Socioeconomic indicators for sustainable design and commercial development of algal biofuel systems
Abstract
Social and economic indicators can be used to support design of sustainable energy systems. Indicators representing categories of social well-being, energy security, external trade, profitability, resource conservation, and social acceptability have not yet been measured in published sustainability assessments for commercial algal biofuel facilities. We review socioeconomic indicators that have been modeled at the commercial scale or measured at the pilot or laboratory scale, as well as factors that affect them, and discuss additional indicators that should be measured during commercialization to form a more complete picture of socioeconomic sustainability of algal biofuels. Indicators estimated in the scientific literature include the profitability indicators, return on investment (ROI) and net present value (NPV), and the resource conservation indicator, fossil energy return on investment (EROI). These modeled indicators have clear sustainability targets and have been used to design sustainable algal biofuel systems. Factors affecting ROI, NPV, and EROI include infrastructure, process choices, and financial assumptions. The food security indicator, percent change in food price volatility, is probably zero where agricultural lands are not used for production of algae-based biofuels; however, food-related coproducts from algae could enhance food security. The energy security indicators energy security premium and fuel price volatility and external trade indicators terms of trade and trade volume cannot be projected into the future with accuracy prior to commercialization. Together with environmental sustainability indicators, the use of a suite of socioeconomic sustainability indicators should contribute to progress toward sustainability of algal biofuels.
Introduction
Socioeconomic and environmental indicators of progress toward bioenergy sustainability are used for designing facilities and assessing effects of scenarios, making comparisons among energy technology options, and quantifying compliance with sustainability targets at facility, regional, and national scales (Efroymson et al., 2013). Biofuel producers use sustainability indicators to make decisions about siting, cultivation, logistics, conversion, and fuel blends based on projections into the future or retrospective measures. Consumers make decisions about biofuel purchases partly based on sustainability.
Sustainability is the capacity of a process or activity to continue while maintaining options and continuing to meet needs of future generations (Brundtland, 1987). Environmental sustainability includes water quality and quantity, soil quality, air quality, greenhouse gas (GHG) emissions, biodiversity, and productivity (McBride et al., 2011). Socioeconomic sustainability considers social well-being, energy security, external trade, profitability, resource conservation, and social acceptability (Dale et al., 2013a).
Environmental sustainability indicators have been evaluated for their applicability to algal biofuels (NRC, 2012; Efroymson & Dale, 2015), and some environmental sustainability effects have been measured. For example, water availability (Venteris et al., 2013) and water use (Gerbens-Leenes et al., 2014) have been evaluated in the algal biofuel context. The designation of several algal-oil-containing renewable diesels and cyanobacteria-generated ethanol as renewable fuel standard advanced biofuels reflects evidence that manufacturing processes reduce GHG emissions (USEPA, 2015a).
Socioeconomic effects of algal biofuel systems have not been considered comprehensively. Zhu et al. (2015) described potential social, economic, and cultural dimensions of algal biofuels but stopped short of recommending or measuring particular indicators. The National Research Council does not address socioeconomic indicators of sustainability in its report on sustainable development of algal biofuels, except for energy return on investment (EROI). Maintaining food security, that is not competing for agricultural land, is often cited as a general benefit (NRC, 2012; Daroch et al., 2013) without formal measurement.
This study evaluates how and to what extent socioeconomic indicators are used to design algal biofuel systems. The indicators are those proposed by Dale et al. (2013a), which represent a scientifically based and practically measurable suite selected from a variety of sources, including the Global Bioenergy Partnership (GBEP, 2011), the Roundtable on Sustainable Biomaterials (RSB, 2011), and the Sustainable Biodiesel Alliance (SBA, 2014). We consider differences between biofuel systems using terrestrial, vascular crops and microalgae or cyanobacteria. We review previous measurements of socioeconomic sustainability indicators, key sustainability target values, and factors that influence indicator values and consider the consistency of assumptions that influence measurements. Factors that could influence unmeasured indicators during commercial development are discussed.
Background
Algal biofuels include biodiesel, renewable diesel, ethanol, and other liquid fuels produced using microalgae or cyanobacteria. As with terrestrial crop supply chains, the algal supply chain includes feedstock production, feedstock logistics, conversion to biofuel, biofuel logistics, and biofuel end-uses. Unlike terrestrial bioenergy, most start-up algal biofuel companies are engaged in the entire supply chain, rather than being focused on just one step. Within supply chain steps are choices that can be informed by measurements of sustainability indicators, for example, siting, selection of algal strain, and management of facilities and processes, including the cultivation system [e.g., open-pond systems or photobioreactors (PBRs)] and the selection of sources of nutrients such as CO2 and nitrogen. Feedstock logistics and conversion can involve decisions about whether to harvest and dry biomass for lipid extraction or pyrolysis, or whether to use a hydrothermal liquefaction (HTL) process, where drying is unnecessary. Biofuel logistics involves choices about transport, storage, and coproducts, and biofuel end-uses require decisions about blend conditions and engine type and efficiency.
Economic and social sustainability is influenced by characteristics of algal biofuel and terrestrial crop biofuel systems, including methods and costs of production. Characteristics of algal biofuel systems that could affect particular aspects of social and economic sustainability are shown in Table 1. High infrastructure and energy costs of algae-based biofuels, compared to those of terrestrial crop-based biofuels, as well as the need for supplemental CO2, underlie the importance of measuring profitability and energy balance.
| Property of algal biofuel supply chain | Important sustainability indicator(s) |
|---|---|
| Expensive infrastructure | Profitability indicators |
| Probable use of nonagricultural land | Social well-being indicators, such as food security |
| High energy inputs | Resource conservation indicators |
| Need for CO2 supplements | Profitability indicators |
| Wide range of species and strain options | Profitability indicators |
| Potential use of genetically modified algae | Social acceptability indicators, such as public opinion |
| Potential pond crashes and need for monitoring and management | Profitability indicators, social acceptability indicators such as risk of catastrophe |
| Wide range of potential coproducts | Profitability indicators |
| Early stage of commercialization | All indicators, especially profitability |
| Potential occupational hazards from toxins or other water contaminants | Social well-being, such as workdays lost to injury |
| Potential susceptibility to hurricanes and natural disasters | Social acceptability indicators, such as risk of catastrophe |
Indicators of socioeconomic sustainability of algal biofuels
We review how socioeconomic indicators have been applied to design sustainable algal biofuel supply chains, how indicator values have been compared to targets, and which additional indicators should be measured to form a more complete picture of socioeconomic sustainability. Goals for designing sustainable systems with these indicators, including target values, are described in Table 2. Because algal biofuels are not produced at commercial scale, many socioeconomic indicators are projected rather than measured. Below the indicators are considered by category.
| Category | Indicator | Goal for design of sustainable system |
|---|---|---|
| Social well-being | Employment | Provide a large number of high-paying jobs |
| Household income | Provide high-paying jobs and decrease fuel costs so that household income increases | |
| Workdays lost due to injury | Select algal strains and conversion processes to minimize toxin production and toxicant exposure | |
| Food security | Develop algal biofuel systems on nonagricultural land and consider food coproduct options | |
| Energy security | Energy security premium | Maximize energy security dollar benefits of substituting algal biofuel for petroleum fuel |
| Fuel price volatility | Reduce fuel price volatility below value without algal biofuel by contributing to reliable algal biomass and fuel supply with consistent prices | |
| External trade | Terms of trade | Create conditions so that less capital leaves a government entity to purchase petroleum |
| Trade volume | Minimize net imports for fuel | |
| Profitability | Return on investment (ROI) | Create a positive ROI |
| Net present value (NPV)3,4 | Create a positive NPV | |
| Resource conservation | Depletion of nonrenewable energy resources | Reduce amount of petroleum extracted per year, with a goal of zero |
| Fossil energy return on investment (fossil EROI) | Increase fossil EROI above 1 and eventually above 3 | |
| Social acceptability | Public opinion | Demonstrate high % favorable opinion |
| Transparency | Show a progressively increasing or high value | |
| Effective stakeholder participation | Show a progressively increasing or high value | |
| Risk of catastrophe | Maintain frequency of catastrophic events at current incidence or based on similar technology |
Indicators of social well-being
Social well-being denotes fulfilled human needs such as food, health, and shelter. Employment, income, workdays lost to injury, and food security are indicators of social well-being for bioenergy with their units being number of full-time equivalent jobs, household income in dollars per day, average number of workdays lost per worker per year due to injury, and percent change in food price volatility, respectively (Dale et al., 2013a). Employment and food security are particularly important in siting algal biofuel facilities and are discussed below.
Net job creation is often viewed as the principal economic benefit of the algal biofuel supply chain (Zhu et al., 2015). New companies garner political, economic, and social support in a region by projecting the number of hires (e.g., Muradel, 2014). Providing investors with cost-efficient supply chains attracts them to a region and leads to new jobs (Ekşioğlu et al., 2009). Local governments may provide incentives to companies to relocate to an area based on projected hires. Many algal biofuel companies already have reportable employment numbers for pilot plants. Indirect employment (Wei et al., 2010) includes suppliers of resources and technology necessary for algal biofuels, such as nutrients, CO2, polyethylene liners, PBRs, pumps, as well as personnel from plants that provide colocated resources (e.g., CO2, nutrients) to biofuel facilities. An analysis of projected employment from the algal diesel sector in China found that employment numbers were sensitive to technical parameters such as biomass productivity, lipid content, project duration, lipid extraction rate, and lipid conversion rate (Yang et al., 2015).
Both employment and household income are indicators of progress toward sustainable algal fuel production that relate to rural development (GBEP, 2011; RSB, 2011). Yang et al. (2015) found considerable variation in projected regional employment in the algal biodiesel sector in China, with model parameters reflecting marginal land resources, temperature, sunlight, and local supply rates of diesel and crude oil. However, given the cost of infrastructure and industrial cultivation expertise required to produce algal biofuels, employment and income indicators are unlikely to be incentives for small landholders (Florin et al., 2014) who may cultivate terrestrial feedstocks. Instead, deployment of enabling technologies may be a prelude to social improvements such as increased employment and income associated with algal-based biofuels in a region. For example, the use of saline water for cultivation might lead to the development of algal biofuel production in regions where freshwater is scarce, such as nonarable regions of Australia, northwestern Africa, the Arabian Gulf, and deserts in the United States (Nair & Paulose, 2014).
Statistics on workdays lost to injury should be available for laboratories and pilot plants, at least for internal company purposes. However, few types of health issues or injuries are specific to algal biofuel production. Potential hazardous scenarios that could lead to workdays lost include algae being dried without mitigations for airborne particulate matter; the use of unfamiliar, toxin-producing algae; risk from volatile organic compounds emitted from unventilated algal cultivation systems; or the use of wastewaters or waste CO2 streams that lead to accumulation of metals in algal cultivation systems (NRC, 2012, Sullivan, 2013).
Biofuel operations should facilitate ‘the human right to adequate [and nutritious] food and improve food security in food insecure regions’ (RSB, 2011). Measuring food security can be fraught with disagreements about definitions and methodologies (FAO, 1996). Percent change in food price volatility (FAO, 2011) is a potential unit for the food security indicator that would generally be zero if nonagricultural land is used (NRC, 2012; Daroch et al., 2013). Under this constraint, biomass and prices of algae and algal fuel should not be impacted by food price volatility (Ziolkowska & Simon, 2014), nor should they affect it. This result is consistent with the European Union's decision not to impose an indirect land use change factor on algal biofuels (EU, 2012). Nonetheless, it is important to include food security in the set of indicators so that comparative assessments can use a consistent set of indicators.
A possible factor affecting food price volatility could be the use of pastureland, if algal biofuels grown on those lands reduce meat production (NRC, 2012) and if coproducts are not used as feed. Under a scenario where 41.5 × 109 L yr−1 of second-generation biofuels are produced on US pastureland, the most likely land type where both algae grown in open ponds and terrestrial biofuel crops might be deployed, two to five percent of the pastureland could be converted to algae as feedstock (Langholtz et al., 2016). Competition between food crops and algae for fertilizer (Jacquin et al., 2014) could also affect food security if fertilizer is not derived from process waste or wastewater.
Some researchers suggest that algal biofuels could have a net positive impact on food security if defatted, high-protein residual algal biomass is used as a coproduct to replace corn and soybean meal in swine and poultry diets (Gatrell et al., 2014) and if the boundaries of sustainability analyses include coproducts. Food additives for humans, livestock feed, aquaculture food, biochar for fertilizer, polyunsaturated fatty acids, and recombinant protein extracts (e.g., astaxanthin) (Brennan & Owende, 2010; Kiron et al., 2012; NRC, 2012; Austic et al., 2013) are coproducts that could reduce food price volatility. However, this indicator would not capture short-term, temporary disruptions in food supply or contamination (if caused by or alleviated by algal biofuels production), even though they could also have implications for social well-being. Any hypothesized link between algal biofuels and food price volatility cannot be realistically assessed until algal biofuels are in large-scale commercialization.
Indicators of energy security
Another category of socioeconomic progress toward sustainability is energy security, which refers to the relationship between national security and the availability of natural resources for energy. Energy security is one of the primary motivations for the development of algal biofuels, for it provides a response to the need for diversification of energy supply chains (Kirayama & Kajikawa, 2014) and energy supply continuity (Winzer, 2012). During the current early stage of technology development, the flexibility to respond to changing markets, new technologies, and competition is important (Nair & Paulose, 2014). Two indicators of energy security are energy security premium, with units of dollars per gallon of biofuel, and fuel price volatility, with units of standard deviation of monthly percent price changes over 1 year (Dale et al., 2013a). Algal biofuels have the potential to be a hedge against price volatility of diesel, ethanol, or other fuels if they provide a significant fuel source in areas with high fuel imports and flexible supply. Biofuels would not be susceptible to geopolitical challenges that have influenced fossil fuel price volatility and energy security premium in the USA.
Energy security indicators can be classified as ex-post indicators (calculation after price formation in fuel markets) or ex-ante indicators (estimate of future changes in energy security) (Lӧschel et al., 2010). Years or decades are required before ex-post indicators can be measured for algal biofuels. Projecting fuel diversity and market concentration in particular geographic areas (and estimating when and to what extent algal biofuels might make a significant contribution to fuel supply) has substantial uncertainty.
As the most geographically isolated and fossil fuel-dependent US state, Hawaii is at high risk from supply interruptions and fuel price volatility (Bennett et al., 2014). This risk justifies the state's emphasis on renewable energy with a 2030 mandate of 40% of the state's electrical generation being from renewable resources and a concurrent 70% reduction of petroleum use for ground transportation (State of Hawaii, 2013). The climate of Hawaii is suitable for high-productivity algal biofuel production. Therefore, Bennett et al. (2014) estimated land and water resources available across the state and the quantity of potential biofuel that could be produced.
Aviation fuel supply chains have special needs with respect to energy security, including the goal to develop locally based supply chains on every continent, and these fuel supply chains can begin with algae. Furthermore, aviation requires liquid fuels and hence cannot use many other renewable energy alternatives (Dale et al., 2014). Fixed price contracts for aviation fuel are important, but poor timing can result in noncompetitive prices if fossil fuels are used. In addition to political instabilities, fluctuating prices, and decreasing resources, the petroleum supply chain is not sufficiently integrated from production to the airplane, because of geographically distant resources and inefficient transportation by tankers (Nair & Paulose, 2014). Algae production is suited to the need for reliable fuel supplies at many locations around the world. Algal biofuel producers are typically the same companies that produce the feedstock, so supply chain steps are well integrated.
However, threats to the continuous supply of algal biofuels could affect fuel price volatility. Factors include pathogens and predators that cause algae pond crashes and extreme weather events that could affect cultivation. For example, Hurricane Katrina in 2005 in the Gulf of Mexico had a negative effect on energy supply (Reed et al., 2010), and the ability to establish the necessary infrastructure and culture for algae cultivation systems to be able to withstand future disturbances is uncertain.
Indicators of external trade
Two key indicators of external trade (i.e., movement of goods and services across borders) are terms of trade and trade volume. Terms of trade, expressed as percent ratio of price of exports to price of imports, has a value of more than 100% if more capital is coming in from imports than is going out. Trade volume represents net exports or balance of payments and has units of dollars. Prior to large-scale commercialization, algal fuels would likely be used regionally. However, if ethanol were a significant product of a number of algae companies, local surpluses might lead to large-scale exports. New algae projects are sometimes advertised for their ability to improve terms of trade (Muradel, 2014). Until there is commercialization as well as trade, there can be little confidence in projections of trade indicators.
Indicators of profitability
Profitability is an important category of economic sustainability for producers in a nascent industry. Prospects for industry profitability lead to investment capital and help determine which technologies should be pursued through research and development and during scale-up. Key indicators are return on investment (ROI), measured as a ratio or percent (net investment/initial investment), and net present value (NPV) in dollars, the present value of benefits minus costs. Factors that affect profitability and are described below include the cost and availability of infrastructure and capital equipment, availability of resources, productivity, the policy environment, the biofuel system, the price of biofuels, coproducts, and factors affecting the future price of petroleum products (Table 3).
| Factor influencing profitability | References |
|---|---|
| Infrastructure and capital equipment | |
| Use of pond or photobioreactor | Davis et al. (2011), Amer et al. (2011), Richardson et al. (2014b) |
| Use of liner or unlined ponds | Rogers et al. (2014) |
| Availability of natural gas | Venteris et al. (2014b) |
| Availability of electricity | Venteris et al. (2014b) |
| Plant production capacity | Brownbridge et al. (2014), Jones et al. (2014) |
| Scaling of downstream capital equipment to production | Abodeely et al. (2014) |
| Availability of oil pipelines | Venteris et al. (2014b) |
| Use of existing petroleum refineries (or not) | Nair & Paulose (2014) |
| Proximity to rail lines | Venteris et al. (2014b) |
| (Under)utilization of equipment during off-peak seasons | Davis et al. (2014) |
| Number and scale of conversion equipment trains scaled to seasonal production | Davis et al. (2014) |
| Installation and operation costs | Jonker & Faaij (2013), Ramos Tercero et al. (2014) |
| Region (e.g., affecting capital costs) | Huntley et al. (2015) |
| Depreciation | Ramos Tercero et al. (2014) |
| Rate of amortized capital costs | Campbell et al. (2011) |
| Resource requirements | |
| Source of nutrients (recycling, colocation with wastewater, fertilizer) | Slade & Bauen (2013), Jonker & Faaij (2013) |
| Source of CO2 (pure gas, colocation with power plants or industry), purity, delivery method, and distance to source | Campbell et al. (2011), Oilgae (2011), Slade & Bauen (2013), Jonker & Faaij (2013), Bennett et al. (2014), Rogers et al. (2014), Orfield et al. (2014a) |
| Source of water (groundwater or utility purchase) | Davis et al. (2011), Slade & Bauen (2013) |
| Energy needs | Davis et al. (2014), Orfield et al. (2014b), Béchet et al. (2014) |
| Cost of land | Ramos Tercero et al. (2014) |
| Policy environment | |
| Production of coproducts and their market saturation | Soratana et al. (2014) |
| Potential carbon taxes | Gallagher (2011) |
| Carbon price increase rate | Brownbridge et al. (2014) |
| Carbon capture legislation | Sayre (2010) |
| Approved biofuel pathways under ‘advanced biofuels’ mandate of US Renewable Fuel Standard under the Energy Independence and Security Act | USEPA (2015a) |
| Excise taxes | Campbell et al. (2011) |
| Cultivation | |
| Areal growth rate | Benemann & Oswald (1996), Williams & Laurens (2010), Nagarajan et al. (2013), Jonker & Faaij (2013), Rogers et al. (2014), Abodeely et al. (2014), Mata et al. (2014), Quinn & Davis (2015) |
| Temperature control system | Béchet et al. (2014), Ramos Tercero et al. (2014) |
| Temperature variability and associated productivity fluctuations | Béchet et al. (2014) |
| Cell lipid concentration | Gallagher (2011), Rogers et al. (2014), Abodeely et al. (2014), Brownbridge et al. (2014) |
| Frequency of culture crashes | Richardson et al. (2014b) |
| Energy needed for pumping | Slade & Bauen (2013) |
| Continuous vs. seasonal or diurnal operation of paddlewheels | Rogers et al. (2014) |
| System monitoring required for pathogens, nutrients, growth | Hanson (2011) |
| Labor and overhead | Jonker & Faaij (2013) |
| Processing | |
| Harvesting, including energy and potential dewatering, drying, flocculation, centrifugation, etc. | Williams & Laurens (2010), Slade & Bauen (2013), Richardson et al. (2014a), Rogers et al. (2014), Abodeely et al. (2014), Brennan & Owende (2010) |
| Extraction method and efficiency, including potential solvent recovery | Amer et al. (2011), Richardson et al. (2014a), Rogers et al. (2014) |
| Extraction of protein (for protein hydrolysate coproducts) prior to hydrothermal liquefaction | Barreiro et al. (2014) |
| Conversion method | Amer et al. (2011) |
| Concentration and temperature of slurry (which influence reactor size), and operating pressure in hydrothermal system | Elliott et al. (2015) |
| Coprocessing with other types of biomass | Jones et al. (2014) |
| Oil yield | Jones et al. (2014) |
| Offsite or onsite upgrading | Jones et al. (2014) |
| On-stream operating factor (fraction of time process operating) | Quinn & Davis (2015) |
| Distribution | |
| Off-take partnerships | Neste Oil Corporation (2013, 2014) |
| Use of conventional diesel fuel distribution networks | Borkowski et al. (2012) |
| Product longevity | |
| Shelf stability of fuel | Borkowski et al. (2012) |
| Prices of related commodities | |
| Price of animal feed and algal protein as animal feed | Williams & Laurens (2010) |
| Price of petroleum, diesel, biodiesel | Shen et al. (2009) |
| Project financials | |
| Installation and operation costs | Jonker & Faaij (2013), Ramos Tercero et al. (2014) |
| Facility lifetime | Quinn & Davis (2015) |
| Equity rate of return | Hassannia (2009) |
| Interest rate (debt rate of return, risk financing) | Hassannia (2009), Davis et al. (2015) |
| Discount rate | Taylor et al. (2013), Davis et al. (2015), Quinn & Davis (2015) |
| Depreciation assumptions | Ramos Tercero et al. (2014), Quinn & Davis (2015) |
| Rate of amortized capital costs | Campbell et al. (2011) |
| Other income streams and credits | |
| Quantity and type of coproducts, including market saturation | Williams & Laurens (2010), Soratana et al. (2014) |
| Wastewater treatment credits | Orfield et al. (2014a), Zhou et al. (2014) |
| Subsidy for greenhouse gas emissions reductions | Gutiérrez-Arriaga et al. (2014) |
| Collaborations with major oil companies | Ziolkowska & Simon (2014) |
| Competition with petroleum | |
| Price of oil, gasoline, diesel, and price increase rate | Gallagher (2011), Brownbridge et al. (2014) |
| Subsidies and tax incentives for oil exploration | ELI (2009), Campbell et al. (2011) |
Techno-economic analyses (TEAs) can be used to estimate profitability indicators and to identify factors that affect them. As with other social and economic indicators, profits are not measurable for algal biofuels until the industry grows commercially, but scenario analysis provides estimates. During research and development and at the pilot and commercial scales, TEAs can be performed to compare costs and benefits of a project or facility, to compare multiple technologies, to evaluate how a production target can be met, to identify largest contributors to cost, and to evaluate economically feasible scales of technology implementation. For example, Davis et al. (2015) performed TEAs to determine how to reach a specified biomass cost target (and related productivity target) by 2022. Primary sources of differences in TEA results are differences in cultivation infrastructure, production pathways, financial assumptions, and productivity estimates (Quinn & Davis, 2015). Factors affecting profitability, as identified in TEAs and other studies, are described below. TEAs can be highly uncertain because of a lack of data availability and reliance on parameters extrapolated from laboratory studies and from old data (Slade & Bauen, 2013).
Previous uses of ROI and NPV
Return on investment has been projected in TEAs of algal biofuel systems. For example, Brownbridge et al. (2014) evaluated the economic viability of a biorefinery with naphtha and carbon sequestration as coproducts. In this case, the 30-year ROI was most sensitive to algae oil content, the rate of increase in crude oil price, and annual productivity (Table 3). The 5-year ROI was sensitive to algae oil content, the crude oil price when the plant began operation, and annual productivity (Brownbridge et al., 2014). ROIs for several pond design cases were presented in Davis et al. (2015).
Researchers have calculated NPV using different supply chain assumptions. Gallagher (2011) calculated NPV for algae-to-biodiesel scenarios at a pilot plant, assuming different productivities and lipid concentrations. CO2 costs, as well as the real cost of crude oil, influenced profitability (Table 3). Richardson et al. (2014a) compared NPV and cost of producing lipid while varying two harvesting technologies and three extraction technologies. They found a 64% improvement in NPV and a 90% cost reduction for the most promising combination of harvesting and extraction systems compared to a defined baseline. Mata et al. (2014) estimated NPV of microalgae grown in brewery wastewater and found that NPV increases with areal productivity, although internal rate of return (IRR) approaches a limit value of around 26%, as simulated for pond areas between one and three hectares. Gebreslassie et al. (2013) developed a multiobjective model for designing an algal biorefinery using NPV to measure the economic objective. Multiple technologies were considered, with global warming potential as an additional objective and energy balance as a constraint. To achieve the maximum NPV in the model, flue gas, a tubular PBR, a filtration dewatering unit, and a hydroprocessing pathway were used.
Discount rates, used to convert future prices to present prices, are assumed in profitability calculations. Example discount rates used for algal biofuels are 10%, which assumes that technologies are already developed (Davis et al., 2015), and 15%, which assumes that technologies have low maturity and high risk (Taylor et al., 2013). Investors require higher returns before a technology is fully developed and typically need a high ROI within 3–5 years (Lundquist et al., 2010). Projections of minimum algal biomass selling prices are sensitive to the after-tax discount rate (Davis et al., 2015).
Factors affecting costs and prices
Capital costs for algae production are high, compared to costs of farms that grow terrestrial crops for biofuels. The need for capital for permanent infrastructure is a significant risk for investors and financers, as well as project developers, especially if infrastructure is not portable (Hanson, 2011). Capital costs for PBRs are much higher than those for open-pond systems (for which operational costs are primary), and production costs from PBRs are largely due to capital costs of the reactor (Richardson et al., 2014b). However, PBRs may be relocated, minimizing the risk of this capital expense. In open-pond systems, liners have a large impact on costs (Davis et al., 2013), with estimates ranging from 24% (Rogers et al., 2014) to 75% (Coleman et al., 2014) of capital expenses (Table 3). In some locations algal biofuel companies can construct open-pond, freshwater cultivation systems without liners (Rogers et al., 2014), but state or local regulations may require liners for saline systems. To minimize costs of processing, the capacity of capital equipment should be designed so that it is not idle for long time periods (Abodeely et al., 2014). Additional infrastructure cost issues include whether existing refineries can be used (Jones et al., 2014; Nair & Paulose, 2014) and whether fuel pipelines are available, both of which are influenced by whether drop-in fuels are produced (Table 3).
Freshwater, CO2, and fertilizer can account for up to 30% of production costs for algal biofuels (Clarens et al., 2010; Chen et al., 2011; Zhou et al., 2014), all of which influence profitability (Table 3). Costs of nutrient inputs can be reduced by using CO2 waste streams from colocated industry or power plants, using nitrogen and phosphorus from colocated wastewater, or recycling nutrients. Resource costs depend on the source and purity. Pure CO2 is produced by ammonia, hydrogen, and ethanol plants, whereas processing of flue gas from power plants may require capital and operational expenses for preprocessing, desulfurization, heavy metal removal, and other purification steps (Oilgae, 2011), as well as transport capacity for mixed gases. Transport costs limit the use of waste CO2 streams (Quinn et al., 2013). Pipeline transport costs depend on the capacity of the pipeline and, for longer distances, on the length of the route and number of highways and water crossings (Oilgae, 2011). The use of power plant flue gas can have additional cost considerations. Metals in flue gas could increase or decrease productivity of some algae species (Napan et al., 2015) or possibly render refinery catalysts for hydrotreating ineffective (Borkowski et al., 2012). Infrastructure is not yet in place to commodify waste CO2 (Liu et al., 2013).
The use of wastewaters as nutrient sources may decrease costs of algal biofuels, which is the subject of feasibility analyses (e.g., Fortier & Sturm, 2012). Furthermore, wastewater treatment credits might be redeemed if wastewaters are used, potentially leading to electricity cost savings of 20% (Lundquist et al., 2010). Orfield et al. (2014a) projected economically viable biofuel production using flue gas and wastewater co-utilization and found that production potential was more sensitive to nutrient than CO2 availability.
Efficiency in the algal biofuel supply chain is important for maximizing profitability. Doubling biomass productivity could reduce the cost to produce algal biodiesel by 26–32% based on Benemann & Oswald (1996) or 40–42% based on Nagarajan et al. (2013). Profits may be impossible below annual yields of 20 g m−2 day−1 (Williams & Laurens, 2010). Greater profits would accrue from mitigating the 5–10% and 10–30% lost productivity from grazers in PBRs and open-pond systems, respectively (Richardson et al., 2014b). Strain selection influences profitability, with different productivities, lipid yields, or potentials to produce coproducts.
Reducing harvesting and extraction or HTL costs would lead to increased profitability (Richardson et al., 2014a). Hydrotreating at a central, conventional refinery would obviate the need for a hydrotreater, hydrocracker, hydrogen plant, and the import of natural gas (Jones et al., 2014). Off-take agreements, that is, agreements of buyers such as gasoline companies to purchase algal biomass or fuel, would also be expected to affect price and profitability of algal biofuels (Neste Oil Corporation, 2013, 2014).
The economics of coproducts affects profitability estimates for algal biofuel systems. According to one company CEO, less than 10% of the biomass drives 80–90% of product value, and fuel and feed can be produced from the remainder (Schultz, 2013). The production of biodiesel, ethanol, and biogas from a single source can improve economics of algal biorefineries (Zhu et al., 2014). However, most coproducts of algae bioenergy are food and feed; thus, at commercial scale, market saturation could lead to reduced profitability. An analysis of the ratio of production-to-consumption quantities of coproducts suggests that animal feed and nitrogen fertilizer markets are less likely to be saturated than ethanol and other markets (Soratana et al., 2014).
Subsidies and the price of carbon will help determine future profitability of algal biofuels. Carbon taxes and carbon trading may affect prices. For example, the 2015 US Clean Power Plan (40 CFR Part 60) allows state plans to implement carbon capture and utilization technologies to reduce CO2 emissions from power plants, as long as emission reductions are monitored, reported, and verified. This is an incentive for utilities to sell waste CO2 cheaply or pay algae facilities to accept it. As algal biofuels are produced in large volumes, fossil gasoline and diesel may become cheaper because of increased biofuels supply. Thus, the profitability of algal biofuel production may depend on market incentives such as wedge taxes that fix the costs of petroleum fuel to match that of algal biofuel (Levitan et al., 2014).
Indicators of resource conservation
Indicators of resource conservation are used where the biofuel supply chain may affect an important resource for sustainability not captured by environmental sustainability indicators, such as water quantity indicators (Dale et al., 2013a). Fossil energy resources are the primary focus, because biofuels substitute for petroleum gasoline and diesel. Also, fossil energy is used to produce algal biofuels in most real or hypothetical systems, but the use of renewable inputs leads to more sustainable systems. Two primary indicators of resource conservation with respect to bioenergy sustainability are depletion of nonrenewable energy resources, with units of MT of petroleum extracted per year, and fossil EROI, the ratio of the amount of fossil energy inputs to the amount of useful energy output in MJ, adjusted for energy quality. Fossil EROI has also been termed the ‘fossil energy ratio’ (Murphy et al., 2011). EROI is recommended by the National Research Council as a first step in assessing the sustainability of an algal biofuel supply chain, because the system must return more energy than it is producing (NRC, 2012). Change in the mass of petroleum extracted per year to support the production of algal biofuels is less commonly measured and is not discussed here.
Fossil EROI considers only fossil energy inputs; solar, wind, and waste biomass used for energy are not included in the denominator because they are renewable resources. Automated technologies used to monitor algae growth, temperatures, and potential harmful strains of algae would facilitate the use of solar photovoltaics in algal biofuel production (Nair & Paulose, 2014). Solar drying is feasible for some algae-based industries but may not be rapid enough for large-scale commercial applications (Sander & Murthy, 2010).
Several studies have estimated EROI for algal biofuels. Most scenarios use fossil energy sources or fossil energy combined with biomass energy, such as biogas. For example, Sills et al. (2013) simulated EROI for algae grown in seawater in hybrid PBR-open-pond systems and found that areal productivities of 34–50 g m−2 day−1 combined with wet extraction consistently resulted in EROI values >1, while productivities between 17 and 33 g m−2 day−1 resulted in EROI values greater than one in 75% of Monte Carlo simulations. Zaimes & Khanna (2014) found fossil EROI to be less than one for many algae supply chain scenarios. Beal et al. (2015) simulated scenarios with EROI values between 3.24 and 8.35 that involved gravity-fed volume transfers, airlift pond circulation, naturally settling species, and efficient extraction and conversion processes. Wind power was used in some of their cases. Orfield et al. (2014b) compared EROI of HTL reaction temperatures and times.
Factors that drive energy usage and EROI are listed in Table 4 and depicted with relevant steps in production pathways in Fig. 1. EROI is highly dependent on assumptions of the analysis and boundary choices for supply chains (NRC, 2012), for example, on whether coproducts such as feed are included (Beal et al., 2015).
| Factor influencing EROI | References |
|---|---|
| Infrastructure | |
| Installation | Slade & Bauen (2013), Lardon et al. (2009) |
| Use of pond/raceway or photobioreactor | Weschler et al. (2014) |
| Type and geometry of photobioreactor | Slade & Bauen (2013), Pegallapati et al. (2013) |
| Geometry of pond/raceway (e.g., baffles) | Huang et al. (2015) |
| Mixing method (e.g., paddlewheel assumptions, airlift pond circulation) | Beal et al. (2015), Slade & Bauen (2013), Rogers et al. (2014), Benemann & Oswald (1996) |
| Number, type, and size of pumps or gravity-fed volume transfers | Slade & Bauen (2013), Beal et al. (2015), Jonker & Faaij (2013), Rogers et al. (2014) |
| Resource requirements | |
| Fertilizer (embodied energy, recycling) | Clarens et al. (2010), Slade & Bauen (2013), Lardon et al. (2009) |
| Source and purity of CO2 and distance to source (e.g., flue gas) | Jonker & Faaij (2013), Brentner et al. (2011), Venteris et al. (2014a) |
| Technology for purifying CO2 | Borkowski et al. (2012) |
| Wastewater use | Sturm & Lamer (2011), Beal et al. (2012), Dassey et al. (2014) |
| Rate of sparging of CO2 | Pegallapati et al. (2013) |
| Source of water and delivery (drilled wells or pipeline) | Beal et al. (2015), Brentner et al. (2011) |
| Cultivation | |
| Areal growth rate, including improvement by species selection, genetic modification or improving growth conditions | Sills et al. (2013), Slade & Bauen (2013), Jonker & Faaij (2013), Dassey et al. (2014) |
| Algal strain–lipid composition and properties, such as ability to settle | Beal et al. (2015), Slade & Bauen (2013), Mata et al. (2014), Ponnusamy et al. (2014), Dassey et al. (2013) |
| Temperature control system | Béchet et al. (2014) |
| Use of artificial lighting at night (or not) | Gebreslassie et al. (2013) |
| Storage of flue gas (or not) | Gebreslassie et al. (2013) |
| Recirculation of water | Richardson & Johnson (2015) |
| Processing | |
| Process boundary for analysis | Slade & Bauen (2013) |
| Preharvesting with settling ponds | Jonker & Faaij (2013) |
| Harvesting (e.g., filtration, flocculation, flocculant choice, centrifugation) | Brentner et al. (2011), Mata et al. (2014), Beal et al. (2015), Ponnusamy et al. (2014), Slade & Bauen (2013), Weschler et al. (2014) |
| Dewatering, drying (including source of heat) | Brentner et al. (2011), Mata et al. (2014), Beal et al. (2015), Razon & Tan (2011), Weschler et al. (2014), Borkowski et al. (2012), Lardon et al. (2009), Slade & Bauen (2013), Rogers et al. (2014) |
| Extraction method (linked to amount of drying required, extraction efficiency, heat required) | Slade & Bauen (2013), Ponnusamy et al. (2014), Borkowski (2014), Lardon et al. (2009), Richardson & Johnson (2015), Sills et al. (2013), Weschler et al. (2014), Beal et al. (2015) |
| Conversion method (HTL, lipid extraction, pyrolysis, direct secretion) | Ponnusamy et al. (2014), Beal et al. (2015), Bennion et al. (2015), Luo et al. (2010) |
| HTL reaction conditions (e.g., temperatures, reaction times) | Orfield et al. (2014b) |
| Heat recapture and efficiency (HTL, pyrolysis) | Liu et al. (2013), Bennion et al. (2015), |
| Integration of HTL and catalytic hydrothermal gasification | Jones et al. (2015) |
| Scale of HTL or pyrolysis process | Bennion et al. (2015) |
| Oil yield | Orfield et al. (2014b) |
| Use of waste algal biomass after lipid extraction (e.g., anaerobic digestion,  landfilling, nutrient recycling) | Brentner et al. (2011), Dassey et al. (2014) |
| Other energy credits | |
| Quantity and type of coproducts, if included in system boundary | Beal et al. (2015), Jonker & Faaij (2013), Sills et al. (2013), Ponnusamy et al. (2014), Soratana et al. (2014) |
| Wastewater treatment credits (and aeration energy offsets) | Orfield et al. (2014a) |
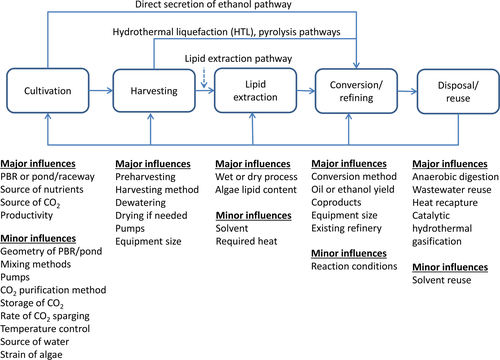
Infrastructure construction for algal biofuel systems is energy intensive (Lardon et al., 2009). Raceway ponds use less energy than flat-plate PBRs (Weschler et al., 2014). Temperature control of PBRs can require a large amount of energy (Béchet et al., 2014). Energy requirements for cultivation are dominated by pumping and mixing water and sparging (bubbling in) CO2 (NRC, 2012; Pegallapati et al., 2013). Power consumption increases with mixing speed by about a cube power factor (Benemann & Oswald, 1996).
Synthetic fertilizers comprise a large fraction of energy use in algae cultivation (Clarens et al., 2010). Wastewater use reduces energy requirements, and an energy credit for nutrient removal may be implemented in energy analyses (Sturm & Lamer, 2011). In one study, combining wastewater treatment with algal biofuel production for the two systems, each with EROI less than one, led to an EROI above one (Beal et al., 2012). Energy requirements associated with CO2 supply can be high (NRC, 2012; Jonker & Faaij, 2013), and fossil EROI is sensitive to the CO2 source (Borkowski et al., 2012). If flue gas is used, the CO2 content determines energy requirements of potential separation and transport (Brentner et al., 2011).
Harvesting and dewatering are energy-intensive processes with many options (Mata et al., 2014) that affect EROI (Beal et al., 2015) (Table 4). The pump power consumed for algae harvest is an important determinant of energy consumption (Jonker & Faaij, 2013). Thermal drying processes have a high energy requirement (Razon & Tan, 2011; Sills et al., 2013; Weschler et al., 2014), and fossil EROI is sensitive to these processes (Borkowski et al., 2012).
The most energy-intensive step of algal biodiesel production may be lipid extraction; therefore, energy requirements are sensitive to the type of extraction process (Ponnusamy et al., 2014) (Fig. 1). In example processes, 70–90% of the energy consumption was used for lipid extraction, depending on whether a wet or dry process was involved (Lardon et al., 2009). Wet extraction is a strategy for reducing energy consumption (Lardon et al., 2009). In one study, fossil EROI was linearly dependent on algae lipid content (Mata et al., 2014).
Hydrothermal liquefaction typically has a higher EROI than lipid extraction and transesterification (Beal et al. 2015). In pilot plant measurements, the EROI of HTL processes was around 1.0, but with efficiencies of scale the value could increase to 2.5–3.0 (Liu et al., 2013). Beal et al. (2015) simulated an HTL system with an EROI above eight. Fossil EROI depends on HTL reaction conditions and whether catalytic hydrothermal gasification is used to recover thermal and electrical energy from aqueous phase products (Orfield et al., 2014b).
Energy return on investment is highly sensitive to the fate of residual algal biomass (landfilling vs. anaerobic digestion and biogas production) (Lardon et al., 2009; Brentner et al., 2011; Sills et al., 2013; Dassey et al., 2014). Razon & Tan (2011) estimated a high energy usage for sewage treatment for the biogas effluent. With respect to energy balance, the use of residual biomass in anaerobic digestion may be superior to the production of animal feed as a coproduct (Sills et al., 2013).
Users of fossil EROI should document all energy inputs and outputs and use consistent assumptions in comparisons. How the EROI is calculated can determine whether energy performance is viewed as capable of supporting a sustainable society (Hall et al., 2009) or as ‘marginal to fair’ (Zhang & Colosi, 2013).
Indicators of social acceptability
Social acceptability indicators quantify judgments. Judgments about algal biofuel systems relate to siting, operations, resource use, and fuel use and can be made by any stakeholder group (e.g., local residents, industry leaders, or citizens). Wolfe et al. (2003) defined acceptability as ‘a feasible set of conditions under which the technology would be ‘on the table’ for serious public discussion’. In the algal biofuel context, technologies are already ‘on the table’, and the questions are which technologies and types of facilities have a future.
Social acceptability indicators proposed by Dale et al. (2013a) for bioenergy sustainability include public opinion (percent favorable opinion), transparency (percent of indicators for which timely, relevant performance data are reported), effective stakeholder participation (percent of documented responses addressing stakeholder concerns and suggestions), and risk of catastrophe (annual probability of a catastrophic event). Below, we focus most on factors that affect public opinion. While effective stakeholder participation cannot be projected, we generally address that indicator as well as transparency and risk of catastrophe.
Public opinion could include potential for new employment opportunities, esthetics and odor, recent media reports about algae, perceptions of genetically modified organisms (GMOs) (if used), perceptions of renewable fuels, and perceptions regarding sustainability. Characteristics of algal biofuel production that could affect public opinion are listed in Table 5.
| Property of algal biofuel production | Sustainability indicator category potentially affected that in turn affects public opinion |
|---|---|
| Renewable fuel development | Energy security↑ |
| Need for research and development | Employment ↑ |
| Potential for automated processes | Employment ↓ |
| Probable use of nonagricultural land | Food security ↑ |
| Potential use of CO2 from utilities or other industries |
Profitability↑ Greenhouse gas emissions ↓ |
| Possible toxins as occupational hazards or perceived hazards | Workdays lost to injury ↑ |
| Possible odorous emissions from cultivation systems | Air quality ↓ |
| Possible breaches from natural disasters | Risk of catastrophe ↑ |
| Expected use and possible release of GMO | Biodiversity ↕ |
| Blooms, a property of some algae | Water quality ↓ |
| Large volumes of freshwater used by some cultivation systems | Water quantity ↓ |
| Possible use of wastewater |
Water quality ↑ Profitability ↑ |
Little information has been published on public opinions of algal biofuels, specifically. Most existing studies of stakeholder perceptions relate to barriers and opportunities that algal fuels provide, rather than concerns about environmental or social effects (Oltra, 2011). An exception is a dialogue among northwestern European algal biofuel stakeholders (industry, nongovernmental organizations, and government institutions) regarding benefits and risks from algal biomass production. Participants in a workshop recommended that nonarable lands should be used for cultivation, GMOs should not be used because of difficulty preventing leakage, and more attention should be devoted to water requirements (Rӧsch et al., 2014).
One consumer trial addressed judgments about fuel. In four cities in California, sustainability factors apparently drove the preferred purchase of a 20% algae-derived biodiesel (Danko, 2013). Sales volume increased 35% at fuel stations offering the algae-derived fuel Ninety-two percent of those surveyed noted that environmental benefits made them likelier to purchase algae-derived fuel, compared to other fuels without those benefits (Propel Fuels, 2013). Forty percent of consumers indicated they would be willing to pay a premium for algae-derived fuels (Danko, 2013), which indicates a high positive public opinion.
Sustainability factors affecting public opinion of bioenergy are pertinent to algal biofuels. Attributes affecting public opinion about Eucalyptus, another bioenergy feedstock, are potential invasiveness and high water use (Dale et al., 2013b). Water scarcity might lead to a poor public opinion of water-intensive bioenergy in locations where current consumptive water use is already close to the available amount (Chaudhry & Barbier, 2013). Potential effects on food prices and deforestation, as well as perceptions of risk of climate change, affected Canadians’ support for advanced biofuel policies (Dragojlovic & Einsiedel, 2015), but food and deforestation issues may be less important determinants of public opinion for algal biofuels. Economic development was a significant factor contributing to the level of community support for a new forest-based biorefinery in Maine, USA (Marciano et al., 2014).
Also important to social acceptability is the extent to which a company's sustainability goals are implemented in facility operations, products, and general strategies (Nair & Paulose, 2014). These goals include safety of operation (Perlaviciute & Steg, 2014) and, therefore, workdays lost to injury (Table 5). Moreover, trust affects social acceptability of energy technologies (Perlaviciute & Steg, 2014).
Perception of a fuel as renewable affects public opinion. The renewable feedstock was an influential variable in determining the level of community support for the forest-based biorefinery in Maine, USA (Marciano et al., 2014). However, in one cross-national study, almost half of respondents did not recognize biomass as a renewable energy source (Devine-Wright, 2003). People evaluate renewable fuels as having lower adverse environmental impacts than fossil fuels, although the environmental effects of concern are not always clear (Perlaviciute & Steg, 2014). Perceived energy independence associated with renewable resources leads to a higher public opinion of them (Culley et al., 2011). Public opinion may be tied to specific biofuels; opinions about corn-based ethanol could be different from those about ethanol (or renewable diesel) derived from algae (Cacciatore et al., 2012).
Place attachment can affect public opinion, depending on whether the project is thought to pose a local threat or opportunity (Devine-Wright, 2007). However, place attachment has been examined in very few renewable energy contexts (e.g., hydropower in Norway, Vorkinn & Riese, 2001).
The use of GMOs in algal biofuel production is expected to grow, especially in PBRs. We are unaware of public opinion surveys that focus on acceptability GMOs for producing algal biofuels. However, the Sustainable Biodiesel Alliance asserts that only biodiesel that is derived from non-GMO feedstock is sustainable (SBA, 2014). Concerns about GMOs in other contexts have been expressed in surveys and the popular press, and concerns about microorganisms (in contrast to plants) relate more to potential environmental effects than to health or ethical issues (Hagedorn & Allender-Hagedorn, 1997). Henley et al. (2013) discussed the probability, albeit low, of released algae altering natural ecosystems. In the USA, anecdotal evidence reveals little unfavorable public opinion regarding uses of GMOs in beneficial products (Glass, 2015). Uncertainty regarding effects of GMOs on the environment and human health is a large concern in developing countries that could affect public opinion of algal biofuels (Adenle et al., 2013). Trust is also important for social acceptability of gene technology (Siegrist, 2000). Some industry and other stakeholders believe that US regulatory review of open-pond uses of GMO algae could impede plans for commercialization, given concerns in the 1980s over outdoor testing of GMOs and current debates over transgenic food crop plants (Glass, 2015). Mesocosm experiments and monitoring of algal cultivation systems could lead to greater understanding of ecological and health risk and to greater public acceptance of the use of GMOs (Henley et al., 2013).
Public perceptions of algae in general, especially in western nations, are sometimes negative. The mention of algae can conjure images of green tides or toxic algal blooms (Jacquin et al., 2014). This perspective is especially pertinent if adverse effects of algae have recently occurred in a region and been covered and potentially exaggerated by the media, for example, Lake Erie ‘being choked each summer by thick mats of algae, much of it poisonous’ (Wines, 2014). Even claims unsupported by science have the potential to affect social acceptability of a nascent industry.
Another indicator of social acceptability is transparency in how indicators are reported. In one study, European algal biofuel experts felt that life cycle analyses lacked transparency regarding data sources (Slade & Bauen, 2013). The study that the US Department of Energy funded to harmonize engineering assumptions for techno-economic modeling of algal biofuels (Davis et al., 2012) was intended, in part, to make the basis for cost estimates be more transparent. If transparency is lacking among scientists and engineers, then it is likely to be lacking among other stakeholders as well.
Effective stakeholder participation is equivalent to what Perlaviciute & Steg (2014) term ‘perceived procedural fairness’. For example, in an effort to make its risk assessment process for GMO algae ‘open and transparent’, the US Environmental Protection Agency is developing a ‘Considerations for Biotechnology Algae’ document (with stakeholder input via a workshop) to clearly describe scientific and technological issues that drive the agency's evaluation of risks from production and use of GMO algae (USEPA 2015b).
Risk of catastrophe can be estimated for well-defined scenarios. For bioenergy sustainability purposes, a catastrophic event has been defined as an event or accident that has more than 10 human fatalities, affects an area greater than 1000 ha, or leads to extinction or extirpation of a species (Dale et al., 2013a,b). Risks to infrastructure, for example, can be estimated based on properties of the materials and frequency of extreme weather events, such as hurricanes. If saline water were extracted, nearby freshwater aquifers could become contaminated (McGraw, 2009), which is why liners are generally required for saline ponds. Where data on risk are not available, indicators might be selected that are related to potential exposure; for example, proximity of cultivation systems to streams or groundwater should relate to risks to aquatic organisms or to humans who drink the water.
The context of a sustainability assessment, including costs and benefits to particular people, is important for assessing social acceptability of energy technologies (Jensen & Andersen, 2013; Perlaviciute & Steg, 2014). While measures may be specific to particular stakeholder groups, to be viable, emerging technologies are generally accepted by producers, markets, the public, and policymakers (Peck et al., 2009). Psychological factors affect how costs and benefits influence acceptability (Perlaviciute & Steg, 2014). Because some technologies will be rejected in some locations and deployed in others (Wolfe et al., 2003), social acceptability indicators are spatially explicit. Moreover, social acceptability of large-scale development of biofuels is hypothetical (Jensen & Andersen, 2013), and social acceptability may change over time (Wolfe et al., 2003). Therefore, these indicators are not as easily modeled as profitability or EROI, which are less sensitive to place and time.
Discussion and conclusions
Sixteen socioeconomic indicators of progress toward social and economic aspects of sustainability of bioenergy were examined in the context of algal biofuels and the industry's early stage of commercial development. The indicators represent categories of social well-being, energy security, profitability, external trade, resource conservation, and social acceptability (Dale et al., 2013a,b). To date, modeled and measured indicators have been focused on sustainable system design.
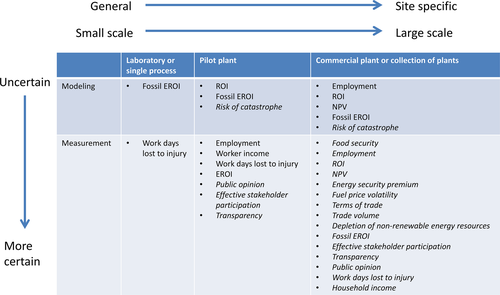
Algal biofuel technologies are still in need of advancement (Lardon et al., 2009). Few indicators have been estimated for pilot-scale systems or projected for commercialization scenarios (Fig. 2). Those that have been modeled include profitability indicators ROI and NPV and the resource conservation indicator fossil EROI. Measured indicators at the laboratory or pilot plant scale are not usually published, but likely include employment, worker income, and workdays lost to injury (Fig. 2).
Some of the estimated indicators have clear target values, and many of the studies assess how close alternative system designs are to those targets and how targets may be met. For example, profits should be positive. Fossil EROI should exceed one at a minimum, so that the process is thermodynamically favorable and then needs to be improving and approaching EROIs of other transportation fuels, that is, a value of around three (NRC 2012). The change in food price volatility should be zero. Public opinion for any algal biofuel scenario should be above 50% and increasing. Facilities, municipalities, and states would have goals for employment and income for incoming industries.
Prior to commercialization, some indicators represent the drivers for the development of an algal biofuel industry, while other indicators and indicator categories are less useful to measure (Fig. 2). Initial stakeholder goals for energy security, resource conservation, and employment may simply be improvement of the indicators through development of a new industry. Energy security indicators (energy security premium and fuel price volatility) as well as terms of trade, trade volume, and stakeholder participation do not have clear target values and cannot be projected into the future with much accuracy. Similarly, workdays lost to injury cannot be projected prior to commercialization (Fig. 2). In early stages of commercialization, employment may be more of an indicator of public opinion than an indicator of projected social well-being.
The attention given to estimating profitability and EROI for algal biofuels suggests their importance in early technology development and to the industry, generally, as well as the relative simplicity of modeling them, compared to social indicators. Public opinion may be just as important for the fate of a facility, but that cannot be estimated without a concurrent survey. Social acceptability and social well-being indicators are sensitive to place. As commercialization proceeds, indicator measurements become much more site-specific and reflect larger scales (Fig. 2). Estimating risk of catastrophic breaches of cultivation infrastructure requires good understanding of the supply chain, as well as the local weather.
Inconsistent assumptions are used for modeling indicators; standard methods and documentation are needed for comparisons. The preliminary examination of 12 estimates of the cost of 1 gallon of triacylglycerides in 2008 revealed a 50-fold range (Sun et al., 2011), because assumptions were not the same and boundaries were not normalized. These estimates would be carried to ROI and NPV calculations. The wide range of EROI values in life cycle analyses of algal biofuels reflect differences in model scope, boundaries, and assumptions, as well as coproduct allocation methods (Sills et al., 2013). Zhang & Colosi (2013) noted that the meaning of EROI determines whether performance of a system is fair or good. Sun et al. (2011) advocated a common framework for comparative cost analysis with agreed-upon definitions, metrics, assumptions, and cost categories.
Social and economic sustainability indicators may have different levels of importance in different contexts. Profitability may be the most important indicator for industry; whereas employment is more important for the general public, and social acceptability is the hallmark for nongovernmental organizations. Gallagher (2011) suggested that IRR would be a better economic indicator than NPV and ROI for longer periods than a 20-year period of returns. Long-term economic sustainability, that is, decades or generations, is not equivalent to profitability measured in the near term.
General indicators may serve as a ‘reservoir’ from which to select indicators for specific contexts and goals (Dale et al., 2015). Contexts of sustainability assessments include the purpose, components of the biofuel supply chain, policy conditions, stakeholder values, place and spatial scale, temporal aspects, baselines, and reference scenarios (Efroymson et al., 2013). With this study, we add the state of development with respect to commercialization (Fig. 2) to the list of contextual factors for assessing bioenergy sustainability.
The early stage of commercial development (i.e., technology development and scaling up) is an important aspect of the context of algal biofuels. Ribeiro et al. (2015) presented estimates of the timing and extent of commercialization, with many experts believing that algae will represent the source of 1–5% of global fuel consumption from 2021 to 2030 and potentially 10–25% of fuel consumption from 2031 to 2050. Profitability may be very different for the first few plants than after many plants are built; the latter assumption is termed ‘nth plant economics’ (Davis et al., 2015). Risk financing for pioneer projects could require higher interest rates than future projects using proven technologies. EROI would also be different for pilot and commercial-scale plants. In one example, the net energy return for two thermochemical processing pathways, HTL and pyrolysis, increased by factors of 2.4 and 2.9, respectively, between pilot-scale and commercial-scale simulations (Bennion et al., 2015). Energy savings at scale-up is uncertain (Rogers et al., 2014), but some cultivation designs are more easily scaled up than others (Benemann & Oswald (1996), with indicators more easy to extrapolate to commercialization in the former case.
Many social and economic sustainability indicators are closely related, and correlations and conflicts are being examined. For example, public opinion and EROI influence profitability. Trade-offs among socioeconomic and environmental sustainability indicators may be necessary. PBRs optimized for biomass productivity do not necessarily have good energy efficiency (Pegallapati et al., 2013). Winter shutdowns reduce GHG emissions but also increase costs (Davis et al., 2014). Centrifugation as a harvesting method minimizes EROI and increases cost (Ponnusamy et al., 2014). Trade-offs between production potential costs are beginning to be evaluated in facility design (Coleman et al., 2014), and optimizations for combined economic and environmental objectives (such as GHG emissions reductions) are being conducted (Gebreslassie et al., 2013).
Together with environmental sustainability indicators (Efroymson & Dale, 2015), social and economic indicators should contribute to the evaluation of sustainability of algal biofuels in particular contexts, including process design, site sustainability assessments, energy technology comparisons, regulatory assessments, and sustainability certification. The potential of algae to contribute to progress toward sustainable solutions for future liquid biofuel needs can be measured.
Acknowledgements
This research was supported by the US Department of Energy (DOE) under the Bioenergy Technologies Office (BETO). Oak Ridge National Laboratory is managed by UT-Battelle, LLC, for DOE under contract DE-AC05-00OR22725. We thank Kristen Johnson and Daniel Fishman of BETO for insights and project sponsorship. We thank Amy Wolfe and two anonymous reviewers for helpful comments on an earlier draft. We appreciate discussions with Andre Coleman, Mark Wigmosta, Shahab Sokhansanj, Susan Schoenung, Val Smith, Melanie Mayes, Terry Mathews, and Ryan Davis.




