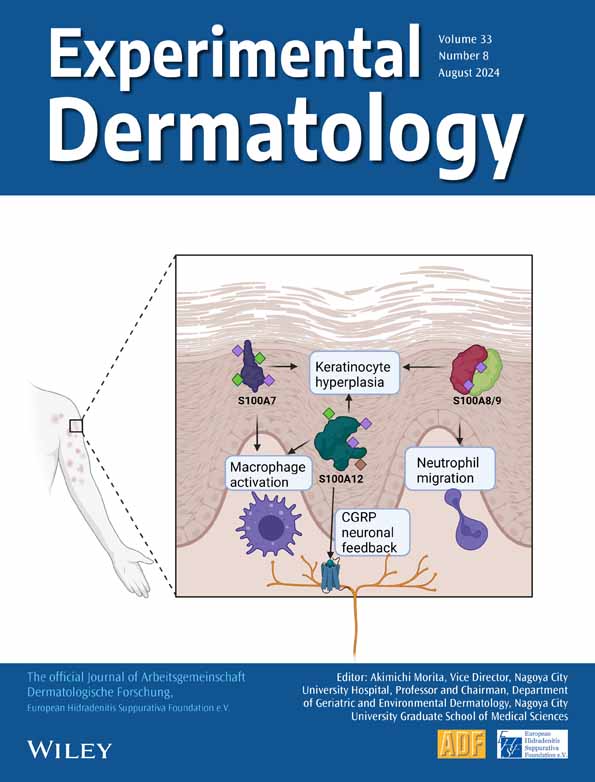
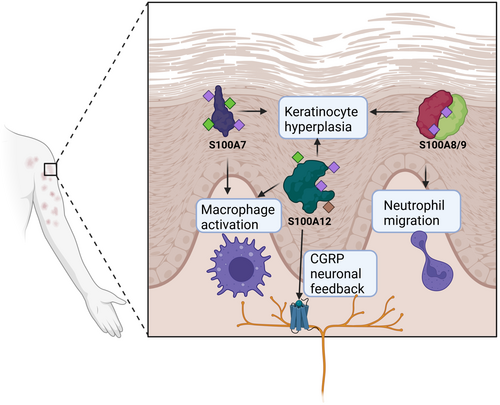
An overview of S100 proteins and their functions in skin homeostasis, interface dermatitis conditions and other skin pathologies
Abstract
S100 proteins comprise a family of structurally related proteins that are calcium-sensitive. S100 proteins have been found to play various roles in regulation of cell apoptosis, cell proliferation and differentiation, cell migration and invasion, energy metabolism, calcium homeostasis, protein phosphorylation, anti-microbial activity and inflammation in a variety of cell types. While the specific function of many S100 proteins remains unknown, some of the S100 proteins serve as disease biomarkers as well as possible therapeutic targets in skin diseases. Interface dermatitis (ID) is a histopathological term that covers many different skin conditions including cutaneous lupus erythematosus, lichen planus, and dermatomyositis. These pathologies share similar histological features, which include basal cell vacuolization and lymphocytic infiltration at the dermal-epidermal junction. In this review, we summarize how the S100 protein family contributes to both homeostatic and inflammatory processes in the skin. We also highlight the role of S100 proteins in neuronal signalling, describing how this might contribute to neuroimmune interactions in ID and other skin pathologies. Last, we discuss what is known about the S100 family proteins as both biomarkers and potential treatment targets in specific pathologies.
1 INTRODUCTION
The S100 proteins comprise a broad family of at least 30 known low-molecular weight proteins characterized by two calcium-binding EF-hand motifs.1 S100 isoforms are highly structurally homologous, but functional discrepancies may be attributed to variations in the C-terminal extension and central hinge region.1 These proteins are known to be expressed only in vertebrates and exert functions and regulatory effects intracellularly, extracellularly, or both.2 There are 5 primary functional groupings of the calcium-dependent roles of the S100 proteins: regulation of phosphorylation by protein kinases, modulation of enzymatic activity, maintenance of cell shape and motility, influence on signal-transduction pathways, and promotion of calcium homeostasis.3 S100 proteins have also been found to play various roles in regulation of cell apoptosis, cell proliferation and differentiation, cell migration and invasion, energy metabolism, calcium homeostasis, protein phosphorylation, and inflammation in a variety of cell types.2 Dysregulation of S100 proteins is a common occurrence in a variety of disorders, including many human cancers, neurodegenerative diseases, and autoimmune disorders.1, 4, 5
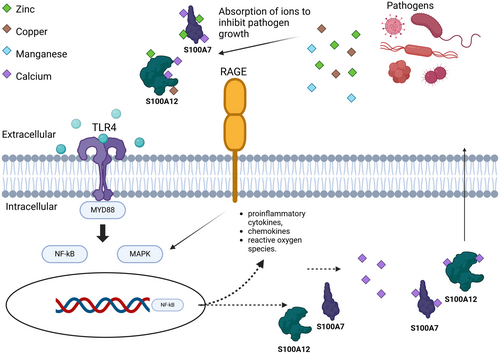
S100 proteins play important roles in both innate and adaptive immunity. After being released into the extracellular space, S100 proteins regulate immune homeostasis as well as post-traumatic injury and inflammation through interactions with RAGE and TLR4.6 Certain S100 proteins (S100A8, S100A9, and S100A12) are known for their chemoattractant properties. Other S100 proteins act as antimicrobial agents by chelating zinc and magnesium, which are metals that are essential for the growth of bacteria and fungi.7 The S100 protein family is highly expressed in the skin and is implicated in antimicrobial defence as well as many inflammatory disease processes. While the specific function of many S100 proteins in skin remains unknown, some of the S100 proteins serve as disease biomarkers as well as possible therapeutic targets, which we will discuss below.8
2 S100 PROTEINS AND THEIR HOMEOSTATIC FUNCTIONS IN THE SKIN
Multiple members of the S100 family are expressed on the epidermal differentiation complex (EDC) of chromosome 1q21, a region of particular interest for encoding many genes that are expressed in epidermal cell types including keratinocytes.3 At least eleven S100 proteins that have been cloned to date, including S100A2-4, S100A6-12, and S100A15, are expressed in the human epidermis or in cultured keratinocytes.3 Furthermore, S100B is expressed in Langerhans cells and melanocytes, and S100P is expressed in Meissner's corpuscles.3 S100 proteins serve several functions. For example, S100A7, called psoriasin, promotes keratin production and aids in maintaining the integrity of the epidermal barrier.9 Together, these features highlight the importance of S100 genes in the skin during homeostasis and disease. In this section, we will describe the various functions of S100 family members and how they relate to skin homeostasis.
2.1 S100 proteins as antimicrobial agents
The antimicrobial properties of the S100 proteins are attributed to the chelation of Zn2+ and Mn2+, which reduces the essential transition metals needed for growth of bacteria and fungi.7 For example, keratinocytes that express S100A8/A9 (calprotectin) bind three-fold fewer bacteria and have 10 fold fewer microorganism invasions.7 If a pathogen manages to gain access in a calprotectin-expressing cell, there are intracellular mechanisms in place to combat infection: intracellular calprotectin can inhibit the cytoplasmic growth of the bacteria.7 Many different fungi and bacteria have mutated and evolved to override calprotectins' mechanism of action via increased expression of metal transporters.10 These metal transporters enable the bacteria to grow and handle the stress of a metal-starved environment due to calprotectin.11
S100A8 and S100A9 are mainly released in response to infection-induced inflammation or tissue damage due to these antibacterial properties.12 Expression is normally weak or absent in healthy skin. The S100A8/S100A9 heterodimer, calprotectin, modulates the release of cytokines, reactive oxygen species, and nitric oxide.13 Calprotectin has two binding sites: one site binds both zinc and manganese and the second binding site binds only zinc.14 Multiple zinc binding sites allow for an increased binding affinity to calcium.12 A tetramer may form in a calcium-independent fashion.15 Tetramer formation is important for local restriction of sterile inflammation by hiding the TLR4/MD2 binding site on the dimer form.16 S100A8/A9 may also promote keratinocyte proliferation and regulate the activation of dermal fibroblasts in dehydrated human skin, consistent with evidence that S100A8/A9 promotes wound healing.17
S100A12 also plays a significant role in skin homeostasis. S100A12 differs from calprotectin because it utilizes zinc and copper at its binding site.18 S100A8/S100A9 and S100A12 are constitutively produced in neutrophils, monocytes and early macrophages.7 Expression is induced in epidermal keratinocytes, fibroblasts and gastrointestinal epithelial cells in response to inflammation.7
The skin microbiome is heavily regulated by antimicrobial proteins that are meant to protect the host from pathogens, but antimicrobial overexpression has been shown to also harm our skin.19 For example, S100A7/Psoriasin displays antimicrobial properties and is a key protector against E. coli by disrupting their cell membranes, but it is also associated with keratinocyte hyperplasia and poor prognosis in psoriasis (reviewed in20). Its production is induced in wounded skin through the activation of the epidermal growth factor receptor (EGFR), and S100A7 is well-known to be over-expressed in inflammatory epidermal lesions, particularly in psoriasis, atopic dermatitis, mycosis fungoides, Darier's disease, inflammatory lichen sclerosus, and cutaneous lupus erythematosus.3, 21 S100A7 overexpression in a keratinocyte cell line led to the upregulation of Keratin 16, which is physiologically upregulated in response to barrier breach to induce inflammation, as well as IL1B.1 These findings support the idea that the concentration level of S100A7 can function as a modulator of keratinocyte differentiation and/or mediate inflammation. These functions are summarized in Figure 1.
2.2 S100 proteins as chemoattractants and immuno-activating agents
The immune response to vulvovaginal Candida albicans is partially mediated by polymorphonuclear leukocytes (PMNs), which are recruited by S100 ‘alarmins’ S100A8 and S100A9 produced by vaginal epithelial cells in response to infection.22 S100A8 is upregulated by lipopolysaccharides (LPS) that induce TNF-α and IFN-γ.23 S100A9 (formerly known as migration inhibitory factor-related protein 14 (MRP-14)) activates beta-2 integrins on neutrophils.24 While S100 alarmins are not necessary for PMN migration to the vagina, they do cause significant induction of migration.25 In addition to neutrophil recruitment, S100A8 (formerly known as CP-10) is a chemoattractant for monocytes that induces migration in a Gai, Ca2+-independent manner.26 These alarmins are also upregulated in other inflammatory diseases such as inflammatory bowel disease, rheumatoid arthritis, and some cancers.27
S100 proteins not only act as chemoattractants, but also function as macrophage activators and modulators of cell proliferation and function.28 S100 proteins primarily serve to activate macrophages through stimulation of TLR4 and RAGE receptors.6 Both receptors trigger NF-kB-mediated release of inflammatory cytokines.6 In addition to pattern recognition receptors, oxidative stress induces S100A8 expression in macrophages in an IL-10-dependent manner.29 The induction of S100A8, S100A9, and S100A12 heterodimers in macrophages is induced by proinflammatory factors, such as LPS and Th2 cytokines.30 S100A8 and S100A9 mediate TNF-α's inhibitory effect on myeloid-derived suppressor cell (MDSC) differentiation, which is important given MDSC's role in tumour growth.31 In contrast, macrophage-derived S100A4 enhances protumor macrophage polarization through peroxisome proliferator-activated receptor-gamma (PPAR-gamma).32 Similarly, S100A10 mediates protumor macrophage migration towards tumours, evidenced by reduced tumour growth and fewer intratumoral macrophages in S100A10-null mice.33 This intricate interplay underscores the significant role of S100 proteins in shaping macrophage behaviour and immune responses. Further research is needed to understand how this contributes to ID.
2.3 S100 proteins as calcium binding/signalling proteins
S100 proteins are the largest subgroup of EF-hand Ca2+-binding proteins and regulate a variety of intracellular processes, usually in a Ca2+ and sometimes Zn2+ and Cu2+-dependent manner.34-36 Calcium can also induce expression of S100 proteins in keratinocytes.37 S100 proteins are typically symmetric dimers, in which both of the subunits contain two Ca2 + -binding domains. Upon Ca2+ binding, a hydrophobic cleft required for target binding is revealed by conformational change, with target binding results in a 5–300 fold increase in Ca2+-binding affinity relative to its weak affinity to Ca2+ in the absence of a target (Kd ~ 10–50).4 Sometimes, S100 proteins are also involved in Ca2+-independent signalling, although these interactions are often less well understood, as in the case of dopamine D2 GPCR activation by S100B and serotonin receptor activation by S100A10.34
An important role of S100 proteins is in signalling regulation by influencing the phosphorylation of kinases in a calcium-dependent manner. Upregulated S100B targets and activates Ndr, a nuclear serine/threonine protein kinase that is known to be important for cell division and morphology regulation, and one study suggests that hyperactivation of Ndr through Ca2+/S100B regulatory overactivation could be implicated in the development of melanoma and may explain an effect of high S100B levels seen in a majority of melanomas. S100A4, which is closely related to S100B, could thus also be implicated.38 S100B has also previously been shown to induce inflammation in neural cells by binding to RAGE in a strictly calcium dependent manner.39
3 S100 PROTEINS IN NEUROIMMUNE INTERACTIONS
Mounting evidence supports the involvement of neuroimmune interactions as drivers of skin diseases. For example, neurogenic itch can drive atopic dermatitis (reviewed in40), alloknesis41 and chronic pruritus.42 Neuroimmune responses can also govern immunity to C. albicans.43, 44 S100 proteins are pivotal players in the intricate communication between the immune and nervous systems. They are expressed not only in immune cells, but also in diverse components of the nervous system, including central and peripheral neurons as well as glial cells. Notably, S100B levels are known to increase in response to stress,1 and it is known that stress can trigger skin disease flares.45 Studies have demonstrated the neuroprotective effects, apoptosis-inducing capabilities, and other modulatory functions of S100 proteins in the bidirectional communication between the immune and nervous systems. For example, S100B promotes neurite extension, modulating long-term potentiation, and protecting neuron survival. Additionally, S100B has been associated with counteracting neurotoxic insults and increasing scavenger activity of reactive oxygen species (ROS).46 Here, we will describe documented roles of S100 proteins in sensory neuron function and how expression in the skin may contribute to neuroimmune feedback during ID.
3.1 An introduction to skin neuroimmunology
The skin originates from the ectoderm, the same embryonic layer as the nervous system. The skin's innervation is primarily sensory, with various nerve fibres that are categorized based on their size, myelination, and conduction speed. Neuroimmune cell units (NICU) are the nerve fibres in the skin and immune cells that work together to maintain skin homeostasis.47 The skin has specific mechanisms that aid in neurogenic inflammation. The skin cells possess functional receptors for neuropeptides, allowing them to receive signals from the nervous system.48 These skin cells produce neuropeptides that stimulate nerve fibres, creating a positive bidirectional feedback loop that can enhance the inflammatory response.49
3.2 S100A12 encodes CGRP, which is associated with skin diseases like atopic dermatitis
Neurogenic inflammation is facilitated by the release of neuropeptides such as calcitonin gene-related peptide (CGRP) and substance P (SP) from nociceptors. CGRP is a peptide product that can be encoded by multiple genes, including S100A12.50 Neuropeptides directly impact vascular endothelial and smooth muscle cells, with CGRP causing vasodilation and SP increasing capillary permeability, leading to plasma extravasation and edema.51, 52 CGRP in the epidermis can stimulate the proliferation of keratinocytes and the expression of cytokines. Research using a keratinocyte cell line identified receptors for SP and CGRP on keratinocytes, revealing positive autocrine stimulatory effects in keratinocytes including neuropeptide signalling, cell proliferation, and the expression of various cytokines: when the keratinocytes are stimulated with SP or CGRP, the expression of neuropeptide receptors in keratinocytes are enhanced, which in turn leads to the enhanced secretion of SP and CGRP.53
Atopic dermatitis is a chronic inflammatory skin condition that presents with dry skin and severe itching. It frequently presents with asthma and allergic rhinitis as a triad. Studies have shown that there is an increased density of CGRP-positive nerves in the affected skin of individuals with atopic dermatitis (AD), along with heightened contacts between mast cells and nerve fibres, in comparison to unaffected skin.54 CGRP has also been suggested to play a role in mood disorders such as anxiety and depression.55 Studies have found that the presence of CGRP-positive nerve-like fibres in lesional skin is linked to higher scores of depression and anxiety in AD.56
While the direct roles of S100 family proteins in neuroimmune interactions during ID have not been fully studied, we postulate that these will be important for conditions in which pain, itch or other sensations are prominent. For example, pain is a prominent feature in Hidradenitis suppurativa (HS), and patients exhibit altered skin sensory perception.57 Cutaneous lupus is highly photosensitive,58 though neuroimmune mechanisms underpinning this response remain unprobed.
4 S100 PROTEINS IN INFLAMMATORY SKIN DISEASES
4.1 Lessons learned from studies of psoriasis
Psoriasis is a chronic, inflammatory disease that currently has no cure. Treatments mainly focus on symptom management and addressing the underlying inflammatory process.59 One characteristic abnormality in psoriasis is the excessive production of antimicrobial peptides and proteins. S100A15 (also known as koebnerisin) is elevated in psoriatic lesions and participates in the disease phenotype, known as ‘koebnerized’ psoriatic skin.60 The Koebner phenomenon refers to the development of skin lesions in areas of skin trauma or injury in individuals with psoriasis.60 Liang et al. found S100 proteins including S100A7, S100A8, S100A9, S100A12, and S100A15 to be upregulated in psoriatic skin.20, 61
Histopathological features of psoriasis include excessive keratinocyte proliferation and infiltration of immune cells. The proliferation of keratinocytes is regulated by the intracellular Ca2+ concentration and the binding of calcium binding proteins such as S100A7, known as psoriasin.37 The term ‘psoriasin’ was first used to describe S100A7 because the protein was first identified as a 11.4 kDa cytoplasmic and secreted protein isolated from skin involved by psoriasis,62 reflecting its strong association with psoriasis and its potential role in the pathophysiology of this disease. The elevation of S100A7 is a potential explanation for the excessive proliferation of keratinocytes found in psoriatic patients.63, 64 S100A7 has been found to be upregulated by Th1 and Th17 cytokines in epidermis of psoriasis,24 further compounding hyperplasia as inflammatory cells are recruited.
S100A8 and S100A9 are strongly implicated in multiple autoimmune disorders, including psoriasis.65 They play a key role in regulating inflammation especially through TLR4. S100A8 and S100A9 are significantly increased in psoriatic keratinocytes and leukocytes, and hence, their expression levels directly correlate with the severity of psoriasis, suggesting their active involvement in the disease's progression.20
Similar to S100A8/A9, S100A12 also exhibits cytokine-like pro-inflammatory activities through the activation of the TLR4 pathway and antimicrobial actions. In untreated psoriasis patients, S100A12 serum levels showed the closest association with the disease activity, and when treated with etanercept, Wilsmann-Theis et al found a corresponding decrease in S100A12 levels for psoriatic patients.61
In conclusion, the diverse role of the S100 protein family in psoriasis pathogenesis underscores the potential of these proteins as future therapeutic targets and exemplifies ways in which S100 proteins can contribute to skin pathologies.
4.2 S100 proteins in interface dermatitis conditions
Interface dermatitis (ID) is characterized by lymphocytic infiltrate at the dermal-epidermal junction that can be complicated by secondary infection by bacteria or fungi as a result of disruption of the skin barrier. S100 proteins can help combat these infections through their antimicrobial properties, but may also drive autoinflammation. Often, ID is driven by T cell infiltration of the basilar epidermis. The most prominent changes in ID are basal keratinocyte vacuolization and lymphocytic infiltration of the epidermis (Figure 2).66 Apoptotic keratinocytes can also be seen in different layers of the epidermis and papillary dermis projections.66 The apoptotic basal cells are also known as civatte bodies, and they are comprised of keratin filaments covered in immunoglobins.67 Melanocytes, which normally reside in the basal layer, die as a result of basal layer damage and release their contents into the upper dermis. This pigment is then taken up by macrophages, resulting in melanophages. Changes in pigmentation can help determine how long the damage has been going on, and is often referred to as pigmentary incontinence or pigment dropout. There are also changes in the dermo-epidermal junction itself due to inflammation, including thickening of the basement membrane or obfuscation, depending on the stage of the process or type of inflammation. Other histopathological features of ID include hyperkeratosis, hypergranulosis and acanthosis.68
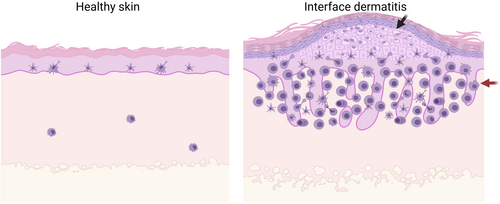
There is an algorithm that pathologists can utilize when analysing ID lesions. There are five categories in this algorithm that can help identify the lesion.69 The first criterion is determining the amount of necrotic keratinocytes, whether there are a few or numerous amounts.70 The second criteria is to determine the level of premature terminal differentiation.70 The third criteria are to consider the prominent immune infiltrate, which can vary from lymphocytic, to eosinophilic or plasma cell infiltrates. The fourth criteria to consider is the type of epidermal hyperplasia, and whether it is hypertrophic, which is seen in lichen planus (LP), or verrucous, which is seen in discoid lupus, or psoriasiform as in psoriasis.70 The fifth criteria is to consider epidermal atrophy.70 These specific epidermal changes help differentiate the different types of ID.
S100 proteins play an important role in mediating innate and acquired immune responses and are thus thought to contribute to inflammation in ID. S100 proteins may induce inflammation and damage by promoting the release of cytokines and chemokines that mediate the attraction of other immune cells.1 S100 proteins also lead to the development of fibrosis, by promoting the production of extracellular matrix proteins. The overproduction of these proteins results in the thickening and lichenifying of the skin, as well as development of scar tissue. Elevation of S100 family proteins are observed in a number disease processes, such as subacute cutaneous lupus erythematosus (SCLE), discoid lupus erythematosus (DLE), LP, dermatomyositis (DM) and drug eruptions. In this section, we will describe specific S100 proteins in different disease entities, highlighting how they might be driving ID (summarized in Figure 3; Table 1).
| S100 proteins/complexes | Associated skin diseases | Role in diseased skin | Selected references |
|---|---|---|---|
| S100A1 | Typically not expressed in the skin, but is expressed in the vascular system | May be involved in vascular-related skin conditions | [71, 72] |
| S100A2 | Basal cell carcinoma, calcifying epithelioma of Malherbe, cylindroma, cutaneous mixed tumour, eccrine poroma, langerhans cell histiocytosis, malignant melanoma, sebaceous adenoma, sebaceous carcinoma, squamous cell carcinoma, steatocystoma, syringocystadenoma papilliferum, syringocystadenoma, trichoepithelioma | Specific role in inflammatory skin disease unknown; tumour suppressor gene significantly down-regulated in stage III and IV malignant melanomas, suggesting role in cutaneous neoplasm development/progression | [73] |
| S100A3 | Pilomatrixoma | S100A3 plays a role in calcium-dependent processes leading to hair shaft formation. Specific role in skin disease unknown. | [74] |
| S100A4 | Dermatomyositis, epidermoid cysts, melanoma | The S100A4 protein is crucial for promoting a protumor phenotype in tumour-associated macrophages (TAMs) by upregulating peroxisome proliferator-activated receptor γ (PPAR-γ), which, in turn, enhances fatty acid oxidation (FAO) and lipid metabolism. | [32, 75] |
| S100A6 (calcyclin) | Melanoma, neurothekeoma, Spitz nevi, squamous cell carcinoma | Specific role in skin disease unknown; present in primary cutaneous melanomas as well as many melanoma metastases; potential diagnostic marker for melanoma and neurothekeoma | [76] |
| S100A7 (psoriasin) | Actinic keratosis, atopic dermatitis, basal cell carcinoma, Bowen's disease, melanoma, psoriasis, squamous cell carcinoma | Promotes keratin production and aids in maintaining the integrity of the epidermal barrier. It also functions as a modulator of keratinocyte differentiation and mediates inflammation, particularly in response to Th17 cytokines | [77] |
| S100A8 (calgranulin A or MRP8) | Chronic cutaneous lupus erythematosus, psoriasis, lichen planus | Activates adjacent keratinocytes to release cytokines and acts as pro-angiogenic and chemotactic factors; can be induced by UVA radiation | [12] |
| S100A9 (calgranulin B MRP14) | Chronic cutaneous lupus erythematosus, psoriasis | Acts as a chemotactic factor for immune cells, inducing pro-inflammatory cytokines and pro-angiogenic mediators in keratinocytes | [12] |
| S100A10 | Melanoma (down-regulated) | May regulate proliferation of melanocytic lesions | [78] |
| S100A11 | Atopic dermatitis (down-regulated), basal cell carcinoma, psoriasis (down-regulated), squamous cell carcinoma | Potentially causes increased cell proliferation by decreasing nuclear concentrations of p21CIP1/WAF1 and p16INK4a; may predispose atopic dermatitis and psoriasis patients to the development of disseminated vaccinia viral skin infections by impairing the ability of keratinocytes to control viral replication | [79] |
| S100A12 (calgranulin C) | Behçet's disease, psoriasis | Secreted by blood or neutrophils in inflamed tissues and interacts with RAGE receptor involved in transducing pro-inflammatory signals | [80] |
| S100A13 | Melanoma | Promotes angiogenesis | [81] |
| S100A14 | Oral squamous cell carcinoma | Tumour-type specific; can function as a tumour suppressor in some cancers while it promotes tumorigenesis in others | [82] |
| S100A15 | Atopic dermatitis, psoriasis | S100A15 (koebnerisin) contributes to the inflammatory processes in psoriasis by stimulating proinflammatory cytokine production in circulating leukocytes (PBMCs), highlighting its role as an ‘alarmin’ in the disease pathology | [83] |
| S100B | Benign peripheral nerve sheath tumours, chondroid syringoma, clear cell sarcoma, dendritic cells in melanocytic matricoma, eccrine spiradenoma, granular cell tumour, indeterminate cell histiocytosis, langerhans cell histiocytosis, malignant peripheral nerve sheath tumours, melanoma, myoepithelioma, plexiform schwannoma, primary cutaneous langerhans cell sarcoma, Rosai-Dorfman disease, sweat gland carcinomas | S100B activates Ndr, which then promotes heightened cell growth and diminishes apoptosis, key features that define cancer cells, including melanoma. This activation pathway underscores how S100B-driven Ndr activity could contribute to the aggressive nature of melanoma by fostering uncontrolled cell division and evading cell death mechanism | [6] |
| S100P | Mycosis fungoides, Sezary syndrome | Specific role in skin disease unknown; plays a role in tumour development, progression, and metastasis | [84] |
4.2.1 Dermatomyositis
Dermatomyositis (DM) is a rare chronic inflammatory disease that affects the skin and muscle. DM presents with characteristic skin findings such as the heliotrope rash, which is a violaceous, or an erythematous rash affecting the upper eyelids with or without periorbital edema. It can also present with gottron papules, which are erythematous macules or patches over dorsal metacarpophalangeal and interphalangeal joints.85 There are various things that can predispose an individual to dermatomyositis, such as genetic factors, immunologic factors, and environmental factors. The genetic factor that is highly associated with dermatomyositis is the HLA-A*68 in White North American and HLA-DRB1*0301 in African Americans.86 There are various environmental factors, such as viruses like Coxsackie B, enterovirus and parvovirus that can trigger dermatomyositis.86
S100A7/8/9 are elevated in the skin of DM patients.87, 88 Of these, S100A8/9 also correlate with the presence of interstitial lung disease (ILD) in DM.88 Interstitial lung disease is a major cause of morbidity and mortality in DM. Serum S100A12 is used to evaluate the clinical severity and prognosis of DM,89 and high levels of S100A12 are positively associated with inflammatory markers, such as C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and ferritin, as well as greater structural changes in the lung in both stable and acute exacerbation (AE) dermatomyositis-associated interstitial lung disease (DM-ILD).90 Increased levels of circulating S100A4 is a biomarker of muscle involvement, and these levels are linked to various indicators of disease activity, particularly involving extramuscular components.75 While S100A4 has been associated with cancer, and DM patients have an elevated cancer risk,91 there is no established connection between the levels of S100A4 and the occurrence of cancer-associated myositis.75, 92 Cerezo et al. found that in all myositis patients, elevated S100A11 was correlated with both pulmonary and cutaneous disease activity.89 S100A11 potentially participated in the local inflammatory and tissue remodelling processes.89 Overall, the S100 protein family was found to be useful for determining the severity of disease progression in DM in multiple organs including the skin.
4.2.2 Lichen planus
LP is an inflammatory disease that localizes on the skin and mucosal membranes. The plaques or papules appear to be violaceous, pruritic and are commonly found on wrists, lower back, and ankles. The immunopathogenesis behind LP is currently unknown. There is a theory that states the process is due to a T-cell mediated autoimmune response.93 Triggers such as drugs or viruses cause a change in the epidermal self antigens, which results in activation of cytotoxic CD8+ T-cells, leading to immune attack of the basal keratinocytes.93
In LP, the inflammatory process may begin with S100A8 as a promoter for the activation of CD8+ T cells.94 To further investigate the role of S100A8 in the pathogenesis of LP, Carvalho et al. compared S100A8 and CD8+ T cell activation between individuals with LP and healthy controls. They found that patients with LP had elevated S100A8 serum levels that corresponded with increased CD8+ T cell activation. This may be due to S100A8 induction of proinflammatory cytokines such as IL-6, TNF-α, and IL-1β.94 However, this may be site-specific, as Ferrisse et al. found no significant difference between S100 protein level expression in LP vs healthy in the oral mucosa.95 Overall, various studies have used S100 protein as a marker to identify the inflammatory process of LP, but minimal literature has reported the direct involvement of specific S100 protein in the pathogenesis of LP.
4.2.3 Systemic and/or cutaneous lupus erythematosus
Systemic lupus erythematosus (SLE) is an autoimmune disease that has a range of effects on different organ systems. It is a complex disease process that is influenced by genetics, environmental triggers and immunological processes. Approximately 80% of SLE patients experience malar rash, which is skin involvement that is characterized by a butterfly-shaped rash on the face that crosses the cheeks and nose but spares the nares. S100 proteins are useful biomarkers in inflammatory disease processes including SLE.96 For example, the S100A8 protein activates granulocytes and monocytes, which promote the cyclical inflammatory response.97 S100A8/S100A9 serum levels are elevated during intensified lupus disease activity.98
Glomerulonephritis (GN) is a severe manifestation of SLE with significant mortality and morbidity. An estimated 60% of individuals with SLE will develop lupus nephritis and 10% of those people will go on to develop end stage renal failure.99 S100A12 serves as significant markers for active disease with kidney involvement, particularly in SLE patients with positive anti-double-stranded DNA antibodies.100 The elevated levels of the S100 proteins in serum, urine, and saliva show strong diagnostic potential, suggesting that using a combination of multiple biofluids, rather than a single biomarker, can be helpful in screening for SLE.96 The S100 protein can be used as a therapeutic target. Hydroxychloroquine modulates the expression of S100 in SLE,101 even when used supplementally in those with low-disease activity.102
Cutaneous lupus erythematosus (CLE) is an autoimmune skin disease that significantly affects quality of life. It can present independently or coexist with SLE. CLE is categorized into acute, subacute, or chronic types based on lesion characteristics.103 About 80% of CLE cases fall under chronic cutaneous lupus erythematosus (CCLE), with discoid lupus erythematosus (DLE) being the primary subtype.104 ACLE, characterized by malar erythema, is almost always associated with SLE. In contrast, localized DLE primarily affects the head and neck, with potential for systemic involvement in 20% of cases, especially in those with generalized lesions.105 CLE patients have increased expression of S100A8 and S100A9 in epidermal cells.106 Additionally, when CD8+ T cells from individuals with lupus are stimulated with S100A9 or S100A8 proteins, there is a significant upregulation of IL-17 expression through TLR4 ligation.107 This implies a crucial role for these proteins in the development of autoreactive lymphocytes during human autoimmunity.
4.2.4 Drug eruptions
Drug eruptions often cause ID, and there are several clinical entities including Erythema multiforme (EM), Stevens Johnson Syndrome (SJS) and toxic epidermolysis bullosa (TEN).108 In addition to drugs, EM is typically induced by a bacterial or viral infection such as herpes or Mycoplasma.109 Specific studies of S100 expression in EM have not been published. However, SJS and TEN have been reported to have elevation of S100A7110 and S100A8/9 in serum,111 the latter of which correlated with the SCORTEN and mortality rate.112 S100A2 is elevated in keratinocytes from patients with drug eruptions,113 and S100A8 is elevated in blister fluid.114 Given their potential roles in pathology and utility as biomarkers of disease severity, studies of S100 proteins and their value as treatment targets warrants further evaluation.
4.3 S100 proteins in skin cancer
S100 proteins generally play a negative role in cancer: they can be induced by soluble factors such as VEGF-A secreted by tumour cells,28 and the upregulation of S100A8/S100A9 promotes cancer cell growth through NF-kB and MAPK signalling.115 In contrast, S100A14 has some tumour suppressing effects in oral squamous cell carcinoma by downregulating the expression of metalloproteinases.116 S100A6 is expressed by squamous cell carcinoma (SCC), but not by the malignant epithelium in basal cell carcinoma (BCC).117 S100A7 appears to be upregulated in dysplastic keratinocytes, but is lost upon development of invasive phenotypes.118 Thus, it will likely be important to catalogue specific S100 family members' roles in specific tumours and stages.
Compared to normal skin, S100A1/S100A13 and S100B were significantly upregulated in metastatic melanoma,119 whereas S100A2, S100A7, S100A8, S100A9, S100A10, S100A11, and S100P were highly expressed in primary melanoma.119 Since the presence of these proteins are associated with different stages of melanoma, they can help with early diagnosis and treatment. Additionally, the presence of certain S100 proteins can determine the efficacy of treatment and prognosis. S100B has been shown to be a valuable prognostic biomarker for melanoma, due to its increased expression.120, 121 Mechanistically, the S100B protein inhibits the tumour suppressor p53. There are treatments that inhibit S100B-p53 interaction for melanoma.122 S100 is correlated with both lymphatic spread and distant melanoma metastasis,119 and is a potential serum marker for advanced malignant melanoma. An analysis of 73 patients who were at different stages of the disease, and 130 healthy controls, found that S100 was significantly upregulated in stage IV in comparison to Stage I/II by immunoradiometric assay.123 The progression of malignant melanoma results in increased levels of S100, and patients in remission have virtually no S100 protein in their serum. Studies have shown that the diagnostic sensitivity for S100 in early stages of melanoma is 15% compared to late stages at 60–85% sensitivity.124
In addition to keratinocyte-derived tumours and melanocyte-derived tumours, S100 proteins are important for haematopoietic-derived tumours of the skin. S100A2 is upregulated in cutaneous T cell lymphoma (CTCL) by specific cell clusters as assessed via single cell RNA sequencing.125 S100A1 and S100B can distinguish CTCL clinical stages.125 S100A7, A8 and A9 are also elevated in CTCL, and correlate with reduced expression of loricrin and filaggrin and higher transepidermal water loss (TEWL).126 Thus, upregulation may occur due to barrier defects. Langerhans cell histiocytosis, which is a clonal expansion of Langerhans cells,127 is characterized by upregulation of S100B.128 While this appears to be non-specific, it may still be a useful biomarker for estimating disease burden.
5 MAJOR OPEN QUESTIONS
Some major open questions that remain are: how can we target specific S100 proteins in ID conditions? The recent success of biologic therapies, such as anifrolumab for CLE, and small molecule inhibitors, such as JAK inhibitors for several dermatologic conditions, means that S100 proteins could likely be targeted with these modalities. However, the complex biology including proteolytic processing to CGRP means that other treatment avenues, such as peptide mimetics or silencing RNAs might be necessary. Another major open question is: how exactly do S100 proteins mediate neuroimmune feedback loops in ID? A sub-question of this is: how does neuroimmune feedback mediated by S100 family proteins result in different sensations in the peripheral nervous system including pain and itch in the skin? Understanding these pathways will likely require both preclinical and translational studies to systematically map each family member, its receptor, and the cell types which express them. This could be achieved by using knockout mouse disease models, CRISPR human cell lines in vitro, and ex vivo studies of human tissues from different ID conditions and healthy controls, among other methods.
6 CONCLUSIONS AND PERSPECTIVES
There are many proteins that can be implicated in driving the inflammatory processes underlying ID and other skin pathologies, but the roles of S100 proteins cannot be understated. The intricate histopathological changes in ID reveals a complex interplay of cellular responses, evident in the basal keratinocyte alterations and immune cell infiltration that characterize this condition. The involvement of S100 proteins in skin homeostasis and their various functions, including antimicrobial activities, chemoattraction, and signalling modulation, highlights their pivotal roles in both skin health and disease. Specifically, the distinct roles of S100 proteins in various dermatoses such as psoriasis, LP, systemic lupus erythematosus, and dermatomyositis provides a deeper understanding of their contributions to inflammatory processes, immune responses, and disease severity in these conditions. Future research is needed to better understand how S100 proteins mediate neuroimmune feedback loops in the skin and peripheral nervous system. The S100 proteins are both biomarkers and potential therapeutic targets of skin pathology, emphasizing their relevance in both diagnosis and treatment strategies.
AUTHOR CONTRIBUTIONS
Conceptualization: JMR; methodology, investigation, validation, formal analysis, resources, data curation: N/A (review article, no experiments performed); visualization: WA, SK, IO, JMR; supervision: JMR; project administration: JMR; funding acquisition: WA, JMR; writing—original draft: WA, AR, DA; writing—review and editing: ES, YZ, SS, IO, WA, JMR; All authors approve of the final submission.
FUNDING INFORMATION
Dermatology Foundation Diversity Research Supplement Award (WA), Dermatology Foundation Women's Health Career Development Award (JMR).
CONFLICT OF INTEREST STATEMENT
JMR is an inventor on patents #63/478900 ‘Diagnosis of skin diseases in veterinary and human patients’ for CTCL, #62489191, ‘Diagnosis and Treatment of Vitiligo’ which covers targeting IL-15 and Trm for the treatment of vitiligo, and #15/851651, ‘Anti-human CXCR3 antibodies for the Treatment of Vitiligo’ which covers targeting CXCR3 for the treatment of vitiligo. Remaining authors declare that they have no competing interests.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.