Lactic acid bacteria and manganese-based additives for improved lucerne, perennial grass, and perennial grass-white clover preservation as silage
Abstract
Lactic acid bacteria (LAB) are commonly used as silage inoculants. Manganese sulphate (MnSO4) has been proposed to protect certain LAB during oxygen exposure and improve silage aerobic stability (ASTA). The aim of the present study was to assess the efficacy of inoculants in preservation of different forages. A short-term screening trial investigated the effect of 24 additive treatments, compared to a negative control, on ensiling perennial grass in mini-silos (2.75 L) for 7 d. The additive treatments comprised different ratios of Lactiplantibacillus plantarum [IMI 507026, IMI 507027, and IMI 507028] and Pediococcus pentacaceus [IMI 507024 and IMI 507025], with or without MnSO4. Target parameters, such as low levels of fresh matter loss, pH, acetic acid, ammonia-nitrogen fraction (NH3–N/Total N), and high levels of lactic acid and ASTA, were considered for treatment selection. Eight treatments were selected based on a principal component analysis and hierarchical clustering of the target parameters. These selected treatments subsequently underwent a 100 d long-term trial using lucerne, perennial grass, and perennial grass-white clover forages. All silages treated with the eight additives exhibited an improved homolactic fermentation and a lower (p < .001) pH, acetic acid, and ethanol content than the control. Although DM losses and ASTA markedly improved in the treatments containing MnSO4 in the short-term trial, these effects were not observed in the long-term trial. These outcomes suggest that the selected additives can improve silage fermentation quality across a broad range of forages, but further studies are required to assess the impact of MnSO4 on ASTA.
1 INTRODUCTION
Ensiling is a preservation method to ensure sufficient livestock feed resources throughout the year, whether silage is prepared from annual or perennial grasses, cereals, maize (Zea mays L.) or legumes. Microbial inoculants have been extensively studied for their effectiveness in silage conservation and minimisation of feed loss during storage. In particular, homofermentative LAB, such as Lactiplantibacillus plantarum (L. plantarum), Pediococcus pentosaceous (P. pentosaceous), Pediococcus acidilactici, and Enterococcus faecium, as well as heterofermentative LAB, like Lentilactobacillus buchneri and Leuconostoc spp., are commonly used as silage inoculants (Carvalho et al., 2021; Fabiszewska et al., 2019). Homofermentative inoculants act as forage fermentation stimulants, facilitating rapid utilization of available nutrients (e.g., sugars), rapidly lowering pH and establishing anaerobic conditions, in turn reducing the proliferation of endophytic microbiota and limiting fermentation via less efficient pathways (McDonald et al., 1991). In contrast, heterofermentative inoculants are employed as inhibitors of aerobic deterioration, due to their high acetic acid (AC) production compared to uninoculated forages; however, this is often correlated with an increased DM loss (Borreani et al., 2018).
Silage additives can consist of single strains or a mixture of different microbial strains, and their efficacy in improving silage quality has been investigated primarily in single forage species, with limited studies involving multiple species (Gonda et al., 2023; Jatkauskas et al., 2013; Okoye et al., 2023). Although inoculants can be useful tools for managing silage quality, the success of silage fermentation is still dependent on other factors, such as crop composition, ensiling conditions, and management factors. Whereas forages treated with combinations of microbial inoculants, acids, salts, or molasses have been studied to maximize the efficacy of inoculant treatments, evidence suggests that the growth of some LAB members may be enhanced where specific trace elements are available (Archibald, 1986; Bomba et al., 2002). However, studies involving the addition of trace minerals for ensiling purposes, such as manganese (Mn), are scarce and it is unclear whether their supplementation improves ensiling quality when applied as part of an inoculant dressing (Gonda et al., 2022; Haag et al., 2016; Jatkauskas et al., 2013).
Despite lacking a complete electron transport chain (ETC) or the Krebs cycle, numerous Lactobacillus species, including L. plantarum, are aerotolerant facultative anaerobic microorganisms (Watanabe et al., 2012). They can colonize different niches, which explains their wide range of industrial applications where oxygen (O2) stress is inevitable. However, under aerobic conditions, for example, the first aerobic stage of the ensiling process, reactive oxygen species (ROS), such as superoxide (O2−), hydrogen peroxide (H2O2), and hydroxyl radical (HO˙), can be produced in the ETC, leading to DNA and cellular damage (Archibald & Fridovich, 1981; van de Guchte et al., 2002), potentially reducing fermentation efficiency and lactic acid (LA) production.
Mn, in a dialyzable form, has been reported to possess pseudocatalase properties, acting as an efficient O2− scavenger, similar to superoxide dismutase found in aerotolerant organisms (Archibald & Duong, 1984). L. plantarum, like most LAB, lack superoxide dismutase and is catalase-negative, while the catalase-like effects of Mn are well established (Watanabe et al., 2012). Mn(II), at concentrations of 20–25 mM, effectively scavenges O2−, comparable to the micromolar levels of superoxide dismutase found in various microorganisms (Archibald & Fridovich, 1981).
Understanding and consistently achieving optimal fermentative efficacy for silage preservation is important in maximizing the preservation of forage for more sustainable agricultural systems. To achieve this, a comprehensive evaluation of different silage additive compositions across various forage species and climatic conditions throughout the year is required (Fijałkowska et al., 2020; Seppälä et al., 2016). Therefore, the aim of the present study was to identify effective and innovative silage additives and assess their silage preservation performance across different forage types. The first short-term trial focused on studying the efficacy of 24 combinations, consisting of one out of three L. plantarum (IMI 507026, IMI 507027 and IMI 507208) and one out of two P. pentosaceous (IMI 507024 and IMI 507025) strain combinations at two ratios (50:50 and 75:25), with or without the addition of 25 mg/kg MnSO4, compared to negative control, after 7 days of ensiling and 7 days of aerobic stability (ASTA) testing. In the second, long-term trial, the eight best-performing combinations out of 24 from the first trial, selected based on fermentation efficacy, reduced nutrient loss, and improved ASTA, were evaluated for their performance in the preservation of three forage types: Medicago sativa L. (lucerne), Lolium perenne L. (perennial grass), and Lolium perenne: Trifolium repens L. (50:50 perennial grass: white clover) after an ensilage period of 100 d and a subsequent 10 d of ASTA testing.
2 MATERIALS AND METHODS
2.1 Treatments
Five lactic acid bacteria strains produced by Alltech (Alltech Inc., Kentucky, KY, USA) were used in the preparation of silage additives, namely three strains of Lactiplantibacillus plantarum (IMI 507026, IMI 507027, and IMI 507208) and two strains of Pediococcus pentosaceus (IMI 507024 and IMI 507025). These strains were deposited at the Centre for Agriculture and Bioscience International (CABI) Culture Collection. Their identity and safety information have been recently published (Nikodinoska et al., 2022a, 2022b, 2022c, 2022d, 2022e; Nikodinoska, Heikkinen, & Moran, 2023; Nikodinoska, Spohr, et al., 2023). Strains of both species were combined with each other at two different ratios, with or without the addition of MnSO4, resulting in 24 different silage additive treatments (Table 1). MnSO4, combined with microorganisms herein used, was previously tested and the outcome was summarized in a conference abstract (Gonda et al., 2022).
| Treatment | Cfu/g FM | MnSO4 | Abbreviation | Trial |
|---|---|---|---|---|
| Negative control (untreated: water) | - | - | T1 | ST, LT |
| Lactiplantibacillus plantarum (LP) IMI 507026: Pediococcus pentosaceous (PP) IMI 507024 (50:50) | 1 × 106 | - | T2 | ST, LT |
| LP IMI 507026: PP IMI 507024 (75:25) | 1 × 106 | - | T3 | ST |
| LP IMI 507026: PP IMI 507025 (50:50) | 1 × 106 | - | T4 | ST, LT |
| LP IMI 507026: PP IMI 507025 (75:25) | 1 × 106 | - | T5 | ST |
| LP IMI 507027: PP IMI 507024 (50:50) | 1 × 106 | - | T6 | ST, LT |
| LP IMI 507027: PP IMI 507024 (75:25) | 1 × 106 | - | T7 | ST, LT |
| LP IMI 507027: PP IMI 507025 (50:50) | 1 × 106 | - | T8 | ST |
| LP IMI 507027: PP IMI 507025 (75:25) | 1 × 106 | - | T9 | ST |
| LP IMI 507028: PP IMI 507024 (50:50) | 1 × 106 | - | T10 | ST |
| LP IMI 507028: PP IMI 507024 (75:25) | 1 × 106 | - | T11 | ST |
| LP IMI 507028: PP IMI 507025 (50:50) | 1 × 106 | - | T12 | ST |
| LP IMI 507028: PP IMI 507025 (75:25) | 1 × 106 | - | T13 | ST |
| LP IMI 507026: PP IMI 507024 (50:50) + Mn | 1 × 106 | 25 mg/kg FM | T14 | ST, LT |
| LP IMI 507026: PP IMI 507024 (75:25) + Mn | 1 × 106 | 25 mg/kg FM | T15 | ST, LT |
| LP IMI 507026: PP IMI 507025 (50:50) + Mn | 1 × 106 | 25 mg/kg FM | T16 | ST |
| LP IMI 507026: PP IMI 507025 (75:25) + Mn | 1 × 106 | 25 mg/kg FM | T17 | ST, LT |
| LP IMI 507027: PP IMI 507024 (50:50) + Mn | 1 × 106 | 25 mg/kg FM | T18 | ST |
| LP IMI 507027: PP IMI 507024 (75:25) + Mn | 1 × 106 | 25 mg/kg FM | T19 | ST |
| LP IMI 507027: PP IMI 507025 (50:50) + Mn | 1 × 106 | 25 mg/kg FM | T20 | ST |
| LP IMI 507027: PP IMI 507025 (75:25) + Mn | 1 × 106 | 25 mg/kg FM | T21 | ST |
| LP IMI 507028: PP IMI 507024 (50:50) + Mn | 1 × 106 | 25 mg/kg FM | T22 | ST |
| LP IMI 507028: PP IMI 507024 (75:25) + Mn | 1 × 106 | 25 mg/kg FM | T23 | ST |
| LP IMI 507028: PP IMI 507025 (50:50) + Mn | 1 × 106 | 25 mg/kg FM | T24 | ST, LT |
| LP IMI 507028: PP IMI 507025 (75:25) + Mn | 1 × 106 | 25 mg/kg FM | T25 | ST |
- Abbreviations: FM, fresh matter; Mn, MnSO4.
2.2 Study design
Two non-randomized silage trials were conducted, both including a negative control: a short-term trial (7 days) to assess 24 silage additive treatments in one forage type, and a long-term trial (100 days) to screen eight treatments in three different forage types. The fresh forage materials were harvested at the Research Farm Bottelare (HOGENT-UGent; Belgium; 50.96251° N, 3.761193° E). The forages were mown at cutting height of 6–10 cm using a Krone EasyCut mower-conditioner (Krone, Spelle, Germany), pre-wilted in the field, and chopped to 6–8 cm length with a New Holland 719 chopper (CNH Industrial Europe, Turin, Italy) before ensiling. For the ensiling experiments, mini-silos consisting of polyvinyl chloride tubes and rubber sealing caps with a capacity of 2.75 L were used. The mini-silos were equipped with a Bunsen valve to allow the release of fermentation gases while preventing air entry. Silage additive solutions were sprayed onto a thin, evenly spread layer of chopped and homogenized forage material using handheld sprayers, in a ratio of 10 mL per kg fresh matter (FM) for both the microorganism suspensions and the MnSO4 solution; the negative control was treated with 20 mL of water per kg FM. The mini-silos were then filled manually with a predetermined amount of fresh material in two stages, with intermediate and final pressing using a hydraulic press.
2.2.1 Short-term trial
For the short-term trial, a negative control (T1) and 24 silage additive treatments (T2-T25) were considered (Table 1). First-cut Lolium perenne was harvested at the early earing stage (Biologische Bundesanstalt, Bundessortenamt and CHemical industry – BBCH 51) and prewilted to 31.3% DM prior to ensiling on 8 June 2021; the water-soluble carbohydrates (WSC) content was 1.76% on FM basis, thus the grass is classified as moderately easy to ensile according to the European Food Safety Authority (EFSA) classification (EFSA et al., 2018); silo density was 160 ± 2 kg DM per cubic meter (m3). Each of the 24 treatments and the negative control were prepared as previously described. The starting material was sampled for subsequent analysis. Per treatment, four mini-silos were filled as replicates, and all mini-silos were stored at 20 ± 2°C for 7 d. Immediately after opening, the silage was sampled for analysis of the fermentation characteristics and ASTA determination.
2.2.2 Long-term trial
Eight out of the 24 treatments tested in a short-term trial were selected for the long-term trial, namely T2, T4, T6, T7, T14, T15, T17, and T24, complemented with a negative control (T1) (Table 1). For treatment shortlisting, in addition to the outcomes from hierarchical clustering and principal component analysis (PCA), various criteria were considered: low FM loss, pH, and NH3–N/Total N (at silo opening and the end of Honig's protocol for ASTA determination) and high LA and ASTA. Three forages were considered in three sub-trials for testing the performance of eight shortlisted treatments against the control. The following crops and related conditions were considered: (1) lucerne: third cut lucerne harvested on 17 September 2021 at early flowering (BBCH 50) and prewilted to 37.1% DM; WSC content was 0.75% on FM basis, classifying the crop as difficult to ensile according to the European Food Safety Authority (EFSA) classification (EFSA et al., 2018); silo density was 230 ± 1 kg DM/m3; (2) perennial grass (‘grass’): third cut perennial grass harvested on September 9th 2021 at early earing (BBCH 51) and prewilted to 41.8% DM; WSC content was 3.45% on FM basis, classified as easy to ensile according to the EFSA classification (EFSA et al., 2018); silo density was 265 ± 4 kg DM/m3; (3) perennial grass-white clover (‘grass-clover’). in a ratio of ~50/50 on (FM basis): third cut grass-clover harvested on 9 September 2021 at 30% flowering of the clover (BBCH 63) and prewilted to 49.9% DM; WSC content was 1.0% on FM basis, classifying the crop as difficult to ensile according to the EFSA classification (EFSA et al., 2018); silo density was 246 ± 7 kg DM/m3. The starting materials of all three crops were sampled for subsequent analysis. For each crop, five mini-silos were prepared per treatment as replicates. Mini-silos were stored at 20 ± 2°C during 100 d. At silo opening, samples were taken for the determination of fermentation characteristics and ASTA.
2.3 Chemical and microbiological analyses
2.3.1 Fresh forage
Duplicate samples of fresh material were collected from each of the four forage crops to determine: DM, NDF, crude ash, crude protein, NO3−N, and WSC concentration. Additionally, pH and buffering capacity were measured, along with colony-forming unit (cfu) enumeration of LAB, Clostridia, yeast, and moulds.
2.3.2 Silage
Mini-silos were opened after an ensilage period of either 7 days (d) (short-term trial) or 100 d (long-term trial). Fermentation losses were calculated throughout the ensiling period by subtracting the weight of the mini-silo each week from the initial mini-silo weight on 0 d. Sampling was conducted at silo opening to measure DM, DM losses during ensiling, crude protein, ammonia, NH3–N/Total N, LA, AC, propionic acid (PA), butyric acid (BA), ethanol (EtOH), WSC concentration, and pH. ASTA was assessed following the protocol outlined by Honig (1990) during a period of 7 d (short-term trial) or 100 d (long-term trial).
2.4 Analytical methods
The DM content of both the fresh and ensiled forages was determined by air drying at 60°C until a constant weight was achieved. The DM content of the silage samples obtained at silo opening was adjusted for volatiles lost during drying using the method described by Dulphy and Demarquilly (1981). Neutral Detergent Fibre (NDF) content was determined as described by Van Soest et al. (1991). Crude ash content was determined according to ISO 5984, and crude protein content was determined according to ISO 16634-1. Nitrate nitrogen (NO3−N) was analysed using ion chromatography according to the CMA 2/Ι/C.3:5/1996 method. WSC were determined using Luff-Schoorl's method (NEN3571). Buffering capacity was determined using a modified method based on Jasaitis et al. (1987), involving titration with 0.1 M HCl to pH 4.0, and recalculated to LA. The pH was measured using a watery extract in accordance with De Boever et al. (2013). Total N was determined according to ISO16634-1, the ammonia nitrogen content was determined using the Kjeldahl method (Kjeldahl, 1883), and the NH3–N/Total N was calculated as the ratio of ammonia nitrogen to total nitrogen.
LA, AC, PA and BA were analysed using high-performance liquid chromatography (HPLC) (Waters Inc., Milford, MA, USA) following the procedure outlined by Ohmomo et al. (1993). Briefly, the organic acids were detected by HPLC-UV in a watery silage extract (1:10 wt/vol) incubated for 24 h at 20°C and filtered through a 0.22-μm syringe filter. The HPLC device (Waters 2695) was equipped with an Aminex HPX-87H pre-column and column (Bio-Rad, Boulder, USA), a thermostat at 35 +/− 1°C (Waters) and a UV detector (Waters 2487). The mobile phase consisted of an 8 mM sulphuric acid solution with 2.5% acetonitrile, at a 0.6 mL/mL flow rate. The detection wavelength was 210 nm.
The EtOH content was determined in the aqueous extract using near-infrared absorption, based on the method described by Sørensen (2004). Enumeration of LAB was performed according to ISO 15214, while Clostridia enumeration followed ISO 15213. Yeast and mould counts were enumerated according to ISO 21527. The Mn content of silage samples from the short-term trial was measured after microwave digestion (n = 2) using an inductively coupled plasma optical emission spectrometry method; the analysis was outsourced to Dairyland Laboratories Inc. (Arcadia, WI, USA).
The ASTA was determined immediately after silo opening according to Honig (1990), by placing silage loosely into 1-L aerated recipients. Temperature data loggers (EL-USB-1, Lascar Electronics) were positioned at the geometric centre of each silage mass. A double layer of cheesecloth was placed on top of each recipient to prevent drying and contamination. Silage temperature was recorded at 20-min intervals for 7 d (short-term trial) or 10 d (long-term trial) and averaged over 2-h periods. The recipients were placed in insulated polystyrene boxes and allowed to aerobically deteriorate at 20 ± 2°C. Aerobic instability was indicated by a 3°C rise in temperature above the temperature registered in an acid-treated reference sample as defined by Driehuis et al. (2001). DM content and pH at the end of Honig's protocol were determined, and the DM loss calculated.
2.5 Statistical analysis
Statistical evaluation was conducted using the R v4.0.3 (R Core Team, 2020). The normality of the residuals was assessed using Shapiro–Wilk's test, and homogeneity of variances was checked using Levene's test. If these two assumptions were met, an analysis of variance (ANOVA) was performed. In the case of significant treatment effects (p < .05), Dunnett's test was used to determine whether individual treatments differed pairwise from the control treatment. If the conditions for ANOVA were not met, a non-parametric Kruskal-Wallis test followed by a post hoc Dunn test was carried out.
To cluster the treatments, the Euclidean distance was calculated as a measure of similarity, following which hierarchical clustering with complete linkages was performed. The resulting dendrogram presents the outcomes.
Boxplots were used to represent the data, providing a graphical depiction of the median (represented by a horizontal line) and quartiles (Q1–Q3, displayed as a box). The upper whisker is positioned at the smaller of the maximum × value and Q3 + 1.5 × interquartile range, while the lower whisker is positioned at, the larger of the minimum × value and Q1–1.5 × interquartile range. An outlier is a data point located beyond the boxplot whiskers, exceeding 1.5 times the interquartile range, above the upper or lower quartile.
3 RESULTS
3.1 Short-term trial
Table 2 presents the chemical and microbiological compositions of the fresh grass forage used in the short-term trial. The epiphytic LAB counts were higher than yeast and mould counts. In addition, Mn content ranged from 71 to 81 mg/kg Mn in untreated forages and from 91.5 to 110.5 mg/kg Mn in MnSO4-treated silages (Table S1).
| Starting material | Mean | SEM |
|---|---|---|
| DM (g/kg FM) | 31.30 | 1.53 |
| pH | 6.09 | 0.04 |
| Crude protein (g/kg DM) | 120.00 | 1.15 |
| NDF (g/kg DM) | 544.00 | 10.58 |
| WSC (g/kg FM) | 1.76 | 0.20 |
| LAB (log cfu/g FM) | 7.51 | 0.09 |
| Yeasts (log cfu/g FM) | 4.37 | 0.10 |
| Moulds (log cfu/g FM) | 5.36 | 0.06 |
- Abbreviations: cfu, colony-forming units; DM, dry matter; FM, fresh matter; LAB, lactic acid bacteria; NDF, neutra-detergent fibre; WSC, water-soluble carbohydrates.
The short-term trial involved grass forage ensiled for 7 d at 20°C, and the results are summarized in Figure 1. The concentrations of PA and BA were below the detection limits. The pH in all treated silages ranged between 3.89 and 4.01 and was lower than that in the control (average pH 4.25) (p < .001). FM losses were lower (p < .001) compared to the control (0.42% on average) in all treated silages (0.29%–0.38%) except for T25. Additionally, all treatments excluding T12, T14, T18, and T20–T25 showed (p < .05) reduced NH3–N/Total N compared to the control. The combination of strains, ratio, and MnSO4 addition affected LA and AC contents (p < .05), but there was no significant interaction between these factors. All treatments exhibited higher (p < .05) LA content (increased by 19%–36%) and lower AC content (reduced by 15%–46%) compared to the control (p < .05). ANOVA results indicated that, excluding AC, one or more interactions among the strain combination (Combo), strain ratio, and MnSO4 were significant.
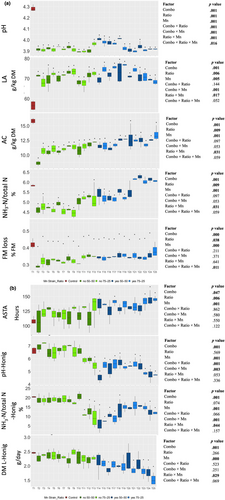
The ASTA values as shown in Figure 1 were higher (p < .05) in silages treated with T14, T17, and T23–T25, indicating ~33 h longer stability compared to the control. At the end of Honig's protocol, the pH after Honig's test (pH-Honig) in T13 and all silages treated with MnSO4 (T14–T25), ranging from 4.50 to 5.70, was lower (p < .001) compared to the control (pH-Honig = 6.63). Similar as pH-Honig, the ammonia nitrogen fraction after Honig's test (NH3–N/Total N-Honig) was lower (p < .001) in treatments T11–T18 and T24–T25, ranging between 6.53% and 13.23%, compared to the control (20.43%). Additionally, DM losses during Honig's protocol were lower (p < .001) in silages treated with MnSO4 (T16, T21, T23–T25) than in the control. Factorial ANOVA indicated that apart from ASTA, one or more interaction effects were significant for all other parameters.
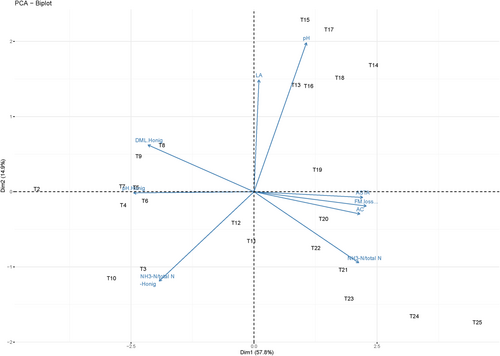
PCA results for the short-term trial are presented in Figure 2. The first two principal components (PCs) explained a cumulative variance of 77.9%. PC1 clearly distinguished treatments with and without MnSO4, where treatments with MnSO4 (T13–T25) exhibited high ASTA values and fermentation-related parameters such as AC, FM loss, and NH3–N/Total N. However, for ASTA-related parameters (pH-Honig, NH3–N/Total N -Honig, and DM loss after Honig [DM loss-Honig]), MnSO4 treatments exhibited low values because of the opposite direction of the loading vectors for these parameters.
Hierarchical clustering analysis was conducted based on FM loss, pH, AC, LA, NH3-N/Total N, ASTA, pH-Honig, DM loss-Honig, NH3-N/Total N-Honig, and the results are illustrated in Figure 3. This analysis resulted in three clusters: the first cluster included the control, which was markedly different from the treatments with silage additive application; the second cluster comprised most treatments without MnSO4 (T2–T10); and the third cluster consisted of all treatments with MnSO4 (T14–T25) and two treatments without MnSO4 (T11, T13).
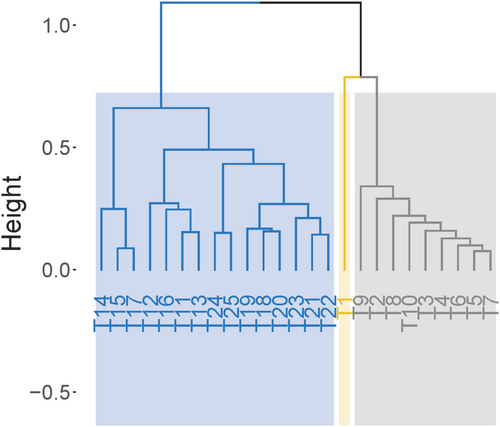
Based on screening criteria described in section 2.3.2, the following treatments were selected: T2, T4, T6, and T7 for their low FM loss, pH, NH3–N/Total N, and AC content; T14, T15, and T17 for high LA content, low pH-Honig, and NH3–N/Total N- Honig; and T24 for high ASTA and low DM loss-Honig, pH-Honig, and NH3–N/Total N- Honig.
3.2 Long-term trial
Table 3 summarizes the chemical and microbiological characteristics of the fresh forages used as starting material in the long-term trial. The DM contents of the lucerne, grass and grass-clover were respectively 31.1%, 41.8% and 49.9%. According to EFSA's classification based on WSC content, the grass is classified as easy to ensile, and the lucerne and grass-clover as difficult to ensile. LAB counts were similar in all three forages, whereas yeast and mould counts were below the limit of detection in lucerne, and Clostridia counts were higher in grass-clover.
| Starting material | Lucerne | Perennial grass | Grass-clovera | |||
|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | |
| DM (g/kg FM) | 37.10 | 1.50 | 41.80 | 2.80 | 49.90 | 4.50 |
| pH | 6.00 | 0.00 | 6.20 | 0.00 | 6.05 | 0.04 |
| Buffering capacity (meq HCl/kg DM) | 123.42 | 11.27 | 48.50 | 5.65 | 76.01 | 3.60 |
| CP (g/kg DM) | 188.36 | 0.22 | 137.90 | 2.50 | 152.60 | 2.08 |
| Ash (g/kg DM) | 114.24 | 2.28 | 95.02 | 3.42 | 120.25 | 5.60 |
| NO3-N (mg/kg DM) | 2378.04 | 42.02 | 421.33 | 38.20 | 200.18 | 2.61 |
| NDF (g/kg DM) | 489.00 | 11.00 | 574.06 | 0.44 | 551.83 | 5.04 |
| WSC (% of FM) | 0.75 | 0.82 | 3.45 | 0.13 | 1.00 | 0.41 |
| LAB (log cfu/g FM) | 5.10 | 0.02 | 5.11 | 0.00 | 5.85 | 0.01 |
| Yeasts (log cfu/g FM) | <2.00 | 0.00 | 6.40 | 0.00 | 6.85 | 0.47 |
| Moulds (log cfu/g FM) | <2.00 | 0.00 | 5.93 | 0.31 | 6.46 | 0.05 |
| Clostridia (log cfu/g FM) | 2.40 | 0.10 | 2.02 | 0.17 | 4.12 | 0.87 |
| EFSA classification | Difficult to ensile | Easy to ensile | Difficult to ensile | |||
- a Perennial grass-white clover in a ratio of approximately 50/50 on FM basis.
- Abbreviations: CP, Crude protein; cfu, colony-forming units; DM, dry matter; FM, fresh matter; LAB, lactic acid bacteria; NDF, neutral detergent fibre; WSC, water-soluble carbohydrate; EFSA classification: easy to ensile (>3% WSC), moderately difficult to ensile (1.5–3% WSC), and difficult to ensile (<1.5% WSC), all on an FM basis.
In the long-term trial, eight treatments were tested to assess their efficacy in preserving the quality of the lucerne, grass and grass-clover as silage. Four treatments were selected from each cluster in the PCA (Figures 3 and 4), which included T1, T2, T4, T6, T7, T14, T15, T17, and T24. The results of the long-term trial are presented in Figure 4. Application of a silage additive significantly influenced fermentation-related parameters. Specifically, pH in all treated silages was lower (p < .001) compared to the control, with values for treated lucerne, grass-clover, and grass silage resp. in the ranges 4.61–4.73, 4.14–4.18, and 3.87–3.94. LA content was higher (p < .001) in all treated silages than in the control, with the highest range observed in grass-clover (76.26–85.84 g/kg DM), followed by grass (69.13–75.95 g/kg DM) and lucerne (65.60–70.03 g/kg DM). AC content was affected (p < .001) by the silage treatment only in grass silage, showing a decrease compared to the control. However, AC content of lucerne and grass-clover treated with T2 or T7 did not differ significantly from that of the respective controls. EtOH content was lower (p < .001) in all treated silages than in the controls, ranging from 14.7 to 18.2 g/kg DM in lucerne, 10.6–11.4 g/kg DM in grass-clover, and 13.3–15.3 g/kg DM in grass silage.
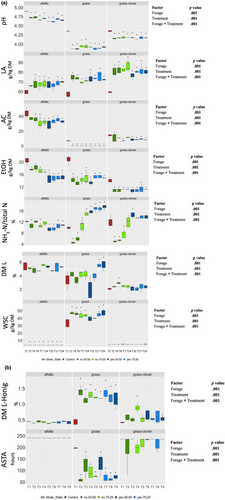
The proteolytic indicator NH3-N/Total N showed a highly variable response. NH3-N/Total N was lower (p < .001) in treated lucerne silages than in the control, except for T4 and T7. Moreover, lower (p < .001) values were observed in treated grass silages for T2 and T4. However, NH3-N/Total N was higher (p < .001) in T7, T14, T15, T17, and T24 than in the control grass silage. In grass-clover treated with T4 or T6, NH3-N/Total N was lower (p < .001), compared to the control, whereas silage treated with T14 had a higher (p < .001) NH3-N/Total N than the control. WSC content was lower in lucerne and grass-clover silages than in grass silage, which was also the case prior to ensiling. However, the WSC content was higher (p < .001) in all treated lucerne and grass silages (except for T14 and T15 in grass) than in the corresponding controls, but not in the grass-clover silages (except for T24). PA and BA concentrations were below the detection limits (0.1 g/kg of DM) in all samples (data not shown).
Figure 4b depicts the ASTA and DM loss after 10 d of aerobic exposure during Honig's protocol (DM loss-Honig). ASTA was higher in lucerne and grass-clover silage than in grass silage. However, ASTA was not affected significantly by treatment in lucerne silage. Conversely, all silage additive treatments reduced ASTA in grass silage, while in grass-clover silage ASTA was reduced only by T14 compared to the control. DM loss-Honig did not differ significantly between the control and the silage additive treatments for lucerne silage. In contrast, compared with those in the control group, the corresponding losses were significantly higher in all treated grass silages, but only in grass-clover treated with T6.
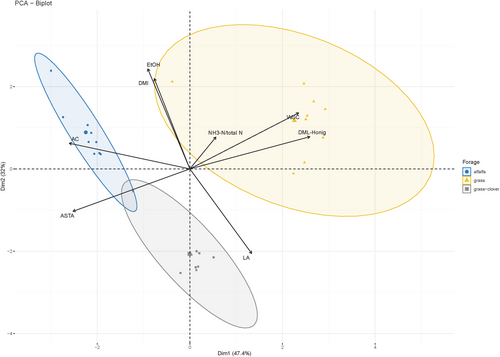
A biplot summarizing the results of the PCA for the long-term trial with the three forages is shown in Figure 5. The first two PCs explained a cumulative variance of 79.4%. PC1 and PC2 clearly differentiated the different forages under study. Overall, for the three forages included in the long-term trial, a very strong positive correlation was observed between DM loss and EtOH, which were both negatively correlated with LA. WSC was positively correlated with both NH3/Total N and DM loss-Honig, and all these three parameters were negatively correlated with ASTA.
4 DISCUSSION
Efficient silage additives improving fermentation are expected to exhibit the following desirable forage improvement patterns: (a) low fermentation losses, indicating an efficient ensiling process; (b) low pH at end-fermentation point; (c) low NH3–N/Total N and high crude protein content at silo opening, indicating limited protein degradation, especially in grass and legume forages; (d) high LA content and low AC content, indicating homofermentative LAB fermentation, and (e) high ASTA, indicating resistance to aerobic deterioration (McDonald et al., 1991). In this study, the examined silage additives showed the potential to improve one or more of these parameters. Specifically, all silage additive treatments improved silage fermentation, shifting towards a homofermentative pathway compared to the negative control, as demonstrated by high LA and low AC contents. The homofermentative pathway has been associated with lower fermentation losses than the heterofermentative pathway (Borreani et al., 2018). This aspect was confirmed in the short-term study, where fermentation losses were reduced (p < .001) in silages treated with different homofermentative LAB containing silage additives after a 7 days ensiled period. The lower pH values (p < .001) compared to the control for almost all treated silages, with pH values <4.0, indicated good strain compatibility within the strain combinations. Pediococci generally initiate the initial phase of the ensiling process because of their short lag phase, which allows for a rapid decrease in pH and enhances the growth and dominance of lactobacilli during the ensiling period (Fitzsimons et al., 1992; Liu et al., 2019). Interestingly, the aerobic stability during 7 d of aerobic exposure at 20°C was improved when silages were treated with additives containing MnSO4, as evidenced by prolonged ASTA and lower pH, NH3–N/Total N-Honig, and DM loss-Honig compared to the control.
Bacteria employ enzymatic degradation or scavenging to eliminate ROS as a coping mechanism under aerobic stress (van de Guchte et al., 2002). Among the mechanisms for enzymatic degradation-based ROS elimination, superoxide dismutase (sodA), catalases (heme-dependent), and pseudocatalases (non-heme-dependent/Mn-dependent pseudocatalase) have been reported (van de Guchte et al., 2002). L. plantarum, similar to most LAB, lacks superoxide dismutase and is catalase-negative; however, the presence of Mn catalase can compensate for this deficiency (Igarashi et al., 1996). Although the catalase gene (katA) and pseudocatalase have been described in the L. plantarum genome, their catalytic activities are very low unless micronutrients are available in the environment, which leads to high gene expression, H2O2 decomposition, and enhanced biomass production (Yu et al., 2019). This may also explain the high Mn requirement for L. plantarum growth (MacLeod & Snell, 1947). When L. plantarum WCFS1 was grown in De Man, Rogosa, and Sharpe agar (MRS) with and without added Mn (0.04 g/L Mn (II) sulphate monohydrate) and under different growth conditions (fermentative, aerobic, aerobic with haem, and respiratory conditions), the cultures supplemented with Mn reached higher cell density and lower pH values than cultures without Mn (Watanabe et al., 2012). This observation confirms that the required Mn level and availability are crucial for maximum microbial growth and oxygen radical detoxification (MacLeod & Snell, 1947; Watanabe et al., 2012).
Considering the growth of lactobacilli across different silage phases, literature suggests that the initial 1–14 d of ensiling constitute the fermentation phase, characterized by peak metabolic activity in lactobacilli. As the ensiling process advances, the pH stabilizes at a low level, leading to reduced microbial activity and marking the onset of the storage phase. Subsequently, during the feed-out phase, lactic acid is consumed by yeasts and moulds, causing an increase in pH (Okoye et al., 2023). In the 7 d short-term trial, corresponding to the fermentation phase with heightened metabolic activity, Mn potentially stimulated the growth of metabolically active aerotolerant lactobacilli, contributing to a prolonged aerobic stability phase. However, when the silage of the long-term trial was opened after 100 d of ensiling, no such outcomes were observed, possibly due to slower lactobacilli growth and lack of enhancement in the presence of Mn under such conditions, warranting further investigation. In addition, the comparison between the short-term and the long-term trials might be tricky as many factors vary, including the initial DM content in the forages.
In the long-term trial with an ensiled period of 100 d, the preservation of the three forages was evaluated in untreated and treated silages. The lucerne and grass-clover starting materials were classified as difficult to ensile because of their very low WSC contents, respective 0.75% and 1.00% on FM basis, with WSC being an important substrate for the initiation of the fermentation process (EFSA et al., 2018). The grass had a WSC content of 3.45% in the FM before ensiling and classified as easy to ensile. The epiphytic LAB counts were ~1 log cfu/g FM lower than the microbial inoculant application rate (1 × 106 cfu/g FM).
The DM content of the forages is known to affect fermentation-related parameters (McDonald et al., 1991; Wilkinson & Davies, 2013). McDonald et al. (1991) found that grass forages with low DM content had a higher WSC content, an optimal condition for LAB activity, resulting in low pH (~4) and high LA production (165 g/kg DM). Conversely, forages with high DM content exhibit potentially lower LAB activity, leading to higher pH (~5) and lower LA (~34 g/kg DM) than that for low-DM forages. In the present study, the grass, and grass-clover forages from the long-term trial had high DM contents (41.8% DM and 49.9% DM, respectively), and the observed pH values ranged from 3.98 to 4.18, with LA contents ranging from 69.13 to 85.84 g/kg DM. These findings indicate that the high DM contents and low WSC levels in the treated silages did not negatively impact microbial activity, regardless of the treatment applied. Seppälä et al. (2016) observed a similar fermentation pattern was observed when high DM content (500 g/kg DM) timothy, and meadow fescue grasses with WSC levels of 125 g/kg DM were treated with homolactic LAB strains. Based on the optimal range of DM suggested by Seppälä et al. (2016), the DM levels of the ensiled grass-based forages in the present study were quite high; however, testing the efficacy of silage additives under suboptimal conditions is very valuable, as ensiling in practice also occurs outside the optimal range for forages, owing to various factors, including climate conditions.
Compared to spontaneously fermented control silages, all treatments tested improved the fermentation-related parameters, namely low pH, high LA, low AC and low EtOH, regardless of the forage type. A homofermentative pathway, with a high LA/AC ratio and low EtOH content, was confirmed in all treated forages. However, the NH3-N/Total N, indicative of proteolysis, was improved in all forages treated with T2, whereas the performance of the other treatments was observed only in selected forages, with the lowest performance for Mn-added treatments in grass silages. Most treatments did not affect fermentation losses during the 100 d ensiled period.
The expected prolonged ASTA observed in silages fermented for only 7 d in the short-term trial upon MnSO4 addition was not confirmed in the long-term trial with silages fermented for 100 d. This outcome may be influenced by several factors as stressed above, which will be investigated in future studies. However, it is interesting to note that in the lucerne and grass-clover forages, most treatments did not differ from the negative control in terms of ASTA-related parameters. ASTA is generally reduced in silages treated with homofermentative inoculants, owing to a high LA content in combination with a low AC content and a high residual WSC content, providing substrate for spoilage yeast growth (Wilkinson & Davies, 2013). The high residual WSC content in the grass silage (where the starting material was classified as easy to ensile based on the high WSC content in the starting material) influenced ASTA negatively (Figure 5).
5 CONCLUSION
This study identified eight effective microbial combinations with or without MnSO4 addition for application as a silage additive in different forage types. Initial results from a short-term trial with an ensiling period of 7 d, followed by 7 d ASTA testing, suggested enhanced ASTA in certain treatments containing MnSO4. However, these observations were not confirmed in the long-term trial with 100 d ensiled period. Future research will investigate the impact of MnSO4 on silage quality and ASTA improvement in different phases of the ensiling process. In the long-term trial, all tested treatments demonstrated their efficacy in improving the homolactic fermentation patterns in three forages: lucerne, grass, and grass-clover. Some treatments, such as T2, consistently reduced NH3-N/Total N, regardless of the forage type, whereas selected treatments containing Mn increased this parameter in grass forage. Based on these observations, it can be concluded that all silage additive treatments selected for the long-term trial were successful at improving the silage fermentation, without compromising the ASTA in lucerne and grass-clover forages. The short-term model proved to be an effective screening tool for inoculant selection, with good agreement on the fermentation characteristics between the 7 d and 100 d studies. The short-term ASTA model could not be deemed to be representative of the effects post-100 d ensiling, and therefore, the model is not fit for purpose under the conditions used in this study.
ACKNOWLEDGEMENTS
The authors wish to acknowledge Dr. Kamal Mjoun (Alltech, South Dakota) for the manganese analysis and Dr. Kate LeCoq (Harper Adams University, United Kingdom) for her review of the manuscript and commentary.
FUNDING INFORMATION
The study was funded by Alltech SARL (France).
CONFLICT OF INTEREST STATEMENT
The authors IN and CAM are employees of Alltech which produces and markets different silage additive preparations under the Egalis® brand. The authors EW and SL declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
All relevant data were included in this study. The corresponding author can be contacted if further explanation is required.




