Cover crop root contributions to soil carbon in a no-till corn bioenergy cropping system
Abstract
Crop residues are potential biofuel feedstocks, but residue removal may reduce soil carbon (C). The inclusion of a cover crop in a corn bioenergy system could provide additional biomass, mitigating the negative effects of residue removal by adding to stable soil C pools. In a no-till continuous corn bioenergy system in the northern US Corn Belt, we used 13CO2 pulse labeling to trace plant C from a winter rye (Secale cereale) cover crop into different soil C pools for 2 years following rye cover crop termination. Corn stover left as residue (30% of total stover) contributed 66, corn roots 57, rye shoots 61, rye roots 50, and rye rhizodeposits 25 g C m−2 to soil. Five months following cover crop termination, belowground cover crop inputs were three times more likely to remain in soil C pools than were aboveground inputs, and much of the root-derived C was in mineral-associated soil fractions. After 2 years, both above- and belowground inputs had declined substantially, indicating that the majority of both root and shoot inputs are eventually mineralized. Our results underscore the importance of cover crop roots vs. shoots and the importance of cover crop rhizodeposition (33% of total belowground cover crop C inputs) as a source of soil C. However, the eventual loss of most cover crop C from these soils indicates that cover crops will likely need to be included every year in rotations to accumulate soil C.
Introduction
Bioenergy production could provide more sustainable energy and reduce dependency on fossil fuels, while using existing infrastructure for fuel delivery (Department of Energy, 2011). Crop stover, not used for food, represents an important potential feedstock for biofuel production, but its removal could lead to lower soil carbon (C) stocks (Clapp et al., 2000; Anderson-Teixeira et al., 2009) and thereby offset some of the greenhouse gas benefits of biofuel production (Gelfand et al., 2010). Removing 25% or 50% of stover biomass has been estimated to reduce soil C by 3 and 8 Mg ha−1, respectively (Anderson-Teixeira et al., 2009). Studies have reported that maintaining soil C pools under corn requires 6–12.5 Mg ha−1 yr−1 stover input (Zanatta et al., 2007; Pikul et al., 2008; Johnson et al., 2014), depending on edaphic properties and management practices (Wilhelm et al., 2007). For example, Johnson et al., 2006 found that conventional tillage required more stover to maintain soil C than no-till (7.6 vs. 5.3 Mg ha−1 yr−1, respectively). By increasing plant C inputs to soil, cover cropping is one of the more promising management practices that could reduce the effects of stover removal on soil C stocks.
In annual temperate agroecosystems, cover crops are often grown during seasonal windows when there are no cash crops (e.g., fall-spring). Cover crops provide many benefits to agricultural systems including weed suppression and soil aggregation and are also known to promote soil C formation (McDaniel et al., 2014a; Kallenbach et al., 2015; Tiemann et al., 2015). Thus, including them in bioenergy cropping systems may counteract the removal of aboveground crop residues by increasing biomass inputs. Belowground cover crop inputs are known to contribute disproportionately to soil carbon (Puget & Drinkwater, 2001; Rasse et al., 2005; Kong & Six, 2010; Mendez-Millan et al., 2010) and, as a result, cover crops may serve as a useful tool for maintaining soil C even if cover crop shoot biomass is harvested as a biofuel feedstock (Moser et al., 2009). While the role of aboveground cover crop biomass in building soil organic matter (SOM) has been widely discussed in the literature (e.g., Barber, 1979; Hooker et al., 1982; Campbell et al., 1991; Drinkwater et al., 1998; Marriott & Wander, 2006; Calegari et al., 2008; Steele et al., 2012), little attention has been paid to the role of belowground cover crop inputs (but see Puget & Drinkwater, 2001; Kong & Six, 2010), and whether they might be sufficient to offset stover removal.
Several studies have shown that belowground root-derived inputs contribute disproportionately to soil C compared to aboveground shoot inputs (Balesdent & Balabane, 1996; Clapp et al., 2000; Rasse et al., 2005; Kong & Six, 2010; Mendez-Millan et al., 2010; Clemmensen et al., 2013; Mazzilli et al., 2015). Studies using biomarkers specific to root and shoot tissue (Mendez-Millan et al., 2010; Ji et al., 2015) and natural abundance isotopes (Balesdent & Balabane, 1996; Mazzilli et al., 2015) show more root than shoot C in SOM, as do studies of cover crops using isotope labels (Puget & Drinkwater, 2001; Kong & Six, 2010, 2012).
In addition to root biomass, rhizodeposits are an additional source of belowground C. Annual grain crops allocate 30–50% of photosynthate belowground, and 30–50% of belowground C are attributed to rhizodeposits although values as high as 40% of total plant inputs have been reported (Barber & Martin, 1976; Meharg & Killham, 1991; Puget & Drinkwater, 2001; Kuzyakov et al., 2003; Butler et al., 2004; Jones et al., 2009). Up to 75% of soil C inputs to SOM come from belowground sources including root biomass and rhizodeposits, whereas C from plant shoots is mostly lost via respiration (Gale et al., 2000). As for shoots, the majority of annual crop root biomass turns over in a single pulse at the time of plant death. However, during the growing season rhizodeposits are continuously being added to SOM from root turnover, sloughed or border cells, mycorrhizal hyphae, actively released secretions, and passively released exudates (Jones et al., 2009; Bradford et al., 2012). Rhizodeposits thus represent a significant input to soil C that may differ from biomass inputs because they are continuous, differ in chemical composition, and enter SOM in close physical proximity to soil minerals and microbial communities. Continuous rhizodeposition can stimulate microbial biomass production and activity, a key precursor for SOM formation (Grandy & Neff, 2008; Schmidt et al., 2011; Wieder et al., 2014, 2015; Kallenbach et al., 2015), and rhizodeposits may be preferentially protected in aggregates on mineral surfaces (Rasse et al., 2005; Dungait et al., 2012; Mazzilli et al., 2015).
Recent work suggests occlusion in soil aggregates or mineral association may be more important mechanisms for long-term SOM stability than reduced decomposition rates via chemical recalcitrance (Grandy & Neff, 2008; Dungait et al., 2012; Wieder et al., 2014; Kallenbach et al., 2015, 2016). Thus, organic matter occlusion in soil aggregates or association with minerals influences its accessibility to microbes, and thus potential to persist in soil (Six et al., 2002; Grandy et al., 2009). In order to capture functionally different SOM pools, fractionation methods are used to separate SOM into pools with distinct protection mechanisms (Zimmermann et al., 2007). The simplest density fractionation defines two pools: a light fraction containing minimally processed inputs known as particulate organic matter (POM) and the remaining heavy fraction (Gregorich et al., 2006). Occluded POM can be isolated by breaking up aggregates prior to density fractionation, and sonication can disrupt a variety of associations between organic matter and mineral surfaces. Complex fractionation schemes may seive multiple sizes of soil aggregates or use multiple sonication steps to isolate SOM with increasingly strong mineral associations and, presumably, turnover times (von Lützow et al., 2007). Cover crop residues in free POM may represent a short-term SOM pool compared to cover crop inputs in aggregates or in the heavy, mineral-associated fraction.
While past studies highlight the potential utility of cover crops for restoring soil C lost to stover removal, their potential to do so under different agricultural management scenarios has not been well studied. For instance, tillage intensity is likely to impact the relative contribution of root vs. shoot C to SOM (Allmaras et al., 2004). Presumably incorporation of aboveground cover crop biomass via tillage would lead to increased shoot C storage; however, studies of root and shoot contributions to SOM have usually taken place in tilled systems (Puget & Drinkwater, 2001; Kong & Six, 2010). Thus, it remains uncertain whether cover crop root and/or shoot inputs could help counteract the negative effects of residue removal on soil C in no-till bioenergy cropping systems.
Here, we examine the relative contributions of cover crop root and shoot to soil C in order to determine whether belowground cover crop C could help offset the deleterious effects of residue removal in a no-till continuous corn bioenergy cropping system. We labeled cereal rye (Secale cereale), a common winter cover crop, in situ with 13CO2, tracked inputs from rhizodeposits during the growing season, and tracked root and shoot inputs into different soil C pools over the following 2 years. We address four specific questions: (1) What are the relative contributions of different cover crop inputs to soil C? (Cshoot, Croot, and Crhizo; Table 1); (2a) How much Crhizo is incorporated into MBC during cover crop growth; and (2b) is Cshoot or Cbg preferentially incorporated into MBC during cover crop decomposition? (3) How are Cshoot and Cbg distributed among three soil density fractions?; and (4) How long do Cshoot and Cbg persist in soils?
| Abbreviation | Definition |
|---|---|
| Cover crop carbon inputs: Pulse labeling of cover crops in situ provides realistic estimates of above and belowground C inputs without disturbing root-soil interactions. At the time of cover crop termination we measured root biomass and soil C from cumulative rhizodeposits during cover crop growth. However, after termination it is not possible to distinguish between rhizodeposit 13C, which occurred during the growing season, and 13C from decomposing root biomass following cover crop death. We therefore define a fourth pool of belowground C inputs resulting in four specific plant C inputs, defined below. | |
| Cshoot | Carbon input from cover crop shoot biomass |
| Croot | Carbon input from cover crop root biomass, measured at the time of cover crop harvest |
| Crhizo | Carbon inputs from rhizodeposits during cover crop growth (e.g., exudates, sloughed cells, root hair turnover) |
| Cbg | Carbon from all belowground cover crop inputs: root biomass and rhizodeposits. (After cover crop termination, Croot and Crhizo combine into Cbg.) |
| Experiments: We performed an initial labeling experiment in 2013 to assess the fate of cover crop C inputs following cover crop termination. Early results indicated that growing season rhizodeposits represented a significant source of cover crop C inputs. We therefore performed a second labeling experiment in 2014 to measure belowground cover crop inputs during plant growth. | |
| EXP1 | Experiment one: Labeling took place in five pulse labeling events during April and May 2013. Samples were measured at 0, 5, 12, and 17 months following cover crop termination. |
| EXP2 | Experiment two: Cover crops were labeled in five pulse labeling events during April and May 2014. Samples were measured during cover crop growth 24 h following the first and third labeling events, and 0, 5, and 12 months following cover crop termination. |
| Soil density fractions: We define three soil density fractions based on separation with 1.6 g L−1 sodium polytungstate and calculated as a proportion of sand-free soil. | |
| FLF | Free light fraction: particulate organic matter <1.6 g L−1 density |
| OLF | Occluded light fraction: particulate organic matter <1.6 g L−1 density released by shaking to disrupt soil aggregates |
| MHF | Mineral heavy fraction: >1.6 g L−1 density |
Materials and methods
Experimental design and labeling
The Great Lakes Bioenergy Research Center (GLBRC) Biofuel Cropping System Experiment (BCSE, http://glbrc.org/) was established in 2008 at the Kellogg Biological Station LTER site (42° 24′ N 85° 24′ W, 288 m asl) in Southwest Michigan, USA. Temperature at the site ranged from −26.5 °C to 34.4 °C during the period of experimentation (2013–2014), and mean annual air temperature was 8.8 °C in 2013 and 7.6 °C in 2014. Precipitation was 1177 mm in 2013 and 933 mm in 2014. The dominant soil series is Kalamazoo (fine-loamy, mixed, mesic Typic Hapludalfs; Muñoz & Kravchenko, 2011; Tiemann & Grandy, 2015). Cover crops were added to the no-till continuous corn treatment in 2012. The treatment was replicated in five 30 × 40 m replicate plots with a subplot (4.6 × 13.1 m) in which cover crops were terminated via glycophosphate application. The winter cover crop Secale cereale (winter rye) was planted November 10, 2012 and October 29 and 30 of 2013. The cover crop in the subplot was terminated with herbicide just prior to planting of corn (Zea mays, Pioneer P8906AM Corn Hybrid) on June 5, 2013 and May 30, 2014.
To assess the fate of cover crop root and shoot C in bulk soils, soil density fractions, and microbial biomass over a 2 year period, we established a reciprocal litter transfer experiment with 13CO2 labeled winter rye in the spring of 2013 (EXP1, Table 1). Early results indicated that rhizodeposition could be an important component of belowground C inputs, and we therefore established a second labeling experiment following the same methods in adjacent plots in 2014 (EXP2, Table 1) to estimate belowground inputs to bulk soil and microbial biomass during the growing season. At the start of each experiment (EXP1 and EXP2), we established three 1 m2 plots in each block of the BSCE. One plot in each block was randomly designated for 13CO2 pulse labeling as described below. We chose to establish the plots within the herbicide treatment to minimize transfer of cover crop residues between subplots by farm equipment. However, cover crops in our plots were clipped prior to glycophosphate application (further described below) and thus not terminated by herbicide.
Pulse labeling was carried out five times between snow melt in early April and cover crop termination in late May. At the start of each labeling event, the designated plot was enclosed under a 1 m2 adjustable height chamber constructed of PVC and clear vinyl sheeting. To seal the chamber to the soil, we placed sandbags along the vinyl where it met the ground. We monitored the concentration of CO2 in the chamber continuously using a portable infrared gas analyzer (Qubit CO2 Analyzer, Model S-151; Qubit Systems, Kingston, ON, Canada) and deployed a small fan inside the chamber to maintain an even distribution of CO2. We recorded initial CO2 concentration in the chamber and added 99 atom percent enriched 13CO2 at the rate of 1 L min−1 for 2–3 min to a maximum level of roughly double ambient CO2 concentration (actual mean 853 ppm). The chamber was left in place until the CO2 concentration returned to ambient levels, the duration of the period between peak CO2 concentration and removal of the chamber ranged from 18 to 96 min (mean 41 min). Because photosynthetic rate varies throughout the day, all labeling occurred between the hours of 10 : 00 and 15 : 00 and the blocks were visited in random order each time. Labeled plots were re-covered with the chambers at night to capture 13CO2 lost from nighttime respiration for re-assimilation the following morning, thereby increasing our labeling efficiency.
Treatment establishment and plant sampling
Following pulse labeling and prior to corn planting, we terminated rye cover crop by clipping aboveground biomass to ground level on May 24, 2013 in EXP1 and May 24, 2014 in EXP2. We collected rye and weeds separately, air-dried, weighed, and cut the shoots into 2.5 cm pieces before returning the material to the soil surface. Aboveground biomass was transferred among three plots within each block to create a root plot containing labeled roots and unlabeled shoots, a shoot plot containing unlabeled roots and labeled shoots, and a control plot containing unlabeled roots and shoots.
We estimated root biomass at the time of rye termination in EXP2 by isolating roots from bulk soil cores; four soil cores (5 cm diameter, 15 cm deep) were collected per plot, and roots larger than 2 mm diameter were isolated by sieving fresh soil collected at the rye termination date. To ensure we were accurately estimating root biomass, two air-dried soil samples from control plots were later wet sieved to 250 μm and fine roots and all discernible root material were collected under a dissecting microscope. Because wet sieving resulted in negligible increases in root biomass estimates, we consider soil sieved to 2 mm root free. Root biomass in EXP1 plots were estimated using the aboveground biomass measures in EXP1 and the ratio of total root biomass to shoot biomass in EXP2 plots. Additional rhizodeposits were estimated using the δ13C values of bulk soil collected from the root plots at the time of cover crop termination and sieved to 2 mm. Belowground C inputs based on bulk soil δ13C ‰ values at the time of cover crop termination were calculated as described below. The final isotopic composition in the EXP1 rye was 757 (±105) δ13C ‰ for the shoots and 701 (±83) δ13C ‰ in the roots; and 787 (±149) δ13C ‰ in shoots and 719 (±60) δ13C ‰ in roots for EXP2 rye.
Soil sampling
To assess rhizodeposits, we collected soil 24 h after the first and third labeling events during the spring cover crop labeling period in EXP2. To calculate the relative contribution of rhizodeposit C at the time of rye termination, we collected soils at the time of rye termination and treatment establishment in both EXP1 and EXP2, and to evaluate changes in cover crop C from aboveground or belowground sources over time, we sampled after 5 months of cover crop residue decomposition in both EXP1 and EXP2, and after 12 and 17 months of residue decomposition in EXP1. We calculated bulk density using four 5 cm diameter, 10 cm long cores taken from each plot.
Density fractionation
To assess the contribution of root and shoot C to different soil fractions, we performed sodium polytungstate (NaPT) fractionation on EXP1 soils collected 5 and 17 months following rye termination. We followed a standard protocol for density fractionation (Sohi et al., 2001) with modifications described below. Air-dried soils were first rewetted using capillary action (Haney & Haney, 2010). About 50 g of air-dried soil from each sample was added to a beaker with holes drilled in the bottom, which was placed in a glass jar (473 mL) with 10 mL deionized water on a glass microfiber filter (Whatman, GF/D 1823 - 043, GE Healthcare Life Sciences, Buckinghamshire, UK). Soils were monitored and in the case that the soil surfaces were dry after 1 h, water was added in 1 or 2 mL increments up to 5 mL (15 mL added total) until moisture had permeated the soil sample.
Following an 8 h incubation, a soil subsample was dried at 70 °C to assess gravimetric water content, and 10 g of moist soil were added to each of three 50 mL centrifuge tubes along with 30 mL of NaPT at 1.7 g mL−1 density (final density after addition of wet soil was 1.68 g mL−1). Tubes were then rolled along the counter one full rotation to promote mixing of the soil with the NaPT and then allowed to settle overnight. The floating light fraction (hereafter free light fraction (FLF, Table 1)) was then vacuumed from the surface and collected on preweighed, ash-free 8 μm pore size filter paper (Whatman, 1540- 055, GE Healthcare Life Sciences, Buckinghamshire, UK). The centrifuge tubes with soil were then placed on a shaker at 250 rpm for 3 h to break apart aggregates, and tubes were subsequently removed from the shaker and placed in a rack overnight. The floating particulate organic matter (hereafter occluded light fraction (OLF, Table 1)) was vacuumed from the surface and processed as for the FLF. Approximately 5 g of the remaining soil was rinsed of residual NaPT by adding 25 mL deionized water, shaking for 1 h at 200 rpm and centrifuging at 966 g for 2 min; the supernatant was removed, and the rinse was repeated once more. Following centrifugation, the sample contained the mineral heavy fraction (MHF, Table 1) at the surface, increasing concentrations of sand toward the bottom. A small portion of sand-free MHF collected from the surface was dried and analyzed for 13C and total C and N contents. Sand content was determined on approximately 10 g of air-dried bulk soil after dispersing soil in 2.5 mL 5% hexametaphosphate and collecting the sand on a 53 μm sieve.
Microbial biomass
We measured microbial biomass C (MBC) and 13C content in bulk soil on three sample dates in EXP1, 5, 12, and 17 months following termination, and four sample dates in EXP2, 24 h following the first and third labeling events, at time of termination, and 5 months after termination. Soils were subsampled after sieving to 2 mm, and subsamples were transported on ice to the laboratory where they were refrigerated at 4 °C and analyzed for MBC within 5 days of sampling. MBC was extracted from five grams of field moist soil using a modified chloroform fumigation and extraction with 40 mL of 0.5 m K2SO4 (McDaniel et al., 2014b).
13C measurements
Plant and soil 13C values were analyzed on a Finnigan Delta Plus XP isotope ratio mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) with a peripheral Costech 4010 elemental analyzer (Costech Analytical Technologies, Valencia, CA, USA) at the University of New Hampshire Stable Isotopes Laboratory at the Institute for study of Earth, Oceans and Space. Samples were ground to a fine powder in a ball mill grinder (SPEX SamplePrep 8000D Mixer/Mill, Metuchen, NJ, USA), and ground, homogenized samples were weighed into Costech aluminum tins (9–11 mg soil or MHF and 2–2.5 mg plant material, FLF, or OLF). Microbial biomass extracts were analyzed for C content and 13C content at the Stable Isotope Facility at the University of California, Davis using an O.I. Analytical model 1030 TOC Analyzer (OI Analytical, College Station, TX, USA) interfaced to a PDZ Europa 20–20 isotope ratio mass spectrometer (Sercon Ltd., Cheshire, UK) with a GD-100 Gas Trap Interface (Graden Instruments, Oakville, ON, Canada).
Calculations
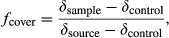 (1)
(1)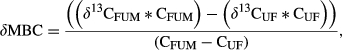 (2)
(2)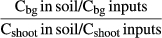 (3)
(3)Statistical analysis
All data manipulation and statistical analyses were performed in r (R Core Team 2014). The following response variables were log-transformed to meet the assumptions of normality: Fcover of bulk soil C, Fcover of MBC, new FLF C, new OLF C, and δ13C in EXP2 bulk soil. To test differences between Fcover in bulk soil or MBC in root and shoot plots at each sampling date after termination (5, 12, and 17 months in EXP1; 2 weeks and 5 months in EXP2), we performed separate one-way anovas at each date. To compare whether Fcover in MBC plots was different from zero, we performed separate one-way t-tests for root and shoot treatments at each date. To compare C inputs from cover crop material in soil density fractions, we performed separate one-way anovas on FLF, OLF, and MHF fractions in EXP1 at 5 and 7 months. To test whether δ13C values increased in bulk soil in root treatment plots during the growing season in EXP2 plots, we compared root and control plots in a two-way anova with date and treatment as discrete independent variables and performed one-way t-tests on the difference in δ13C values (δ13C root plot – δ13C control plot). We report mean values ± standard error and consider α ≤ 0.05 a statistically significant effect.
Results
In EXP1, 151 (±7.3) g m−2 and in EXP2, 123 (±5.9) g m−2 aboveground rye biomass was added to each subplot (oven dry weight), corresponding to 66.9 ± 3.2 and 54.5 ± 2.6 gC m−2 (Cshoot, Table 1). In EXP2, 151 (±37.2) g m−2 of belowground biomass (44.8 ± 11.0 gC m−2; Croot, Table 1) was measured in each subplot and belowground biomass inputs in EXP1 were estimated to be 186 g m−2 (55.0 ± 2.7 gC m−2). When rye was cut in EXP1, plots contained 26.6 (±8.7) g m−2 of rhizodeposit C (in addition to measured root biomass, Cbg, Table 1) and in EXP2, this value was 23.4 (±3.3) gC m−2 (Table 3).
After 5 months, the contribution of Cbg to bulk soil C in EXP1 was approximately four times greater than that of Cshoot (14.5 ± 2.5 gC m−2 vs. 3.5 ± 0.8 gC m−2, Fig. 1a; F(1,8) = 26.3, P < 0.001). One year following cover crop termination, seven times more Cbg remained in bulk soil (14.8 ± 1.3 gC m−2 or 0.7% of total soil C) than Cshoot (2.33 ± 2.2 g m−2 or 0.1% of total soil C; Fig. 1a; F(1,8) = 17.6, P = 0.003). However, after 17 months, there was no difference in bulk soil C derived from Cbg and Cshoot in EXP1 (Fig. 1a, F(1,8) = 0.3, P = 0.63). During the period of cover crop growth in EXP2 plots, Cbg accumulated in bulk soil in the root plots (Fig. 1a, Treatment F(2,66) = 33.2, P < 0.0001; Date F(1,66) = 5.59, P = 0.02). As soon as 24 h following the first labeling event, 12.7 ± 1.9 gC m−2 was attributable to belowground cover crop inputs. This number peaked at 23.4 ± 3.3 gC m−2 at the time of rye termination. Cbg remained more abundant than Cshoot up to 5 months following rye termination, similar to observations in EXP1 (10.9 ± 3.4 g m−2 Cbg and 3.29 ± 2.0 g m−2 Cshoot; Fig. 1a, F(1,9) = 6.7, P = 0.03).
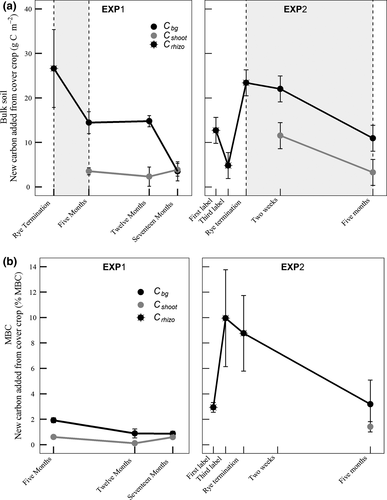
Total MBC in soil ranged from 56.1 ± 19.3 mg MBC kg−1 soil to 240.1 ± 20.9 g MBC kg−1 for the different sampling events across EXP1 and EXP2 (Table 2). Microbial biomass in EXP1 contained over three times more Cbg (1.9 ± 0.2% of MBC) as compared to that of Cshoot (0.6 ± 0.1% of MBC) after 5 months (Fig. 1b, F(1,8) = 58.2, P < 0.001) and seven times more Cbg (0.8 ± 0.4% of MBC) than Cshoot (0.1 ± 0.1% of MBC) 12 months following rye termination (Fig. 1b, F(1,8) = 6.4, P = 0.04). Following 17 months of cover crop decomposition, there was no difference in the fraction of MBC derived from Cbg or Cshoot in EXP1 (F(1,8) = 0.78, P = 0.41) although both Cbg (0.8 ± 0.2%, P = 0.01) and Cshoot (0.6 ± 0.06%, P = 0.002) were present in MBC. We measured significant Cbg inputs to MBC in EXP2 at each sampling date during cover crop growth (P < 0.01 at each date, Fig. 1b), comprising 2.9 ± 0.4% of MBC 24 h following the first labeling event. The fraction of MBC traced to Cbg peaked after the third label at 10.0 ± 3.8% of total MBC. At rye termination in EXP2, 8.8 ± 3.0% of MBC was derived from Cbg. There was no significant difference in the proportion of MBC from Cbg (3.2 ± 31.9%) and the proportion from Cshoot (1.3 ± 0.5%) after 5 months of cover crop decomposition in EXP2 (F(1,8) = 0.27, P = 0.62) although both Cbg (P = 0.004) and Cshoot (P = 0.002) were present in MBC (%MBC > 0).
| Mean (SE) | |
|---|---|
| Total soil C (%) | 1.1 (0.02) |
| pH | 6.7 (0.2) |
| Sand content (%) | 51 (12) |
| Bulk density (g cm−3) | 1.47 (0.03) |
| MBC (mg kg−1 soil) | 115.8 (12.4) |
| FLF (mg g−1 soil) | 7.28 (2.3) |
| OLF (mg g−1 soil) | 6.03 (0.6) |
| MHF (mg g−1 soil) | 476.57 (21.6) |
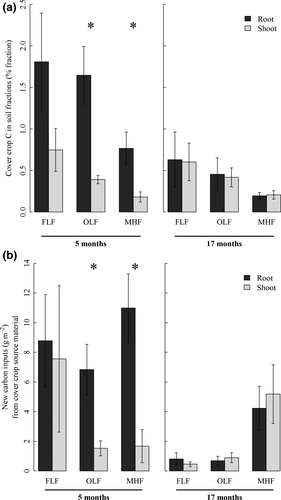
There was more new C incorporated from Cbg, compared to Cshoot, in OLF and MHF 5 months following rye termination in EXP1 (Fig. 2). In the OLF, four times more C was derived from Cbg (6.84 ± 1.7 gC m−2) than from Cshoot (1.54 ± 0.5 gC m−2) representing 1.64 and 0.39% of total OLF, respectively (F(1,8) = 10.4, P = 0.01; Fig. 2). In the MHF, six times more Cbg was present (11.0 ± 2.3 g C m−2) than Cshoot (1.68 ± 1. g C m−2) representing 0.77 and 0.18% of total MHF (F = 15.0, P = 0.005; Fig. 2). A year later, 17 months following cover crop termination in EXP1, there were no differences in Cbg and Cshoot contributions to FLF, OLF, and MHF C (FLF: F(1,8) = 0.004, P = 0.95; OLF: F(1,8) = 0.10, P = 0.76; MHF: F(1,8) = 0.07, P = 0.79; Fig. 2).
Discussion
Our 13CO2 pulse labeling results showed belowground cover crop C inputs (Cbg) present in higher concentrations than cover crop shoot biomass C inputs (Cshoot) in bulk soil, MBC, OLF, and MHF for at least 12 months following cover crop termination. The relative contribution factor suggests that Cbg is three times more likely to be maintained in potentially stable soil pools than Cshoot 12 months following cover crop termination, and Cbg was most abundant in the mineral-associated fraction.
What are the relative contributions of cover crop shoots, rhizodeposits, and total belowground C inputs (Cshoot, Crhizo, and Cbg) to soil C?
Total Cshoot and Cbg inputs (cover crop shoot C inputs and belowground C inputs, Table 1) were similar, although a high proportion of Cshoot may be lost via respiration before entering the soil; one study estimated that approximately 75% of C entering the soil was from Cbg (Gale & Cambardella, 2000). A substantial portion of Cbg inputs were attributed to Crhizo (Table 3). We estimate that rhizodeposition during cover crop growth accounted for 33% and 34% of total Cbg inputs in EXP1 and EXP2, respectively (Table 3). These values are similar to those reported in the literature, which range from 30% to 50% of total Cbg, although values as high as 40% of total plant inputs have been reported (Barber & Martin, 1976; Meharg & Killham, 1991; Puget & Drinkwater, 2001; Kuzyakov et al., 2003; Butler et al., 2004; Jones et al., 2009).
| Biomass (g m−2) | Carbon (gC m−2) | |||
|---|---|---|---|---|
| EXP1 | EXP2 | EXP1 | EXP2 | |
| Cover crop inputs | ||||
| Shoot | 151.3 (7.3) | 123.2 (5.9) | 66.9 (3.2) | 54.5 (2.6) |
| Root | 185.8 (9.0)a | 151.3 (37.2) | 55.0 (2.7)b | 44.8 (11.0) |
| Rhizodeposits | – | – | 26.6 (8.7) | 23.4 (3.3) |
| Belowground total | – | – | 81.6 | 68.2 |
| Total cover crop inputs | 337.1 | 274.5 | 148.5 | 122.7 |
| Corn residues | ||||
| Harvested stover | 367.0 (7.5) | 499.3 (39.5) | 163.2 (3.3) | 222.0 (17.6)b |
| Stover residue | 148.6 (11.4) | 213.3 (15.2) | 66.2 (5.2) | 94.9 (6.7) b |
| Root | 128.1 (14.5) | 177.0 (5.7)a | 57.3 (6.0) | 79.5 (5.7)b |
| Stover total | 515.6 | 712.6 | 229.3 | 316.9 |
| Total corn inputs | 276.8 | 390.4 | 123.5 | 396.4 |
- a Values for belowground productivity were estimated for cover crop in EXP1 and corn in EXP2 based on corresponding allometry (root: shoot) in EXP2 and EXP1, respectively.
- b Values for cover crop root C inputs in EXP1 and corn C inputs in EXP2 were estimated based on corresponding cover crop chemistry in EXP2 and corn in EXP1, respectively.
We observed significantly more Cbg than Cshoot in all measured soil pools, except FLF, for up to 1 year following termination. Our mean relative contribution factor across the two experiments is 3.06 (3.36 in EXP1 and 2.77 in EXP2), indicating that on average 3.06 times more Cbg than Cshoot, per unit C input, was converted to SOM. This value is comparable to others reported in the literature; for example, Puget & Drinkwater (2001) estimated a relative contribution factor of 3.7 using in situ isotopic labeling of a hairy vetch cover crop and Kong & Six (2010) found a relative contribution factor of 3.24 for a hairy vetch cover crop in a maize/tomato rotation. Rasse et al. (2005) compared the relative contribution factor of roots to shoots across various in situ studies of different plant types and found relative contribution factors ranging from 0.77 to 3.7 and an average value of 2.4.
The greater abundance of Cbg vs. Cshoot in SOM, and its higher efficiency of conversion to SOM, is likely due to differences in the size, chemical composition, location, and timing of the two inputs (Rasse et al., 2005; Loecke & Robertson, 2009a,b; Mendez-Millan et al., 2010; Dungait et al., 2012). Greater physical protection of Cbg may result from the small size and close proximity of belowground inputs to soil aggregate formation (Tiemann & Grandy, 2015). Many belowground inputs, especially rhizodeposits, are orders of magnitude smaller in size than shoot inputs that must be shredded, leached, or otherwise broken down prior to incorporation in soil aggregates (Jones et al., 2009). Roots play an important role in structuring soil and contribute to aggregate formation; therefore, rhizodeposits are inherently positioned to be enmeshed in soil aggregates (Puget & Drinkwater, 2001; Denef & Six, 2006; Clemmensen et al., 2013). Increased mineral association of Cbg may be facilitated by the close proximity of rhizodeposits to mineral surfaces and the chemical composition of soluble rhizodeposits.
It has also been suggested that belowground inputs decompose slowly due to the chemically complex components of root biomass (e.g., lipids or waxes such as suberin; Rasse et al., 2005; Mendez-Millan et al., 2012). However, given that long-term SOM storage is not driven by chemical recalcitrance of direct plant inputs (Dungait et al., 2012; Cotrufo et al., 2013) as much as it is by the physical protection of plant and especially microbial products by association with minerals (Grandy & Neff, 2008; Heckman et al., 2013). Thus, an alternative mechanism is that through transformation via microbial consumption, root inputs may enhance microbial processes that result in the preservation of root-derived C. For example, the continuous input of low molecular weight substrates in the rhizosphere may promote greater carbon use efficiency (CUE), which may in turn lead to greater rates of C retention in soils (Puget & Drinkwater, 2001; Kallenbach et al., 2015; Roller & Schmidt, 2015). Further, recent work suggests that the majority of stabilized C in soils has been previously transformed by microorganisms or else is composed of microbial necromass. (Grandy et al., 2007; Kindler et al., 2009; Miltner et al., 2012). The rapid incorporation of root inputs to microbial biomass may further promote its protection by association with minerals (Grandy & Robertson, 2007; Tiemann & Grandy, 2015).
How much Crhizo is incorporated into MBC during cover crop growth and is Cshoot or Cbg preferentially incorporated into MBC during cover crop decomposition?
Given the assertion that microbial belowground inputs may be more rapidly incorporated into microbial biomass and that microbial biomass serves as an important pathway for the C stabilization, we examined the incorporation of Crhizo, Cshoot, and Cbg into MBC. Twenty-four hours following the first labeling event in EXP2, 0.83 (±0.1) g m−2 Crhizo was present in MBC, constituting 2.9% of total MBC. At the time of rye termination, 2.31(±1.1) g m−2 Crhizo was in MBC, which constituted 8.8% of total MBC. Pulse chase labeling studies have found photosynthate in MBC as soon as 1 h following fixation, with peak concentrations occurring roughly 3 h after fixation (Minchin et al., 1994; Rattray et al., 1995; Dilkes et al., 2004). Rhizodeposit C comprised 8.8% of total MBC at time of rye termination, which is lower than the 25–30% of MBC reported by Williams et al. (2006) in a system of ryegrass and clover with belowground biomass of 200–210 g m−2 (compared to 151–186 g in our study, Table 3). Fungi and bacteria in the rhizosphere produce polysaccharides and other binding agents, and transformation by microbial decomposers can be an important precursor to SOM protection on mineral surfaces (Six et al., 2006; Grandy & Neff, 2008; Miltner et al., 2012; Mardhiah et al., 2014; Kallenbach et al., 2015). The greater abundance of Cbg than Cshoot in MBC 5 months to 1 year following termination indicates more belowground C is entering the microbial biomass, which may help explain the higher concentrations of Cbg in mineral-associated fractions.
How are Cshoot and Cbg distributed among three soil density fractions?
In an effort to understand the turnover and stabilization dynamics of Cbg and Cshoot, we measured the incorporation of cover crop C into three different soil fractions. Cover crop C derived from either Cbg or Cshoot accumulated most in MHF C, followed by FLF C, then OLF C (Fig. 2b) and cover crop C comprised the greatest proportion of FLF C, followed by OLF C, and MHF C (Fig. 2a). Slowed decomposition of Cbg due to chemical recalcitrance should result in a buildup of particulate organic matter (POM); thus, if greater retention of Cbg than Cshoot is simply due to greater chemical recalcitrance of roots (which are not likely to result in long-term SOM accumulation), we would expect more Cbg than Cshoot in the FLF. If physical protection within aggregates is primarily driving greater abundance of Cbg than Cshoot in soil, we would expect to find a greater abundance of Cbg than Cshoot in the OLF. More Cbg than Cshoot in the MHF would suggest the possibility that direct mineral association is playing a role in slowing the turnover of Cbg.
We did not observe differences between Cbg and Cshoot in FLF C, where POM is not physically protected and decomposition is primarily driven by chemical recalcitrance. If decomposition of Croot was slower due to chemical recalcitrance, we would expect to find a greater proportion of Cbg in the FLF C pool. We did observe greater quantities of Cbg compared to Cshoot in FLF C, and the lack of statistical significance between these two sources may be due to greater variation in the quantity of cover crop C in FLF C compared to the OLF and MHF fractions. However, recent evidence suggests that chemical recalcitrance and unprotected POM such as that found in FLF contribute little to SOM stability (Carrington et al., 2012; Dungait et al., 2012). Physical protection may result in more Cbg POM in the OLF C pool. Root exudates and secretions may play a role in promoting aggregation, and the small size and close proximity of Cbg POM to aggregate formation may result in greater occlusion of Cbg POM compared to Cshoot POM.
In fact, we did observe a greater content of Cbg than Cshoot in the OLF fraction, which indicates greater abundance of root material than shoot residues as POM in soil aggregates. The belowground POM inputs such as root hairs, mycorrhizal hyphae, and to a lesser extent fine roots are small enough to be incorporated into microaggregates, which are usually defined as <250 μm but may be especially stable at the 2–20 μm scale (Krull et al., 2003), which corresponds to the scale of the most active components of mycorrhizal hyphae and root hairs (Rasse et al., 2005). Conversely, shoot material must be fragmented from the cm to μm scale before incorporation into stable soil aggregates and some C may be lost due to leaching or respiration during that process. Thus, the relatively smaller size of some Cbg components may promote enhanced physical protection of belowground POM in soil aggregates. The decomposition of POM in aggregates may be slowed by physical isolation from decomposers and low oxygen concentrations (Six et al., 2002; Grandy & Robertson, 2007; Dungait et al., 2012).
Mineral association represents another mechanism by which Cbg may be preferentially stabilized in soil (Dungait et al., 2012; Cotrufo et al., 2013; Wieder et al., 2014; Tiemann et al., 2015), and we did observe a greater proportion of MHF derived from Cbg than from Cshoot after 5 months (Fig. 2a). Soluble rhizodeposits include organic acids produced by plants such as lactate, acetate, oxalate, malate, and citrate, which adsorb to clay mineral surfaces via polyvalent cation bonding (Kraffczyk et al., 1984). The highest contribution of rhizodeposits happens at the root tip, especially via mucilages, sloughed cells, and secretions (Dakora & Phillips, 2002; Farrar et al., 2003; Carvalhais et al., 2011), and as the root tip grows between soil pores, organic acids and mucilages are wiped along the surface of soil minerals. Thus, Crhizo, contributing one-third of total Cbg in this system, may have greater likelihood of coming in contact with mineral surfaces. Additionally, root inputs are in close proximity to soil microbial communities, which can facilitate sorption on mineral surfaces. Greater mineral association of Cbg than Cshoot could result in greater long-term storage of Cbg.
How long do Cbg and Cshoot persist in soil C?
Given six times greater abundance of Cbg than Cshoot in soils 12 months following termination, representing three times greater relative contribution (relative to inputs, see eq 3) of Cbg than Cshoot, we would expect this to result in the accumulation of SOM in the long term as has been suggested in previous studies (Puget & Drinkwater, 2001; Kong et al., 2005; Rasse et al., 2005; Mendez-Millan et al., 2010). There are many cases in which the presence of cover crops have led to increases in soil C (Mullen et al., 1998; Mazzoncini et al., 2011; Wang et al., 2012; Higashi et al., 2014; McDaniel et al., 2014a; Tiemann et al., 2015), but others have shown no effect (Kaspar et al., 2006; Steele et al., 2012). We did not detect a difference between Cbg and Cshoot in soils 17 months following termination in EXP1. The majority of both Cbg and Cshoot were mineralized after 17 months. This may indicate short-term persistence of cover crop C in this system despite the lack of physical disturbance from tillage, but further study could reveal long-term stabilization of Cbg in greater proportion than Cshoot as observed in previous studies (Gale & Cambardella, 2000; Puget & Drinkwater, 2001; Rasse et al., 2005; Kong & Six, 2010; Mendez-Millan et al., 2010). A stronger isotopic label may be required to detect the long-term persistence of a single season's cover crop inputs in stable soil C pools, or repeated annual input of labeled materials may reveal the accumulation of cover crop carbon over time. The benefits of cover crop to building soil may thus depend on continuous use of cover crops in annual rotation with main crops.
Cover cropping could support partial harvest of corn stover for biofuel production
Biofuel crop residues and cover crop biomass may provide substantial feedstock for bioenergy production (Perlack et al., 2005; Graham et al., 2007), but this removal of potential soil C inputs could lead to reduced SOM (Anderson-Teixeira et al., 2009; Blanco-Canqui & Lal, 2009). However, several studies have found that decreased aboveground inputs do not necessarily correlate with decreased SOM (Tonitto et al., 2006; Steele et al., 2012; Adler et al., 2015). One potential mechanism for this discrepancy is a disproportionate contribution to SOM from belowground inputs (Balesdent & Balabane, 1996; Rasse et al., 2005; Kong & Six, 2010; Mendez-Millan et al., 2010). A meta-analysis of residue inputs required to maintain soil carbon in corn systems estimated that a mean of 638 ± 219 g m−2 corn stover is required to maintain soil carbon (Johnson et al., 2014), a value roughly equivalent to total annual stover input at the GLBRC BCSE site (Table 3).
We estimate that the use of cereal rye as a winter cover crop in a no-till continuous corn rotation at this site could replace about 80% stover removal based on productivity and carbon content of crops in 2013 and 2014, although conversion rates of stover to soil carbon will vary with litter quality, soil type, management practices, climate, and other factors. Total cover crop C inputs are roughly equal to 80% of harvested stover C, whereas belowground cover crop carbon inputs are roughly equal to 42% of harvested stover C. As Cbg is three times more likely to be stored in soil C than Cshoot, the incorporation of winter cover crop roots could substantially remediate the effects of stover removal in this system in the short term. Including winter cover crops in annual rotation could increase biofuel feedstocks directly and indirectly; aboveground cover crop residues could contribute to biofuel feedstocks and belowground cover crop inputs could offset the C removal associated with the use of main crop stover as a biofuel feedstock.
Acknowledgements
We would like to thank the Grandy Soil Biogeochemistry Lab at The University of New Hampshire and two anonymous reviewers for helpful comments and manuscript revisions. This study was supported by the Department of Energy's Great Lakes Bioenergy Research Center (DOE Office of Science, DE FC02-07ER64494 and Office of Energy Efficiency and Renewal, DE-ACO5-76RL01830), the National Science Foundation LTER program and the University of New Hampshire Agricultural Experiment Station. We are also grateful to the W.K. Kellogg Biological Station, the Great Lakes Bioenergy Research Center, and in particular to Rick Corder and Stacey VanderWulp for assistance in the field.




