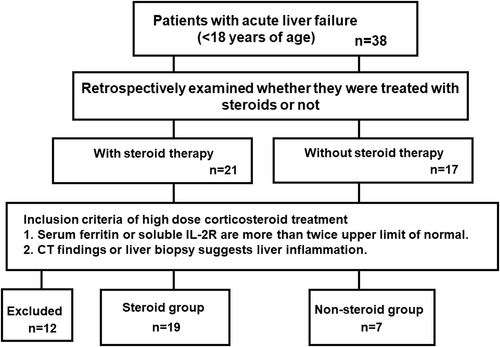
Flowchart of the present study. CT, computed tomography; IL-2R, interleukin-2 receptor.
The clinical and pathological findings of the Steroid and Nonsteroid groups were compared (serum AST, ALT, gamma-glutamyl transpeptidase, creatinine, albumin, ferritin, sIL-2R, total bilirubin, ammonia, platelet count, PT-INR, D/T ratio, stages of hepatic encephalopathy, MELD score, and PELD score) (Figure 1). The MELD and PELD scores were assessed before administering steroids or blood purification therapy prior to liver transplantation. The three patients that did not receive blood purification therapy were in the steroid group, and their scores were calculated before steroid administration. Differences in treatment regimens between the two groups were also assessed. The stages of hepatic encephalopathy were assessed based on the previous guidelines for the management of PALF.20
Liver biopsy and imaging
Pretreatment liver biopsies were obtained from 16 of 26 patients (62%): 15 of 19 patients (79%) in the Steroid group and 1 of 7 patients (14%) in the Nonsteroid group. All liver biopsies were carried out with laparoscopic assistance. Liver biopsies were undertaken less frequently in the Nonsteroid group prior to transplantation; however, histopathological examination of samples resected at the time of liver transplantation was obtained for 6 of the 26 patients (23%): 2 of 19 patients (11%) in the Steroid group, and 4 of 7 patients (57%) in the Nonsteroid group.
Pathological findings from the Steroid and Nonsteroid group liver biopsies were compared in terms of the presence or absence of portal inflammation, lobular inflammation, and massive necrosis. Radiological assessment (by CT imaging) was performed for all patients in the Steroid and Nonsteroid groups. Evaluation of CT imaging included assessment of gallbladder wall thickening and periportal edema, both of which are indicative of acute liver inflammation.
The primary end-points were overall survival and spontaneous recovery; the latter was defined as survival in the absence of liver transplantation up until 24 weeks after initiating treatment for ALF. Indications for liver transplantation were based on guidelines defined by the Acute Liver Failure Study Group in Japan (1996),21 or the criteria for liver transplantation for ALF, proposed in 2011.22
To identify factors useful for predicting responses to steroid therapy, the Steroid group was also divided into a “responder group”, comprising survivors with a native liver, and a “nonresponder group” comprising patients who failed to achieve remission and required a liver transplant or died. The clinical and histological characteristics of these two groups were compared to identify factors predictive of responsiveness to steroid treatment.
Statistical analysis
Results are expressed as the mean ± SD. Continuous variables were compared using the Mann–Whitney test. Two-tailed p values ≤0.05 were considered statistically significant. Categorical variables were analyzed using Fisher's exact test. Statistical analysis was undertaken using GraphPad Prism software version 7.0e (GraphPad Software).
RESULTS
Characteristics of the study cohort
The clinical characteristics of the 26 patients are shown in Table 1. There was no significant difference in age or male : female ratio between the two groups. The causes of PALF in the Steroid group were viral infection (16%; 3/19), hemophagocytic lymphohistiocytosis (16%; 3/19), autoimmune hepatitis (32%; 6/19), drug-induced liver injury (11%; 2/19), and indeterminate (31%; 5/19); these causes suggesting the existence of an excessive inflammatory reaction. Causes in the Nonsteroid group were congenital metabolic disorders (43%; 3/7) and indeterminate (57%; 4/7) (Table 1). Laboratory tests carried out prior to treatment revealed that patients in the Steroid group had significantly higher serum AST (p = 0.01) and ALT (p = 0.012) than those in the Nonsteroid group, and tended to have higher serum sIL-2R, although not significantly so (p = 0.088) (Table 2). Meanwhile, serum total bilirubin levels (p = 0.039) and the D/T ratio (p = 0.03) were significantly lower in the Steroid group than in the Nonsteroid group. The MELD (p = 0.09) and PELD scores (p = 0.076) tended to be higher in the Nonsteroid group. There was no significant difference between the groups with respect to other biochemical markers of liver function, including, platelet count, creatinine, albumin, and ammonia levels, and PT-INR.
| Steroid group (n = 19) | Nonsteroid group (n = 7) | p value | |||
|---|---|---|---|---|---|
| Age (years) | 5.8 ± 5.7 | 4.0 ± 6.8 | 0.12 | ||
| Sex, male/female | 12/7 | 3/4 | >0.99 | ||
| Etiology | Unknown | 5 | Unknown | 5 | - |
| Viral hepatitis | Metabolic | - | |||
| HHV-6 | 1 | Wilson | 1 | - | |
| HSV-1 | 1 | Mitochondrial | 1 | - | |
| Influenza A | 1 | - | |||
| HLH | 3 | - | |||
| AIH | 6 | - | |||
| DILI | 2 | - | |||
- Abbreviations: AIH, autoimmune hepatitis; DILI, drug-induced liver injury; HHV, human herpesvirus; HLH, hemophagocytic lymphohistiocytosis; HSV, herpes simplex virus.
| Steroid group (n = 19) | Nonsteroid group (n = 7) | p value | |
|---|---|---|---|
| Data before plasma exchange | |||
| MELD score | 28 ± 10 | 34 ± 5 | 0.0900 |
| PELD score | 28 ± 21 | 43 ± 18 | 0.0760 |
| AST (IU/L) | 4370 ± 5349 | 681 ± 921 | 0.0100 |
| ALT (IU/L) | 2299 ± 2550 | 263 ± 326 | 0.0120 |
| GGT (IU/L) | 94 ± 41 | 131 ± 96 | 0.1800 |
| Cre (mg/dL) | 0.38 ± 0.20 | 0.36 ± 0.25 | 0.9200 |
| Alb (g/dL) | 3.4 ± 0.6 | 3.1 ± 0.7 | 0.1900 |
| Ferritin (ng/mL) | 18 856 ± 65 956 | 10 106 ± 21 407 | 0.6900 |
| sIL-2R (U/mL) | 3643 ± 2112 | 2016 ± 1898 | 0.0880 |
| PLT (x104/μL) | 19 ± 11 | 20 ± 17 | 0.9600 |
| T-bil (mg/dL) | 12 ± 7 | 28 ± 7 | 0.0036 |
| NH3 (μg/dL) | 105 ± 81 | 95 ± 87 | 0.1900 |
| PT-INR | 4.8 ± 5.5 | 5.1 ± 4.8 | 0.7700 |
| Hepatic encephalopathy | 1.8 ± 1.0 | 1.4 ± 0.5 | 0.5700 |
| D/T ratio | 0.64 ± 0.19 | 0.47 ± 0.18 | 0.0300 |
| Treatment | |||
| Plasma exchange | 79% (15/19) | 100% (7/7) | 0.5500 |
| CHDF or high-flow HDF | 42% (8/19) | 100% (7/7) | 0.0100 |
| Time of CHDF (days) | 2.1 ± 3.7 | 5.7 ± 6.3 | 0.0210 |
| Number of PE | 4.2 ± 2.8 | 5.1 ± 3.5 | 0.4800 |
- Abbreviations: Alb, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CHDF, continuous hemodiafiltration; Cre, creatinine; D/T, direct/total bilirubin; GGT, gamma-glutamyl transpeptidase; HDF, hemodiafiltration; MELD, Model for End-stage Liver Disease; NH3, ammonia; PE, plasma exchange; PELD, pediatric end-stage liver disease; PLT, platelets; PT-INR, prothrombin time – international normalized ratio; sIL-2R, soluble interleukin-2 receptor; T-bil, total bilirubin.
In addition, patients in the Nonsteroid group required significantly greater use of CHDF (p = 0.01) or high-flow hemodiafiltration (p = 0.021) than those in the Steroid group, and the duration of CHDF was longer for the Nonsteroid group. These results could be attributed to the fact that the Nonsteroid group contained a higher proportion of patients requiring liver transplantation (Table 2).
Pretreatment liver biopsy and CT imaging
Mononuclear cell infiltration of the portal area was observed both in the Steroid and Nonsteroid groups (88% [15/17] versus 75% [3/4], respectively; p = 0.49), while lobular inflammation was not observed in the Nonsteroid group (100% [17/17] versus 0% [0/2], respectively; p = 0.0058). Massive necrosis was more common in patients in the Nonsteroid group, although the incidence was not statistically different from that in the Steroid group. Computed tomography imaging revealed gallbladder edema and periportal edema in both the Steroid and Nonsteroid groups (Table 3).
| Steroid group, % (n/N) | Nonsteroid group, % (n/N) | p value | |
|---|---|---|---|
| Liver biopsy before treatment | 79 (15/19) | 14 (1/7) | 0.0053 |
| Histopathological assessment of recipient liver after transplantation | 11 (2/19) | 57 (4/7) | 0.0280 |
| No histological evaluation | 11 (2/19) | 29 (2/7) | 0.2800 |
| Histological findings | |||
| Portal inflammation | 88 (15/17) | 75 (3/4) | 0.4900 |
| Lobular inflammation | 100 (17/17) | 0 (0/2) | 0.0058 |
| Massive necrosis | 35 (6/17) | 80 (4/5) | 0.1500 |
| CT findings | |||
| Gall bladder edema | 79 (15/19) | 57 (4/7) | 0.3400 |
| Periportal edema | 95 (18/19) | 86 (6/7) | 0.4700 |
Corticosteroid dose
All patients in the Steroid group received high-dose corticosteroids, defined as administration of 30 mg/kg (maximum 1 g/day) mPSL, infused on three consecutive days. Because this study was retrospective, the criteria for steroid therapy were not predetermined; the decision was made at the discretion of the attending physician. The steroid dose was based on that used for steroid pulse therapy in cases of pediatric AIH in Japan.23 Recipients of liver transplantation at our institution received steroid administration intraoperatively and postoperatively according to established protocols: mPSL (10 mg/kg) after reconstructing and reflowing the portal vein; 1 mg/kg i.v. until POD 3; 0.5 mg/kg i.v. from POD 4 to POD 6; and 0.3 mg/kg i.v. on POD 7; prednisolone (0.3 mg/kg) was given p.o. until POM 1, and then at 0.1 mg/kg p.o. until POM 3.
Effect of high-dose corticosteroids on ALF
Eighty-five percent (22 of 26) of patients survived, while 15% (4/26) died, and 35% (9/26) underwent liver transplantation. The overall survival rates of patients with steroid treatment were comparable with those of patients without steroid treatment (p = 0.29). The number of patients in the Steroid group who underwent liver transplantation was significantly smaller than that in the Nonsteroid group (21% (4/19) versus 71% (5/7), respectively; p = 0.028). Spontaneous recovery rates among patients who received corticosteroids were significantly higher than those among patients who did not receive steroids (68% [13/19] versus 0% [0/7], respectively; p = 0.0052) (Table 4). Even when excluding metabolic diseases, which have a pathophysiology different from hepatitis (19 patients on steroids vs. 5 on nonsteroids), survival with the native liver was significantly higher in those receiving steroid treatment.
| Steroid group (n = 19) | Nonsteroid group (n = 7) | p value | |||
|---|---|---|---|---|---|
| Age (years); mean ± SD | 5.8 ± 5.7 | 4.0 ± 6.8 | 0.1200 | ||
| Number of survivors | 17 | 5 | - | ||
| Number of LT | 4 | 5 | - | ||
| Number of deaths | 2 | 2 | - | ||
| LT rate | 21% | (4/19) | 71% | (5/7) | 0.0280 |
| Survival rate | 89% | (17/19) | 71% | (5/7) | 0.2900 |
| LT survival rate | 100% | (4/4) | 100% | (5/5) | >0.9900 |
| Spontaneous recovery | 68% | (13/19) | 0% | (0/7) | 0.0052 |
| Infection (bacteremia) | 21% | (4/19) | 51% | (4/7) | 0.1500 |
- Abbreviation: LT, liver transplantation.
Post-transplantation outcome
Effects of corticosteroids on incidence of infection
The overall rate of bacteremia in patients in the Steroid group was not significantly different from that in the Nonsteroid group (22% vs. 46%, respectively; p = 0.25) (Table 4).
Characteristics of nonresponders to steroid treatment
To identify factors associated with clinical responses to steroid treatment, we evaluated differences in the clinical characteristics of nonresponders to steroid treatment (who required liver transplant or died due to failure to achieve remission) and responders (who achieved a clinical remission). Of 19 patients in the Steroid group, 6 were nonresponders and 13 were responders. Of the six nonresponders, four underwent liver transplantation and remained alive, whereas two who did not undergo liver transplantation died (Table 5). One of the two who died was a 7-day-old boy who suffered from ALF caused by hemophagocytic lymphohistiocytosis associated with an HSV-1 infection. Despite treatment with acyclovir, high-dose gamma globulin therapy, and exchange blood transfusion, he died due to renal failure and pulmonary edema. The second patient, an 11-month-old boy with ALF associated with drug-induced hypersensitivity syndrome, died from acute respiratory distress syndrome caused by methicillin-resistant Staphylococcus aureus-related pneumonia (Table 6).
| Nonresponders (n = 6) | Responders (n = 13) | p value | |||
|---|---|---|---|---|---|
| Age (years); mean ± SD | 0.9 ± 1.0 | 8 ± 5 | 0.0013 | ||
| Number of survivors | 4 | 13 | - | ||
| Sex | |||||
| Male/female | 5/1 | 7/6 | 0.3300 | ||
| LT rate | 67% | (4/6) | 0% | (0/13) | 0.0039 |
| Survival rate | 67% | (4/6) | 100% | (13/13) | 0.0870 |
| Spontaneous recovery | 0% | (0/6) | 100% | (13/13) | <0.0001 |
| Infection (bacteremia) | 33% | (2/6) | 15% | (2/13) | 0.5600 |
- Abbreviation: LT, liver transplantation.
| Age | Sex | Encephalopathy | Etiology | MELD score | PELD score | Prognosis | |
|---|---|---|---|---|---|---|---|
| 7 days | M | 2 | Viral hepatitis (HSV-1) | 43 | 55 | Deceased | Renal failure, pulmonary edema |
| 2 months | M | 1 | Unknown | 23 | 20 | LT alive | |
| 11 months | M | 1 | (DILI/DIHS) | 41 | 54 | Deceased | MRSA pneumonia, ARDS |
| 6 months | F | 1 | Unknown | 51 | 65 | LT alive | |
| 1 year | M | 3 | Influenza A | 25 | 22 | LT alive | |
| 2 years | M | 3 | HLH (caused by EBV) | 33 | 22 | LT alive | |
- Abbreviations: ARDS, acute respiratory distress syndrome; DIHS, drug-induced hypersensitivity syndrome; DILI, drug-induced liver injury; EBV, Epstein–Barr virus; F, female; HLH, hemophagocytic lymphohistiocytosis; HSV, herpes simplex virus; LT, liver transplantation; M, male; MELD, Model for End-stage Liver Disease; MRSA, methicillin-resistant Staphylococcus aureus; PELD, pediatric end-stage liver disease.
Factors contributing to the clinical efficacy of steroid therapy
Nonresponders were significantly younger than responders (p = 0.0013; Table 5). All nonresponders were infants, and 80% (4/5) were in the Steroid group. Baseline laboratory tests undertaken before initiating treatment revealed that the following values for nonresponders were significantly higher than those for responders: PELD score (p = 0.023), MELD score (p = 0.021), PT-INR (p = 0.012), and serum ferritin level (p = 0.029). Serum creatinine levels in nonresponders were significantly lower than those in responders (p = 0.049; Table 7). There was no significant difference between nonresponders and responders with respect to platelet counts, albumin and ammonia levels, stage of hepatic encephalopathy, or the D/T ratio. There was no significant difference in the duration of plasma exchange and CHDF (Table 7).
| Nonresponder (n = 6) | Responder (n = 13) | p value | |
|---|---|---|---|
| Data before plasma exchange | |||
| MELD score | 36 ± 12 | 24 ± 8 | 0.021 |
| PELD score | 40 ± 20 | 18 ± 15 | 0.023 |
| AST (IU/L) | 4186 ± 3659 | 4455 ± 6109 | 0.770 |
| ALT (IU/L) | 2062 ± 2746 | 2408 ± 2564 | 0.700 |
| GGT (IU/L) | 124 ± 43 | 82 ± 36 | 0.072 |
| Cre (mg/dL) | 0.24 ± 0.19 | 0.44 ± 0.17 | 0.049 |
| Alb (g/dL) | 3.1 ± 0.9 | 3.6 ± 0.5 | 0.640 |
| PLT (x104/μL) | 13 ± 9 | 21 ± 11 | 0.150 |
| T-bil (mg/dL) | 11 ± 3 | 13 ± 9 | 0.470 |
| NH3 (μg/dL) | 105 ± 81 | 137 ± 87 | 0.170 |
| PT-INR | 8.5 ± 7.3 | 3.1 ± 3.6 | 0.012 |
| Ferritin (ng/mL) | 51 092 ± 116 939 | 3978 ± 8259 | 0.029 |
| sIL-2R (U/mL) | 4643 ± 3347 | 3269 ± 1544 | 0.500 |
| Hepatic encephalopathy | 1.8 ± 1.0 | 1.8 ± 1.0 | 0.970 |
| D/T Ratio | 0.59 ± 0.26 | 0.66 ± 0.16 | 0.900 |
| Treatment | |||
| CHDF time (days) | 4.3 ± 5.6 | 0.8 ± 1.7 | 0.310 |
| Number of PE | 4.3 ± 3.6 | 3.5 ± 2.8 | 0.510 |
| Plasma exchange | 67% (4/6) | 84% (11/13) | 0.560 |
| CHDF or high-flow HDF | 50% (3/6) | 38% (5/13) | >0.990 |
- Note: Values are shown as mean ± SD unless otherwise indicated.
- Abbreviations: Alb, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CHDF, continuous hemodiafiltration; Cre, creatinine; D/T, direct/total bilirubin; GGT, gamma-glutamyl transpeptidase; HDF, hemodiafiltration; MELD, Model for End-stage Liver Disease; NH3, ammonia; PE, plasma exchange; PELD, pediatric end-stage liver disease; PLT, platelets; PT-INR, prothrombin time – international normalized ratio; sIL-2R, soluble interleukin-2 receptor; T-bil, total bilirubin.
Histopathological findings in the liver revealed that a higher proportion of patients in the nonresponders group showed massive necrosis (80% [4/5] versus 17% [2/12] in the responder group; p = 0.028), although there was no significant difference in the proportion of patients with portal inflammation and lobular inflammation (Table 8).
| Nonresponder, % (n/N) | Responder, % (n/N) | p value | |
|---|---|---|---|
| Liver biopsy before treatment | 33 (2/6) | 77 (10/13) | 0.130 |
| Histopathological assessment of recipient liver after transplantation | 50 (3/6) | 8 (1/13) | 0.071 |
| No histological evaluation | 17 (1/6) | 15 (2/13) | >0.990 |
| Histological findings | |||
| Portal inflammation | 60 (3/5) | 100 (12/12) | 0.074 |
| Lobular inflammation | 100 (5/5) | 100 (12/12) | >0.990 |
| Massive necrosis | 80 (4/5) | 17 (2/12) | 0.028 |
| CT findings | |||
| Gall bladder edema | 83 (5/6) | 77 (10/13) | >0.990 |
| Periportal edema | 100 (6/6) | 92 (12/13) | >0.990 |
DISCUSSION
High-dose steroid therapy improved survival of PALF patients with hepatic inflammation, even in the absence of liver transplantation, and had no adverse effect on survival after liver transplantation. There was no difference in posttransplant survival rates between the Steroid and Nonsteroid groups, nor was there a difference in the incidence of infection. These results suggest that the treatment strategy used at our institution is both timely and appropriate for patients treated with steroids, as well as those not treated with steroids.
Patients in the Steroid group had significantly higher levels of serum ALT, which correlate with the grade of liver injury and hepatic inflammation; thus, ALT levels are used as surrogates of inflammatory responses during ALF.24 The high levels of transaminases and sIL-2R (the latter may be an indicator of lymphocyte activation), meant that the Steroid group was characterized by a higher degree of inflammation than the Nonsteroid group. Histopathological examination of the liver revealed lobular inflammation in all patients in the Steroid group, but not in the Nonsteroid group, although portal inflammation was observed in both groups. Hepatocytes were almost entirely absent from liver biopsy samples taken from patients in the Nonsteroid group; therefore, it was impossible to determine whether there was any lobular inflammation. These differences could also be attributed to the fact that most of the Nonsteroid group samples comprised recipient livers resected before transplantation.
At the referring hospitals, most patients in the Nonsteroid group who met the criteria for detecting liver inflammation did not receive steroid treatment or died until transfer for liver transplantation. The absence of established guidelines for steroid therapy in PALF, along with concerns regarding infantile cases and posttransplant complications, might have contributed to the hesitance to administer steroids. This is the reason why patients in the Nonsteroid group had more severe diseases than those in the group that prioritized medical intervention with steroids. Indeed, markers of hepatic synthetic function (e.g., bilirubin levels and the D/T ratio) were lower in the Nonsteroid group, but the MELD and PELD scores (markers of disease severity and mortality, respectively) were higher. Our study also reveals that among the steroid-treated group, patients who did not respond to steroids had impaired liver function and extensive liver necrosis. This suggests that earlier administration of steroids might have prevented disease progression and saved patients with a native liver.
Administration of corticosteroid to patients with ALF remains controversial.3-10 Early studies suggested no survival benefit after steroid administration.3, 8 However, more recent research indicates that patients with ALF related to an inflammatory process may respond to corticosteroid administration.6 Karkhanis et al. undertook a retrospective study of 361 adult patients with ALF to evaluate the efficacy of steroids for improving overall survival and spontaneous recovery.9 Their findings indicated that steroids did not improve overall survival or spontaneous recovery in many patients. Nonetheless, patients who received steroid therapy and showed ALT in the higher 50% of values, did show improved spontaneous recovery compared with those not receiving steroid therapy. Meanwhile, treatment with corticosteroids was significantly associated with lower overall survival in patients with a high MELD score.1, 3 These results suggest that ALF patients with higher ALT levels, a surrogate marker for liver inflammation, and lower MELD scores may be more likely to respond favorably to steroid treatment. A Japanese prospective study also reported the efficacy of high-dose corticosteroid for early-stage adult ALF; the drugs suppressed liver inflammation and trended to increase survival, although the effect did not reach statistical significance.15
In terms of PALF, the efficacy of steroids remains unclear. Few studies have reported the effectiveness of steroid therapy for PALF.5, 12 A retrospective study of patients with indeterminate PALF (median of 4 years of age [range, 1–16 years]) reported that 12 of 28 patients who received intravenous mPSL recovered with a native liver.5 Cases were defined as having indeterminate ALF if, despite a thorough diagnostic work-up, no identifiable cause for their disease could be determined. However, this study did not compare indeterminate PALF patients who received intravenous mPSL with Nonsteroid patients who did not. As a result, it is unclear whether steroid therapy contributes to the improvement of recovery of patients with a native liver. Here, we demonstrate that steroid treatment improves survival of PALF patients with high serum ferritin and sIL2-R levels, coupled with histopathological or imaging findings suggestive of immune activation.
The etiology of ALF in children is different from that in adults. In Japan, a greater proportion of cases are attributed to metabolic disease (10%–11.1% vs. 0%, retrospectively) or remain indeterminate (47%–49% vs. 29%–34%) and fewer cases are caused by viral infection (6%–7% vs. 46%–50%).1, 25-30 Congenital metabolic disorders, which are also important causes of PALF, are not typically associated with hepatic inflammatory cell infiltration17; hence, the use of corticosteroids in PALF should be approached with careful consideration. By contrast, indeterminate ALF characterized by CD103+ CD8+ T cell-predominant hepatic infiltrate31 stands as the most common cause of PALF; therefore, steroids or other immunosuppressive therapies might effectively mitigate CD103+ CD8+ T cell-mediated inflammation.31 Another study focusing on biomarkers related to PALF shows that four circulating lymphocyte activation markers (i.e., perforin- or granzyme-positive CD8 cells, total CD8 cells, and sIL-2R), are associated with immune activation and can predict the outcomes of early targeted immunosuppression.32 However, the activation of peripheral blood lymphocytes does not always reflect inflammation in the liver. Further investigations are needed to identify more accurate biomarkers that can be used for early detection of immune activation in PALF.
In some situations, it is more prudent to use biomarkers linked to liver inflammation to identify ALF candidates suitable for early immunosuppressive therapy (including steroid administration); this is because it is not safe to wait until all full work-up results are available. In our institution, we used steroids for PALF cases showing elevation of serum sIL-2R or serum ferritin levels, both of which are indicators of lymphocyte or macrophage activation; we also base our assessment on imaging findings suggestive of hepatitis, or inflammatory cell infiltration (mainly lymphocytes), on liver biopsy. As a matter of fact, the “Steroid group” in the present study, which was classified according to this protocol, later turned out to have immune activation-based disease caused by viral infection, HLH, AIH, or drug-induced hypersensitivity; all these conditions respond to steroids. By contrast, the “Nonsteroid group” had metabolic diseases (e.g., Wilson disease and mitochondrial hepatopathy), which do not respond to steroid treatment. Therefore, the reasonable approach is to detect early inflammation-mediated disease using our protocol; in such cases, immunosuppressive therapies, including steroid administration, are effective.
We also focused on identifying factors that predict response to steroid treatment.
Patients in the nonresponder group were younger than those in the responder group. In particular, the nonresponder group contained a higher percentage of infants. Also, patients in the nonresponder group were more severely ill, with higher MELD or PELD scores and more severe coagulopathy, than those in the responder group. Moreover, a significantly higher proportion of patients in the nonresponder group had massive necrosis on liver biopsy. These results are consistent with those of a previous study showing that corticosteroid administration to adult ALF patients led to significantly lower overall survival in those with high MELD score.9
Furthermore, higher levels of serum ferritin were found in the nonresponder group, even though there was no difference in transaminase or sIL-2R levels between responders and nonresponders. Serum ferritin level is a widely available surrogate marker of macrophage activation, as well as of iron overload.33 Hyperferritinemia in PALF is common in those with neonatal hemochromatosis, viral infections, and HLH,24, 34 which is consistent with our finding that PALF in some nonresponders was caused by HLH and HSV-1 infection. High levels of serum ferritin prior to liver transplantation in patients with chronic liver failure are associated with increased mortality following liver transplantation.35 Hence, hyperferritinemia in PALF could be a poor prognostic factor.
Taken together, the data suggested that liver transplantation should be a priority for infant patients with ALF coupled with severe coagulopathy or massive necrosis of liver tissue, as they are unlikely to respond to steroid therapy.
The present study has several limitations. First, the study is retrospective, and steroid use was not randomized or standardized. Second, the sample size is small. Finally, there were few available pretreatment liver biopsies, especially from the Nonsteroid group. Histopathologic analysis of the liver provides more accurate information about hepatitis, but biopsy increases the risk of bleeding, especially in patients with coagulation abnormalities. Therefore, in PALF patients with severe coagulopathy, liver resected at the time of transplantation was used for assessment, if available; failing that, we had to rely on the combination of CT images and serum levels of inflammatory markers.
CONCLUSION
In cases of PALF, high-dose steroid therapy in patients with findings suggestive of inflammation-mediated disease can be an effective treatment and increase the recovery rate with the native liver. Liver inflammation detected by serum ferritin and sIL-2R levels, liver biopsy, or imaging are recommended to select candidates for early corticosteroid treatment.
In severe infant PALF with severe coagulopathy, steroids are not recommended, and early liver transplantation should be prioritized.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST STATEMENT
Authors declare no conflict of interests for this article.
ETHICS STATEMENTS
Approval of the research protocol by an institutional reviewer board: This retrospective study was approved by the Institutional Review Board of the Kyoto University Hospital and by the Ethics Committee of Kyoto University Hospital (registered under protocol numbers R2481 and G1233).
Informed consent: Informed consent was obtained in the form of opt-out on the website.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.