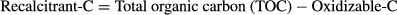Investigating the biochar effects on C-mineralization and sequestration of carbon in soil compared with conventional amendments using the stable isotope (δ13C) approach
Abstract
Biomass-derived black carbon (biochar) is considered to be an effective tool to mitigate global warming by long-term C-sequestration in soil and to influence C-mineralization via priming effects. However, the underlying mechanism of biochar (BC) priming relative to conventional biowaste (BW) amendments remains uncertain. Here, we used a stable carbon isotope (δ13C) approach to estimate the possible biochar effects on native soil C-mineralization compared with various BW additions and potential carbon sequestration. The results show that immediately after application, BC suppresses and then increases C-mineralization, causing a loss of 0.14–7.17 mg-CO2–C g−1-C compared to the control (0.24–1.86 mg-CO2–C g−1-C) over 1–120 days. Negative priming was observed for BC compared to various BW amendments (−10.22 to −23.56 mg-CO2–C g−1-soil-C); however, it was trivially positive relative to that of the control (8.64 mg-CO2–C g−1-soil-C). Furthermore, according to the residual carbon and δ13C signature of postexperimental soil carbon, BC-C significantly increased (P < 0.05) the soil carbon stock by carbon sequestration in soil compared with various biowaste amendments. The results of cumulative CO2–C emissions, relative priming effects, and carbon storage indicate that BC reduces C-mineralization, resulting in greater C-sequestration compared with other BW amendments, and the magnitude of this effect initially increases and then decreases and stabilizes over time, possibly due to the presence of recalcitrant-C (4.92 mg-C g−1-soil) in BC, the reduced microbial activity, and the sorption of labile organic carbon (OC) onto BC particles.
Introduction
The rise of anthropogenic carbon dioxide (CO2) emissions in the atmosphere, particularly through the combustion of fossil fuels and changes in land use, is the most noteworthy driver of radiative energy imbalance by positive radiative-forcing of the earth's climate system, which implies the warming of the global atmosphere since the mid-20th century (Meehl et al., 2005; Wigley, 2005; Liepert, 2010; Crombie & Masek, 2015; Houghton et al., 2015; Rogelj et al., 2015; Scott et al., 2015; IPCC, 2016; Tokarska et al., 2016). It may sound strange, but this consequential increase in the mean global temperature has a significant impact on the rise of sea levels due to thermal expansion of ocean water and is also responsible for the shrinking of land ice by the loss of mass from glaciers and polar ice (Oppenheimer, 1998; Raper & Braithwaite, 2006; Hay et al., 2015; Slangen & Church, 2016). Keeping in view the potential deleterious impact of man-made GHG emissions on the global climate system, the first move toward integrated policies to overcome or stabilize this issue was made at the Earth Summit in 1992, under the platform of the UN Framework Convention on Climate Change (UNFCCC, 1992; Liepert, 2010; IPCC, 2016). Later on, this work was extended to the Kyoto Protocol in 1997, which formulated target policies in terms of ‘equivalent carbon dioxide (CO2eq)’ and committed its signatories to mitigating GHG emissions by setting legally binding emission reduction targets (UNFCCC, 1998; Liepert, 2010). As the Protocol was signed, the sustainable strategy of long-term sequestration of atmospheric-C into soil to increase the carbon sink in the terrestrial environment is receiving increasing attention (Zavalloni et al., 2011).
Although the global carbon cycle is an immeasurably complex phenomenon, it has been widely accepted that both soil and atmospheric-C pools strongly interact with each other and that the natural two-way flux between them is one of the most influential processes (Fontaine et al., 2004). Indeed, the soil organic-C pool in the active atmosphere–ecosystem exchange comprises roughly two-thirds of the total carbon in terrestrial ecosystems (Post et al., 1982), which is approximately three times greater (~2400 Pg C to 2 m depth) than the current atmospheric-C (~830 Pg C) and 240 times higher compared with the current annual fossil fuel emission (~10 Pg C) (Schlesinger, 1995; Batjes, 1996; Fontaine et al., 2004; Paustian et al., 2016). Moreover, the long residence times of the relatively large C reservoirs in terrestrial ecosystems make this pool a potentially important sink for atmospheric carbon emitted by fossil fuel combustion, which could act as a buffer against increasing atmospheric CO2 concentration (Post et al., 1982). Therefore, an increase of even a few percent in the net soil C-stock can considerably offset the increase of atmospheric CO2.
Recent research suggests that the proximal controls on the balance of soil-C incorporate the rate of organic carbon input (agricultural wastes, sludge, and compost), subtracting C that is released through decomposition (Paustian et al., 2016). Therefore, the soil-C pool can be increased by adding exogenous sources of organic carbon or by minimizing the rate of decay/decomposition (for example, by minimum or no tillage) or by a combination of both, leading to enhanced sequestration of atmospheric-C (Karlen & Cambardella, 1996; Paustian et al., 1997; Novak et al., 2009; Zavalloni et al., 2011). However, a few long-term studies explain that the soil C-stock does not necessarily increase even if fresh organic matter in the form of manures and agricultural wastes is applied to soil in large quantities (Gill et al., 2002; Fontaine et al., 2004). This could be due to the decreasing rate of soil carbon buildup with time as the stockpile approaches the new equilibrium (West & Six, 2007).
Another novel strategy to increase the soil C-stock for the long term and to mitigate global warming by offsetting the atmospheric-C (up to 9.5 Pg C annually) is to add pyrogenic organic carbon (biochar) (Lehmann et al., 2006; Wardle et al., 2008; Woolf et al., 2010; Zavalloni et al., 2011; Ventura et al., 2015). Biochar (BC) is a carbonaceous material (a coproduct of pyrolysis) that is obtained from biomass pyrolysis and that is generally considered to be chemically and biologically inert due to its recalcitrant-C composition (Singh & Cowie, 2014; Sagrilo et al., 2015). In the context of carbon sequestration, BC can stay in soil for a long time, with mean retention times of several centuries–millennia due to the high stability of the BC (Forbes et al., 2006). The archaic existence of the C-rich dark earth (Terra Preta) in the Amazon basin gives authentic proof of long-term C storage through a slash-and-char system (Lehmann et al., 2006; Singh et al., 2012).
Supplied organic carbon (fresh organic matter or BC) is reported to accelerate or suppress native SOC decomposition by so-called negative or positive priming effects of organic matter, respectively (Wardle et al., 2008; Keith et al., 2011; Luo et al., 2011; Zimmerman et al., 2011). Although many previous studies have reported priming effects (negative or positive) in terrestrial ecosystems, uncertainties still remain about the interactive influence of biochar and various biowaste amendments, such as the use of δ13C unlabeled organic matter or nonuniform labeling of inputs on native SOC carbon mineralization (Zimmerman et al., 2011; Singh & Cowie, 2014; Ventura et al., 2015). To some extent, these experimental uncertainties could represent contradictions between the obtained results, that is, positive and negative priming effects of applied C sources on native SOC mineralization (Cardon et al., 2001).
To test the above-mentioned issue of uncertainty about the intensity and direction (positive or negative) of priming by BC and various BWs on the native SOC, we performed a two-phase experiment to compare the C-mineralization and C-storage potential of BC and various BWs (PrM, FM, CP, SS, and PM) in a laboratory incubation under controlled conditions, supported by a greenhouse experiment over a 120-day time period. The addition of BC and BW amendments with notably different δ13C signatures compared to soil permitted the differentiation of C-mineralization between applied and native stocks. Our study was based on the following hypotheses: (i) at a very early stage, BC will negatively prime native soil C-mineralization/CO2 emission, and then be triggered toward the peak (as positive priming); (ii) BC-induced positive priming effects will be minimized by gradual stabilization of the soil organic carbon pool, possibly due to the depletion of labile carbon content in soil; and (iii) interactive to various BW amendments, BC will show minimum C-mineralization but higher carbon sequestration potential for the long term.
Materials and methods
Soil, BC and BW amendments
The surface soil sample (0–15 cm) was collected from a research station near the peri-urban area of the city in a region that has been cultivated with fruits and vegetables over the past few decades. The collected soil sample was dried in air-shade conditions, ground, passed through a 10 mesh sieve (2 mm), and stored below 4 °C to restrict further biochemical changes prior to analysis. Comprehensive information about the soil characteristics (texture, saturation percentage, cation exchange capacity, pH, moisture content, organic carbon, and electrical conductivity) and nutrient contents (macronutrients: N, P, K) is summarized in Table 1.
| Characteristic | Unit | Soil | Biochar (BC) | Biowaste amendments (BWs) | ||||
|---|---|---|---|---|---|---|---|---|
| PrM | FM | CP | SS | PM | ||||
| Texture | – | Loama | – | – | – | – | – | – |
| Sand | % | 33.48 ± 0.72 | – | – | – | – | – | – |
| Silt | % | 43.72 ± 0.39 | – | – | – | – | – | – |
| Clay | % | 22.80 ± 0.44 | – | – | – | – | – | – |
| SPb | % | 34 ± 1.49 | – | – | – | – | – | – |
| CECc | cmolc kg−1 | 17.06 ± 0.14 | – | – | – | – | – | – |
| pHd | – | 6.82 ± 0.03 | 10.83 ± 0.15 | 7.55 ± 0.29 | 7.69 ± 0.23 | 7.94 ± 0.24 | 7.64 ± 0.23 | 7.1 ± 0.18 |
| MC (105 °C) ! | % | 11.34 ± 0.98 | 4.23 ± 0.27 | 33.48 ± 2.45 | 25.22 ± 2.25 | 17.35 ± 1.86 | 34.35 ± 3.50 | 32.84 ± 2.75 |
| Ash contente | % | – | 27.65 ± 0.28 | 12.35 ± 0.75 | 11.78 ± 0.91 | 13.16 ± 0.95 | 31.58 ± 0.65 | 10.85 ± 1.06 |
| OCf | % | 0.40 ± 0.06 | 63.03 ± 1.35 | 66.03 ± 3.31 | 54.02 ± 2.29 | 61.34 ± 2.85 | 22.01 ± 1.48 | 50.25 ± 3.86 |
| δ13C | ‰ | −16.18 ± 0.05 | −28.16 ± 0.11g | −13.01 ± 0.08 | −22.96 ± 0.07 | −23.35 ± 0.07 | −21.59 ± 0.08 | −22.38 ± 0.06 |
| ECh | dS m−1 | 3.37 ± 0.02 | 0.84 ± 0.21 | 4.37 ± 0.66 | 7.85 ± 1.25 | 6.82 ± 0.42 | 8.62 ± 0.74 | 7.56 ± 0.29 |
| Macronutrients | ||||||||
| Total N | % | 0.19 ± 0.07 | 1.52 ± 0.22 | 3.88 ± 0.31 | 2.24 ± 0.33 | 3.88 ± 0.31 | 1.56 ± 0.35 | 4.46 ± 0.81 |
| P | ppm | 8.45 ± 0.24i | 308.44 ± 5.15 | 426.50 ± 10.45 | 455.35 ± 13.60 | 622.25 ± 25.37 | 386.12 ± 8.45 | 437.41 ± 14.47 |
| K | % | 0.49 ± 0.08i | 0.87 ± 0.04 | 1.05 ± 0.09 | 0.93 ± 0.05 | 1.24 ± 0.12 | 0.96 ± 0.08 | 0.82 ± 0.06 |
- PrM, pressmud; FM, farm manure; CP, compost; SS, sewage sludge; PM, poultry manure.
- a USDA soil classification system.
- b Saturation percentage.
- c Cation exchange capacity.
- d pH of soil saturated paste.
- e Heating at 750 °C.
- f Soil organic carbon contents.
- g δ13C ‰ value for biochar feedstock was –27.63 ± 0.14 ! moisture content (n = 3).
- h Electrical conductivity of soil saturated paste extract.
- i Available.
The biochar (BC) was produced from wood sawdust (C 46.3, N 0.03%) as a feedstock via slow pyrolysis using an Isotemp muffle furnace (550 series; Fisher Scientific, Pittsburgh, PA, USA). The complete process and pyrolysis conditions used for the production of BC are described elsewhere (Yousaf et al., 2016). In brief, the wood sawdust BC was prepared at 450 °C under a continuous flow of nitrogen (50 sccm), with the temperature rising at a rate of 10 °C min−1 for a retention time of 60 min. The produced BC was then ground and sieved through a 2 mm sieve (10 mesh size sieve). Various BW materials (pressmud, farm manure, compost, sewage sludge, and poultry manure) were obtained from a local nursery, air-dried at 65 °C overnight, and passed through a 2 mm sieve. The physicochemical characteristics of the BC and various BW amendments are given in Table 1.
Incubation experiment
Air-dried soil was uniformly mixed with BC at a 2% OC basis (BC: 6.34 g, 200 g-soil−1 DW basis) and various BW amendments, that is, PrM@2%OC (pressmud: 6.06 g, 200 g-soil−1 DW basis), FM@2%OC (farm manure: 7.40 g, 200 g-soil−1 DW basis), CP@2%OC (compost: 6.52 g, 200 g-soil−1 DW basis), SS@2%OC (sewage sludge: 18.17 g, 200 g-soil−1 DW basis), and PM@2%OC (poultry manure: 7.96, 200 g-soil−1 DW basis) in 500 mL plastic jars. Soil treatment mixtures were adjusted to a 65% water-holding capacity using a nutrient solution with ca. 400 mg N-kg−1 soil, 200 mg P-kg−1 soil, 100 mg-K kg−1 soil, and trace elements (Chapman, 1997; Singh & Cowie, 2014). All treatments, including control (without any amendment) and amended soils, were replicated three times. The soils were placed in 500 mL plastic containers in separate sealed experimental units containing subunits: (i) a 50 mL beaker containing 30 mL of double-distilled water (DDW) to maintain constant humidity and (ii) a 50 mL flask containing 30 mL of 2 m NaOH solution to trap CO2–C evolved during C-mineralization. Each experimental unit was placed in dark conditions at a constant temperature of 25 ± 1 °C, and the incubation experiment was carried out over a time period of 120 days. The NaOH (2 m)-captured CO2 was removed and replaced nine (9) times during incubation after 1, 5, 10, 20, 30, 50, 70, 90, and 120 days and was analyzed for total CO2–C and CO2–δ13C. The blanks (triplicate) without soil were also set up to take into account the presence of atmospheric CO2 in sealed units.
Measurement of carbon mineralization and priming effects
The total C mineralized (CO2–C) in various treatments was estimated by precipitating 1 mL of 2 m NaOH with 5 mL of 0.4 m barium chloride (BaCl2) followed by titration against 0.1 m HCl in the presence of phenolphthalein indicator (Singh et al., 2012). To determine the trapped CO2 (as SrCO3) in NaOH (C mineralized) from BC and various BW amendments, a 10 mL aliquot of NaOH (2 m) was precipitated with 10 mL of 1 m SrCl2 (strontium chloride hexahydrate), and then the strontium carbonate (SrCO3) precipitate was washed with double-distilled water (DDW) 7–10 times to obtain a neutral pH value. Finally, the precipitate was dried at 65 °C for 24 h and ground, followed by δ13C analysis (1.5 mg SrCO3 and 3 mg V2O5) by EA-IRMS (Finnigan MAT Delta Plus, Bremen, Germany) (Harris et al., 1997; Ventura et al., 2015).
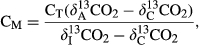 (1)
(1)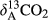 represents the δ13C value of the CO2–C released from BC and various BW-amended soils;
represents the δ13C value of the CO2–C released from BC and various BW-amended soils; 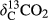 indicates the δ13C value of the CO2–C released from the control (unamended soil); and
indicates the δ13C value of the CO2–C released from the control (unamended soil); and 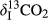 is the initial δ13C value of the BC and various BW amendments (Singh & Cowie, 2014; Ventura et al., 2015).
is the initial δ13C value of the BC and various BW amendments (Singh & Cowie, 2014; Ventura et al., 2015).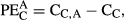 (2)
(2)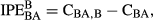 (3)
(3)Pot experiment
A pot experiment was conducted under greenhouse conditions to evaluate the labile organic carbon contents, total carbon sequestration potential, and nutrient behavior of BC and various BW amendments. Six treatments, biochar (BC), pressmud (PrM), farm manure (FM), compost (CP), sewage sludge (SS), and poultry manure (PM), were applied on a 2% organic carbon (OC) basis, with a similar rate as that used in incubation experiments. All the treatments were replicated three times, including an unamended soil as a control, and a total of 28 polyvinylchloride pots were filled with the soil (7 kg soil pot−1, 30 cm height, and 20 cm diameter). The treatments were applied as C = control (unamended soil), BC@2%OC (biochar: 222.17 g pot−1), PrM@2%OC (pressmud: 212.03 g pot−1), FM@2%OC (farm manure: 259.16 g pot−1), CP@2%OC (compost: 228.24 g pot−1), SS@2%OC (sewage sludge: 636.07 g pot−1), and PM@2%OC (poultry manure: 278.61 g pot−1). The soil was fertilized with the recommended rates of nitrogen, phosphorus, and potassium. Half of the nitrogen (0.3 g N pot−1) was applied with a full dose of phosphorus (0.5 g P pot-1) and potassium (0.24 g K pot−1) as the basal dose. The remaining half of the nitrogen was applied in two parts (1st with the first irrigation and 2nd at the milking stage) (Yousaf et al., 2016). Wheat seeds were sterilized for 10 min using 30% H2O2 solution, followed by washing with deionized water (Miché & Balandreau, 2001). The sterilized seeds were soaked in deionized water overnight, and ten wheat seeds were spread in each pot. Then, after sprouting, the seedlings were thinned to four plants pot−1. All necessary agronomic practices were followed, and pots were also randomized in alternate weeks to minimize positional effects.
DOC and MBC analysis
Dissolved organic carbon was determined by UV/persulfate oxidation method: combines the sample with an acid, lowering the sample pH to 2.0. In this process, inorganic carbon (IC) is converted to dissolve CO2–C and eliminate from the sample. Potassium persulfate reagent was added to the sample followed by oxidization of remaining carbon by ultraviolet (UV) radiation, which was detected by the nondispersive infrared detector (NDIR) sensor (Eykelbosh et al., 2015).
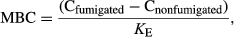 (4)
(4)TOC, oxidizable carbon and recalcitrant-C contents
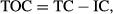 (5)
(5)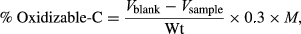 (6)
(6)Statistical analysis and quality control
To ensure accuracy in data with reference to quality control, all the samples were analyzed repeatedly for CO2 emission, C-mineralization priming effects, DOC, MBC, and TOC. All the descriptive data were statistically analyzed by two-way anova using spss 16.0 (SPSS Inc., Chicago, IL, USA) at various incubation times. The BC and BW amendments were used as interactive sources of variance. The predicted mean values were compared using LSD (at 0.05 probability), where F-tests were significant. sigmaplot 11.0 (Systat Software Inc., San Jose, CA, USA) was employed for graph plotting.
Results
Influence of BC and various BW amendments on soil–crop characteristics and nutrient concentrations
The BC and various BW amendments significantly affected many physicochemical characteristics of the soil, including the organic carbon (OC), electrical conductivity (EC), pH, and cation exchange capacity (CEC), and their mean values are given in Table 2. During the course of the study, BC and various BW amendments had a statistically significant (P ≤ 0.05) influence on the soil organic carbon (OC) content, with a highest value of 1.2 ± 0.07% when BC was applied to a 2% organic carbon basis followed by PrM, CP, FM, PM, and SS. However, the minimum OC content (0.39 ± 0.08%) was observed with the nonamended soil (Table 2). Similarly, the highest values of the pH (8.20 ± 0.05) and cation exchange capacity (23.82 ± 2.63 cmolc kg−1) were recorded in the case of biochar (BC) amendment, regardless of other BW amendments, while the electrical conductivity (EC) was extremely high in the PM-amended soil (8.62 ± 0.34 dS m−1), followed by FM (6.02 ± 0.38 dS m−1) and BC (5.59 ± 0.41 dS m−1) (Table 2).
| Soil characteristics | Control | Biochar (BC) | Biowaste amendments (BWs) | ||||
|---|---|---|---|---|---|---|---|
| PrM | FM | CP | SS | PM | |||
| Organic carbon (%) | 0.39 ± 0.08 | 1.20 ± 0.07 | 0.95 ± 0.07 | 0.81 ± 0.14 | 0.90 ± 0.08 | 0.62 ± 0.16 | 0.73 ± 0.20 |
| ECe (dS m−1) | 3.36 ± 0.17 | 5.59 ± 0.41 | 3.96 ± 0.30 | 6.02 ± 0.38 | 4.49 ± 0.44 | 4.50 ± 0.27 | 8.62 ± 0.34 |
| pHa | 7.67 ± 0.33 | 8.20 ± 0.05 | 7.45 ± 0.26 | 8.19 ± 0.05 | 7.53 ± 0.34 | 7.83 ± 0.32 | 7.35 ± 0.41 |
| CEC (cmolc kg−1) | 17.27 ± 0.68 | 23.82 ± 1.63 | 20.68 ± 0.82 | 19.79 ± 0.22 | 20.82 ± 0.41 | 17.40 ± 0.98 | 19.79 ± 0.80 |
- PrM, pressmud; FM, farm manure; CP, compost; SS, sewage sludge; PM, poultry manure (n = 3).
- a pH of soil saturated paste.
Fresh shoot biomass, dry shoot biomass, and grain weight increased significantly (P ≤ 0.01) with BC and most of the BW amendments compared with that of the control, except for with SS and PM. On the other hand, the fresh root biomass and dry root biomass were nonsignificant in the BC and all BW-amended soils (Table 3). Moreover, the plant physiological characteristics, that is, photosynthetic rate (Pn), transpiration rate (Tr), and stomatal conductance (gs), were statistically significant (P ≤ 0.05) with the BC and all BW amendments. However, there was no significant difference in the calculated leaf area (cm2) with all amendments, except for with BC and CP. The mean values of FSB (46.15 ± 3.82 g pot−1) and DSB (30.18 ± 3.09 g pot−1) in the BC-amended soil were 23.7% and 25.2% greater than the control (37.32 ± 3.58 g pot−1), respectively. Similarly, the average harvested grain weight with the BC-amended treatment (15.35 g pot−1) was greater (44.1%) than that observed with the control (10.65 g pot−1). Furthermore, the photosynthetic rate (44.4%), the transpiration rate (36.8%), the stomatal conductance (109%), and the leaf area (18.1%) for BC-amended soil were also greater than that for control soil (Table 3). Soil and plant (root, shoot, and grain) macronutrient (NPK) contents were also significantly (P ≤ 0.05) affected by the application of BC and various BW amendments compared with that of the control (Fig. 1).
| Characteristics | Control | Biochar (BC) | Biowaste amendments (BWs) | ||||
|---|---|---|---|---|---|---|---|
| PrM | FM | CP | SS | PM | |||
| FSB/pot (g) | 37.32 ± 3.58 | 46.15 ± 3.82 | 52.95 ± 6.50 | 52.15 ± 5.13 | 51.75 ± 2.95 | 40.75 ± 4.91 | 48.32 ± 1.05 |
| DSB/pot (g) | 24.11 ± 1.72 | 30.18 ± 3.09 | 30.70 ± 2.57 | 31.44 ± 2.84 | 32.20 ± 5.03 | 26.41 ± 3.45 | 28.57 ± 2.74 |
| FRB/pot (g) | 12.20 ± 1.48 | 13.75 ± 2.96 | 16.50 ± 2.68 | 15.85 ± 4.00 | 12.60 ± 1.33 | 14.80 ± 1.03 | 13.75 ± 1.15 |
| DRB/pot (g) | 6.09 ± 1.18 | 6.82 ± 2.46 | 8.69 ± 2.03 | 7.762 ± 3.21 | 8.15 ± 0.40 | 7.30 ± 2.36 | 6.63 ± 0.75 |
| GW/pot (g) | 10.65 ± 1.33 | 15.35 ± 2.57 | 15.26 ± 1.53 | 15.10 ± 2.23 | 16.25 ± 1.66 | 10.85 ± 1.27 | 12.30 ± 1.20 |
| Photosynthetic rate (Pn) | 26.46 ± 0.95 | 38.21 ± 1.18 | 35.35 ± 0.93 | 38.85 ± 2.55 | 43.18 ± 2.25 | 35.34 ± 1.88 | 36.85 ± 3.54 |
| Transpiration rate (Tr) | 3.18 ± 0.08 | 4.35 ± 0.35 | 3.99 ± 0.45 | 4.16 ± 0.62 | 4.95 ± 0.55 | 3.81 ± 0.21 | 4.05 ± 0.25 |
| Stomatal conductance (gs) | 0.11 ± 0.01 | 0.23 ± 0.03 | 0.20 ± 0.03 | 0.23 ± 0.02 | 0.31 ± 0.05 | 0.19 ± 0.03 | 0.21 ± 0.05 |
| Leaf Area (cm2) | 28.62 ± 2.27 | 33.80 ± 1.54 | 30.88 ± 3.41 | 32.72 ± 2.25 | 32.32 ± 1.14 | 29.22 ± 0.53 | 31.30 ± 1.80 |
- FSB, fresh shoot biomass; DSB, dry shoot biomass; FRB, fresh root biomass; DRB, dry root biomass; GW, grain weight; PrM, pressmud; FM, farm manure; CP, compost; SS, sewage sludge; PM, poultry manure (n = 3).
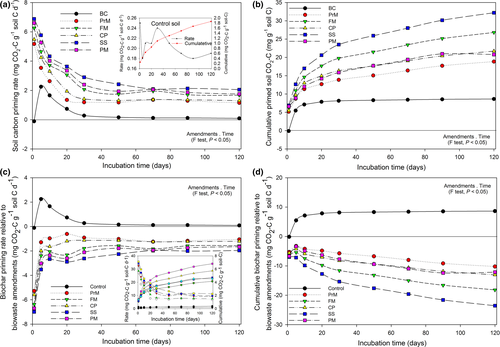
Total carbon mineralization/CO2 emission and interactive priming effects
The δ13C signature of CO2 released form BC and various BW-amended soils are presented in Table S1. Similarly, C-mineralization in soils amended with BC and various BW amendments compared with the control are shown in Fig. 2. The δ13C signature of BC (–28.16 ± 0.09‰) and other BW amendments (PrM, FM, CP, SS and PM) are summarized in Table 1. The δ13C ‰ value of pine wood (–27.23 ± 0.11‰), which was used as the feedstock for BC production and produced BC (–28.16 ± 0.09‰), showed a remarkable difference between the raw material and final product, indicating the significant (P ≤ 0.05) effect of pyrolysis on the δ13C signature (Table 1). On the other hand, the δ13C signature of CO2–C evolved during the incubation period from all amendments on every sampling day and was highly significant (P ≤ 0.01) compared to that of the control. The only exception was observed in the case of BC-amended soil on the first day (Table S1).
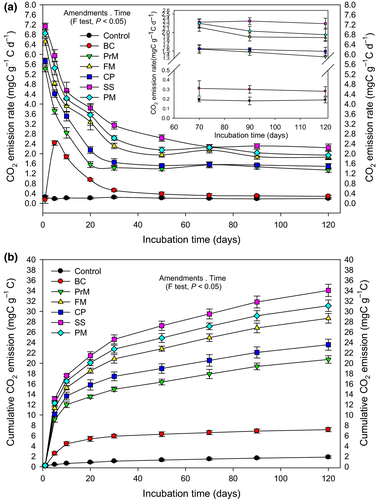
The addition of BW amendments increased the soil respiration rate and cumulative CO2 emissions up to tenfold compared to the unamended soil. However, the total BC-C-mineralization/CO2 emission rate decreased up to 0.14 ± 0.07 mg CO2–C g−1-C day−1 after immediate application (1st day) compared to that of the control (0.25 ± 0.06 mg CO2–C g−1-C day−1), and then increased, causing a loss of 2.57 mg CO2–C g−1-C compared to the control (0.43 mg CO2–C g−1-C) over 1–5 days. After this, the BC-C-mineralization decreased again from 2.43 ± 0.09 to 0.52 ± 0.05 mg CO2–C g−1-C day−1 over 30 days and then stabilized over time (over 120 days). On the other hand, the C-mineralization rates of all BW amendments were higher on day 1 (5.42 ± 0.12, 6.51 ± 0.14, 5.73 ± 0.12, 7.13 ± 0.14, and 6.89 ± 0.13 mg CO2–C g−1-C day−1 for PrM, FM, CP, SS, and PM, respectively) and then decreased in an exponential manner during the experimental time period. Throughout the experiment, the highest C-mineralization rate was observed in the case of SS followed by PM and FM. However, in the case of cumulative C-mineralization, 10.50 ± 0.39 mg of CO2–C g−1-C was released from the BC-amended soil over 120 days (P ≤ 0.05). All the BW amendments significantly increased (P ≤ 0.05) the cumulative C-mineralization (PrM: 20.72 ± 0.73 mg CO2–C g−1-C, FM: 28.67 ± 0.93 mg CO2–C g−1-C, CP: 23.57 ± 1.07 mg CO2–C g−1-C, SS: 34.06 ± 1.11 mg CO2–C g−1-C, and PM: 31.34 ± 0.15 mg CO2–C g−1-C) compared with that of the control (1.86 ± 0.24 mg CO2–C g−1-C) as well as with BC.
Immediately after the application, BC suppressed the native soil C-mineralization (−0.11 mg CO2–C g−1soil-C day−1), indicating a negative priming effect (P ≤ 0.05) (Fig. 3). Afterward, BC triggered the increase of the rate of soil C-mineralization toward the peak within 5–20 days, and this positive priming effect remained over the 120 days of the experimental time period. However, large positive priming effects were observed with all BW amendments (PrM, FM, CP, SS, and PM) relative to the control. Detailed information on a cumulative basis shows that C-mineralization of native soil due to the positive priming effect was higher for SS, followed by FM and CP = PM (Fig. 3).
Dissolved organic carbon and microbial organic carbon
At the beginning, the DOC slightly decreased (2.65 ± 0.42 μg-C g−1 soil) in the BC-amended soil compared to the control (3.18 ± 0.35 μg-C g−1 soil), then gradually increased between 5 and 30 days (up to 16.35 ± 0.85 μg-C g−1 soil) and remained uncertain (12.34 ± 1.19 μg-C g−1 soil to 13.87 ± 2.18 μg-C g−1 soil) from 30 to 120 days (Fig. 4a). However, various BW amendments significantly increased the DOC content (P ≤ 0.05), showing a maximum for SS within the range of 19.75 ± 1.23 μg-C g−1 soil to 35.85 ± 2.16 μg-C g−1 soil, followed by PM, FM, PrM, and CP, compared to that of the un-amended soil as well as to BC during the experimental time period of 120 days. These large DOC contents in various BW-amended soils indicate higher microbial activity, which is responsible for more C-mineralization.
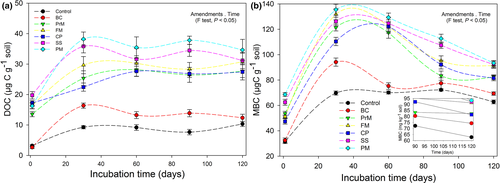
Similar to DOC, BC addition to soil did not influence the MBC on day 1 (32.45 ± 1.90 μg-C g−1 soil) compared to that of the control (31.45 ± 1.54 μg-C g−1 soil). On this other hand, this influence resulted in an increase in BC compared to the control on day 30 and continued to decrease slowly till the end of the experiment (Fig. 4b). However, analogous to the inceptive sequence of the C-priming and the comparative DOC in various amendments, the MBC was significantly higher for the SS (at 1, 30, 60, 90, and 120 days by 51.15 ± 1.28 μg-C g−1 soil, 131.82 ± 4.34 μg-C g−1 soil, 122.35 ± 3.64 μg-C g−1 soil, 95.21 ± 3.87 μg-C g−1 soil, and 91.34 ± 2.01 μg-C g−1 soil, respectively), with a decreasing order of SS < PM < FM < CP < PrM < BC < control. Although MBC was significantly higher in all BW-amended soils, the maximum MBC level was sustained in the SS-amended soil comparative to the control, over 120 days.
C-sequestration potential
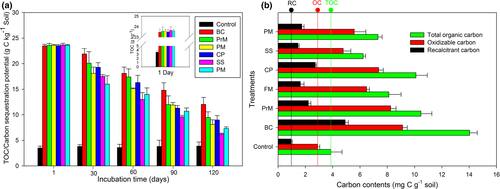
The change in total organic carbon content and the postexperiment soil δ13C value with the addition of BC and various BW amendments, compared to that of the control over an experimental time period of 120 days, are presented in Fig 5a and Table 4. The results obtained from the present study indicate that BC had a clear influence (P ≤ 0.05) on TOC compared with those of various BW amendments, except for PrM, which was not significant on the 30th, 60th, and 90th days. However, on day 120, all the BW amendments showed a significant (P ≤ 0.05) decrease in TOC compared to that of the BC-amended soil. On the other hand, no significant changes were observed in the TOC of the control (un-amended soil) throughout the experimental time period (1–120 days). All the amendments (BC and various BW amendments) had a positive and significant (P ≤ 0.05) effect on TOC, OC, and RC contents, which are presented in Fig 5b. Similarly, a strong, positive relationship (P ≤ 0.05) was observed between TOC and other forms of carbons (OC and RC). TOC, OC, and RC contents were significantly increased with the addition of all amendments with maximum contents for BC (TOC: 262.24%, OC: 214% and RC: 405.98%) compared to that for the control (TOC: 3.88 ± 0.81 mg-C g−1 soil, OC: 2.90 ± 0.15 mg-C g−1 soil and RC: 0.97 ± 0.07 mg-C g−1 soil). It was also observed that BC had a highly significant (P ≤ 0.05) effect on the TOC, OC, and RC contents compared to all BW amendments, except in the case of OC for PrM at the termination of the experiment. The relationships between the incubation study and pot experiment, with respect to total organic carbon and recalcitrant carbon, are presented in Fig. S1. Although both total and recalcitrant carbon contents were higher in the incubation study, the increasing pattern was almost similar to the pot experiment, indicating that the rate of decomposition was fast in the pot experiment compared with incubation.
| Treatments | Incubation experiment (δ13C ‰) | Pot experiment |
|---|---|---|
| Control | −16.67 ± 0.11 | −23.41 ± 0.13 |
| BC | −27.49 ± 0.09 | −25.82 ± 0.17 |
| PrM | −14.32 ± 0.16 | −16.69 ± 0.11 |
| FM | −21.74 ± 0.12 | −20.98 ± 0.19 |
| CP | −22.62 ± 0.15 | −22.08 ± 0.17 |
| SS | −20.22 ± 0.14 | −19.34 ± 0.14 |
| PM | −21.53 ± 0.11 | −20.74 ± 0.18 |
- Control (unamended) and amended soil (biochar and various biowaste amendments); (n = 3).
Discussion
In the case of BC, the higher values of pH and CEC may be attributed to the highly stable organic carbon (OC) content of BC, which provides more surface functional groups and a large surface area due to micropores and aromatic ring structures, which are the main causes of the high pH and CEC values, respectively (Jien & Wang, 2013; Li et al., 2013; Chintala et al., 2014; Gai et al., 2014; Obia et al., 2015). On the other hand, the high pH values of FM- and SS-amended soils could be attributed to the dissolved organic compounds (DOCs) with huge amounts of organic acids, due to the high rate of decomposition of organic matter (OM) (Riffaldi et al., 1998; Stumpe & Marschner, 2010; Liu et al., 2013). These results indicate that BC is composed of a highly persistent form of organic-C having strong carbon–carbon bonds (Singh et al., 2012). In addition, BC has a remarkably lower decomposition rate compared with various BW amendments having the same inceptive organic carbon (OC) contents (Lu et al., 2014; Jiang et al., 2016). Zhang et al. (2012) studied the change in the soil C-stock through the addition of BW amendments. Various BW amendments, such as manures, sewage sludge, and pressmud, contained large amounts of DOCs that were easily mineralized and converted into CO2 and other inorganic forms of carbon, resulting in a reduction of the soil-OC content (Walker et al., 2003; Nett et al., 2010; Romanyà et al., 2012).
The results obtained from this study indicate that addition of BC to soil significantly improved the wheat yield, plant biomass, and various physiological characteristics. Previous studies have shown an increase in the growth and yield of rapeseed, wheat, rice, canola, sweet potato, and maize with the addition of BC (Zhang et al., 2012; Liu et al., 2014; Martinsen et al., 2014; Tammeorg et al., 2014; Ahmed & Schoenau, 2015). In the present study, soils that were amended with carbonized wheat straw and olive tree pruning had a low observed plant biomass and grain yield compared with those that were amended with the application of BC (Antonio et al., 2013). The high value of biomass and greater grain yield of BC-amended soils could be attributed to the various mechanisms that are responsible for the change in soil physicochemical and biological properties (Lehmann et al., 2011; Herath et al., 2013; Jien & Wang, 2013; Mukherjee et al., 2014) and the phyto-availability of numerous macronutrients, that is, N, P, and K.
Soil and plant (root, shoot and grain) N contents increased significantly (P ≤ 0.05) with the application of BC and various BW amendments compared with that of the control (Fig. 1a). Similar results were observed by Saarnio et al. (2013) on the availability and uptake of N when BC spruce chips were applied to soil cultivated with Phleum pretense plants. Khan et al. (2014) described the constructive relationship between the plant uptake of N and the higher available content of N (NO3–N and NH4–N) in BC-amended soil compared with the control. In addition, Saarnio et al. (2013) also argued that the higher uptake of N in BC-amended soil could be attributed to the low availability of N to microbes due to the retention of N in BC-amended soil, which includes numerous mechanisms such as the adsorption of NH3–N and NO3–N onto the BC surface and ion-exchange reactions.
Unlike N, available and plant-uptaken P contents were nonsignificant in BC-amended soil (Fig. 1b). However, the maximum available and plant-uptaken P contents found with CP, PrM, and PM were significantly higher (P ≤ 0.05) than that of the control. This decreased P content in plant parts could be attributed to the adsorption of PO4–P onto BC partials. A few previous studies have shown alternative results, that is, in the BC-amended soils, the release of PO4–P was higher than that of the control, and this could be available to the plants (Hale et al., 2013; Khan et al., 2014).
Among the carbon sources, the maximum concentration of available K was recorded with BC, which was significantly higher compared to that with the control, followed by FM and CP (Fig. 1c). Similarly, the K content of shoots was greater compared with the control, but the K content of roots and grains was observed as being nonsignificant. Overall, BC, FM, and CP significantly (P > 0.05) affected the availability of K as well as its accumulation and uptake by wheat. The relationship between organic carbon and soil macronutrients (NPK) is presented in Fig. 1d. The BC differed significantly in its macronutrient availability and uptakes (Khan et al., 2014). The release of macronutrients with BC could also probably be attributed to various factors, that is, feedstock, pyrolytic conditions, and biochar pH (Nett et al., 2010; Kim et al., 2013; Mukherjee et al., 2014; Ahmed & Schoenau, 2015).
The findings of this study highlight that BC results in manifold stabilization of soil-C and mitigation of CO2 emissions, compared with various BW amendments, while maintaining the C-mineralization rate approximately equal to that of the control. In this study, the results of positive priming effects of BC relative to the control over the short–medium term are entirely consistent with previous investigations of Luo et al. (2011) and others (Farrell et al., 2013; Singh & Cowie, 2014). The priming effects (positive and negative) indicate ancillary mineralization of native soil-C or stabilization with the addition of BC and various BW amendments (Singh & Cowie, 2014; Hernandez-soriano et al., 2016). However, in the case of BC, large negative priming effects were observed relative to various BW amendments [PrM: −10.22 CO2–C (mg g−1 soil-C), FM: −18.17 CO2–C (mg g−1 soil-C), CP: −13.07 CO2–C (mg g−1 soil-C), SS: −23.56 CO2–C (mg g−1 soil-C), and PM: −12.14 CO2–C (mg g−1 soil-C)]. This negative priming effect of BC was large during days 1–5 [−3.24 to −7.18 CO2–C (mg g−1 soil-C)], then slowly reduced over time (Fig. 3). These negative priming effects indicate that BC is highly stable and persistent in soil systems having long-term carbon sequestration potential compared to the other BW amendments (Zimmerman et al., 2011; Tarquis et al., 2014). Based on the C-mineralization/CO2 emission rate, labile-C, and stability, treatments can be ranked in the following order: BC < PrM < CP < SS < FM < PM.
Although the BC used in the present study maintained or slightly increased cumulative CO2 emission compared to the control soil, it was still significantly lower than those of various biowaste amendments. Hernandez-soriano et al. (2016) described that this slight increase in CO2 emission in BC-amended soil may be due to the unexceptional existence of mineralizable carbon content in BC. Furthermore, the initially high respiration rate in BC-amended soil may be attributable to the release of surface-bound CO2 and in precipitated inorganic carbon on BC particles in the form of carbonate during the production of BC (Méndez et al., 2013). Some previous studies also suggest that BC may also stabilize native soil organic carbon by forming organo-mineral interactions as well as through the sorption of DOC on to the surface of BC and within the pore spaces due to the exceptionally high surface area and void fraction (Keith et al., 2011; Lehmann et al., 2011; Zimmerman et al., 2011; Singh & Cowie, 2014).
In contrast to the various BW amendments, DOC was significantly decreased by the addition of BC. The change in aboriginal microbial processes and the decrease in bioavailability were due to the high adsorption affinity of BC for organic compounds and may influence the DOC content of soil (Song et al., 2012; Suresh et al., 2012; Zhou et al., 2013; Shan et al., 2015). However, less MBC in the presence of BC indicates that microbial growth was restricted as a result of low organic carbon availability, which contributed to the decrease in DOC content (Singh & Cowie, 2014). However, previous studies have explained that the soil microbial activities might be an indicator of the influential role of BC in C-metabolism (Lehmann et al., 2011; Farrell et al., 2013). On the other hand, some studies suggest that the addition of BC to soil increases the aromaticity in the form of humic-like substances in DOC (Smebye et al., 2016)
Similarly, many previous studies are consistent with our findings describing that BC could inhibit many enzymatic activities and reduce MBC in soil (Singh & Cowie, 2014; Shan et al., 2015). This inhibition of microbial activity with the addition of BC may be attributable to the variability of soil physicochemical characteristics that are induced by BC (organo-mineral interactions), that is, adsorption of soluble compounds (organic and inorganic) onto the BC surface and change in water retention and microaggregates (Jin et al., 2013; Hernandez-soriano et al., 2016).
Our results highlight the potential increase in recalcitrant-C as a result of the addition of BC to soil rather than various BW amendments. Similarly, Lehmann et al. (2015) have also described that BC has higher recalcitrant-C content than fresh biowaste amendments and uncharred biomass, which are highly stable and not easily decomposable (Singh & Cowie, 2014). Furthermore, the complex structure (aromaticity) of biochar plays an important role in its long-term stay in the soil system. However, the stability of biochar may also be attributed to its low accessibility (Czimczik & Masiello, 2007) due to the existence of microaggregates, which are the indicators of organo-mineral interactions, in soil (Hernandez-soriano et al., 2016). Similarly, Ventura et al. (2015) used isotopic (δ13CO2) measurements to assess the stability and carbon sequestration potential of biochar in two European soils (Italy and UK). They observed less mineralization and a significant increase in soil organic carbon with the addition of biochar in both sites.
In a terrestrial ecosystem, soils act as a major sink for atmospheric carbon and can be helpful for storing atmospheric carbon for an indefinite period (Paustian et al., 2016). Exogenous addition of stable carbon sources, that is, biochar and compost, can significantly contribute to the enhancement of soil C-sequestration and may reduce net CO2 emission/C-mineralization (Lehmann et al., 2015; Ryals et al., 2015). Recent investigations have described that the rate of biochar decomposition is extremely low compared with various conventional organic amendments (fresh manures, sewage sludge, plant residues, etc.). Generally, biochar-C mineralizes several times slower than uncarbonized biomass, with mean retention times (MRT) 10- to 100-fold greater than those of unamended soil (Lehmann et al., 2015). Consequently, a large proportion of additive C from biomass-derived black carbon (biochar) can stay in soil systems for the long term, up to hundreds of years, due to more stable and recalcitrant-C. However, the retention time for sequestration of biochar-C varies depending on various biochar-soil factors such as feedstocks, pyrolytic conditions, and soil physicochemical characteristics (Schmidt et al., 2011).
Our findings describe that although exogenous addition of different sorts of organic amendments may produce varying effects on C-mineralization, microbial activity, and soil C-stocks (labile-C or recalcitrant-C etc.), biochar has a significantly higher influence on sequestration of carbon in soil as compared with various conventional amendments. Recently, the addition of biochar to soil has progressively increased as a potential tool to mitigate global warming (Shan et al., 2015).
In conclusion, the current study presents, for the first time, the priming effect of biochar-C-mineralization with respect to various BW amendments using a stable isotope (δ13C) approach and the possible impact of biochar-C-mineralization on C-sequestration and the labile organic carbon pool. During incubation, BC showed a negative priming effect, indicating a potentially higher carbon sequestration ability compared with those of various other BW amendments. However, recalcitrant-C and incubation time can also significantly affect the route and intensity of the priming effect. Overall, the results of this study suggest that the application of BC reduces C-mineralization compared with various BW amendments through soil carbon stabilization due to the presence of recalcitrant biochar-C and sorption of labile organic carbon onto BC particles, which results in less decomposition and significant increase in sequestration of carbon in soil over the long term (Singh & Cowie, 2014; Hernandez-soriano et al., 2016). In this circumstance, our results have important implications for an understanding of the priming effects of biochar-C-mineralization relative to various BW amendments. However, further studies are needed to evaluate the exact priming mechanism of biochar-C-mineralization in multisource mineralization systems using C-isotopic fractionation and a compound-specific stable isotope approach with a combination of spectro-microscopic techniques.
Acknowledgements
The authors greatly acknowledged the National Basic Research Program of China (973 Program, 2014CB238903) and the National Natural Science Foundation of China (No. 41373110) for financial support for this study. The Chinese Academy of Science, China (CAS), and The World Academy of Science, Italy (TWAS), are also greatly acknowledged for providing the CAS-TWAS President's fellowship (CAS-TWAS No. 2014-179). We also greatly appreciate the thoughtful comments and valuable suggestions from anonymous reviewers for the improvement of this manuscript.