Characteristics and pathogenesis of Koebner phenomenon
Abstract
The Koebner phenomenon, also known as isomorphic reaction, refers to the development of secondary lesions with the same clinical manifestations and histopathological characteristics as the primary lesions in normal skin after trauma or other stimuli. The triggering factors of Koebner phenomenon include physical trauma, chemical stimulation, mechanical stress, iatrogenic stimulation and pathogenic infection. Vitiligo, psoriasis and lichen planus are considered true Koebner phenomenon. Recent studies have shown that immunological disorders, oxidative stress, defective melanocyte adhesion and growth factor deficiency are the main pathological mechanisms of vitiligo Koebner phenomenon. In psoriasis, triggers may drive skin inflammation to induce a psoriatic phenotype through multiple signalling pathways and thereby cause Koebner phenomenon in susceptible individuals. Significantly, keratinocytes mediate the occurrence of Koebner phenomenon in psoriasis through mechano-induced signalling pathways after sensing mechanical signals and explains the high frequency of psoriasis lesions on the extensor side of the elbow and knee joints. On the contrary, TRPA1-driven mechano-transduction, autoimmunity and actinic damage are the underlying mechanisms of Koebner phenomenon in lichen planus. In this review, we have summarized the current understanding of the characteristics and pathogenesis of Koebner phenomenon.
1 INTRODUCTION
The Koebner phenomenon (KP) was first described in 1876 by the German dermatologist Heinrich Koebner. He observed that the non-lesional skin developed psoriatic lesion after scratching, tattooing, or other types of skin trauma. Boyd and Neldner proposed that the secondary lesions of KP had the same clinical manifestations and histopathological features as the primary lesions.1 According to the definition, they have classified KP into four groups: (1) true KP refers to the isomorphic reaction induced by different types of stimuli rather than infection or allergy; (2) pseudo-KP occurs in common skin infections such as HPV warts, molluscum contagiosum and impetigo following a traumatic inducement that allows microorganisms to penetrate the skin barrier and spread to the uninfected tissue; (3) occasional KP occurs after trauma, including Darier's disease, Behcet's disease and Erythema multiforme, and partly meets the criteria for KP; (4) suspected traumatic induction, such as pemphigus, eczema and lupus erythematosus, mostly with single case studies and unreliable data.
It has been known that the true KP occurs in vitiligo, psoriasis and lichen planus, and can be induced by trauma, repeated friction or pressure, scars and tattoos.2, 3 In addition, KP is also instrumental in the occurrence, progression, persistence and relapse of the primary disease. Therefore, it is essential to consider the possibility of KP in patients with skin disorders in order to evaluate disease progression and treatment options and prevent recurrence. For instance, vitiligo patients with KP have a higher age of onset, longer disease course and larger affected area, along with an inadequate response to treatment.4-6 Thus, KP could serve as a reference index for determining the disease stage.
In this review, we have discussed the epidemiology, clinical characteristics and significance of KP in vitiligo, psoriasis and lichen planus and summarized the possible pathological basis for better clinical management and individualized treatment.
2 OVERVIEW OF KP
2.1 Incidence
Due to paucity of data, there is currently no unified method to evaluate KP. Although most of reports are single case, there are some epidemiological studies have been conducted for KP. The incidence of KP varies from 5% to 61% in vitiligo and is more common in non-segmental vitiligo.7, 8 In a survey of 701 children with vitiligo, Martins et al. observed KP in 38.2% of the patients. Moreover, the incidence was higher in non-segmental type (55.9%) compared with that in segmental type (10.6%) and undefined vitiligo (6.4%).9 About 25%–30% of psoriasis patients develop new psoriatic lesions after skin trauma, as well as experimentally induced stimulation (e.g., acupuncture, tape-stripping).10 Sharma et al examined 50 patients with lichen planus and detected KP in 28% of the cases,11 while Nnoruka observed KP in 5 of 13 paediatric lichen planus patients.12 The considerable variation in the incidence of KP reported by these studies can be attributed to the small sample sizes, differences in age, disease typing, disease stability, evaluation criteria and clinical concerns.
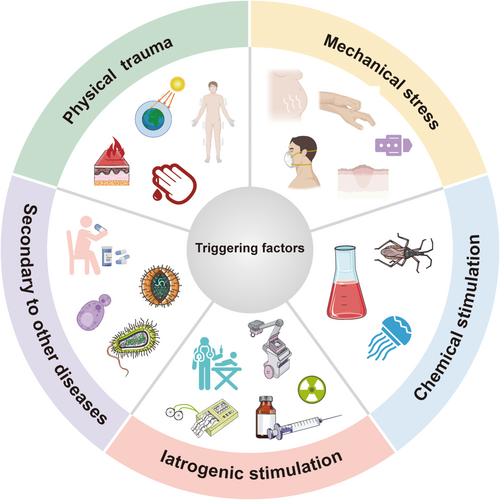
2.2 Triggering factors
The triggering factors for KP are detailed in Table 1 and Figure 1. Trauma is the most common stimulus, even though not all types of physical injury can induce KP in the same patient. Kalayciyan found that the depth, width, duration, area and type of trauma all play a role in determining psoriatic lesions.10 Furthermore, reports have proposed that the incidence of new psoriatic lesions is significantly higher in blisters induced by negative pressure suction than in epidermal damage caused by tape stripping.13 Despite acute deep injury may initiate KP more rapidly, chronic superficial, or minor damage also give rise to it in susceptible individuals once they accumulate to a certain threshold. For instance, vitiligo lesions tend to occur in the waist and wrist, which are prone to repeated friction or pressure due to daily activities. Therefore, lesions in these areas may progress to delayed KP, resulting in chronic and prolonged vitiligo.14 Most known forms of trauma are caused by penetrating injuries. However, mechanical stretch may also induce KP. The continuous stretching tension in a keloid scar can cause a new vitiliginous patch by weakening the adhesion between melanocytes and keratinocytes.3 Additionally, one case study reported that a patient with psoriasis vulgaris developed a new psoriatic lesion following tissue dilator insertion after total mastectomy for right breast cancer. These findings indicate that mechanical stretch-induced lesions as a consequence of KP.15
| Types | Triggering factors | Refs |
|---|---|---|
| Physical trauma | Trauma | [109] |
| Concis | [113] | |
| Burns | [114, 115] | |
| Freezing | [165] | |
| Tattoo | [116-118] | |
| UV radiation | [102] | |
| Facial hair plucking | [119] | |
| Mechanical stress | Scratch | [57, 120] |
| Stretch | [15, 80, 81] | |
| Repeated friction or pressure | [22] | |
| Surgical mask | [121, 122] | |
| Hearing aid | [123] | |
| Use of computer mouses | [124] | |
| Keloid/ Hypertrophic scar | [3, 125, 126] | |
| Striae distensae | [127] | |
| Chemical stimulation | Insect bites | [2] |
| Jellyfish sting | [108] | |
| Exposure to cytotoxic chemicals (Diphenylcyclopropenone, Nickel) | [128] | |
| Iatrogenic stimulation | Vaccination | [129] |
| Needle acupuncture | [130] | |
| Radiotherapy | [131-135] | |
| Cupping therapy | [136, 137] | |
| Hirudotherapy | [138] | |
| Specific Immunotherapy | [139] | |
| Pulsed dye laser | [140] | |
| Laser hair removal | [141, 142] | |
| Platelet-rich plasma injection | [143] | |
| Subcutaneous Methotrexate/Insulin Injection | [144-146] | |
| Purified protein derivative (PPD)/Mantoux test | [31, 147, 148] | |
| Ayurveda | [149] | |
| Colostomy Bag | [150] | |
| Hair transplantation/ skin grafting | [151, 152] | |
| Mohs micrographic surgery | [111] | |
| Electrocardiogram electrode negative pressure suction | [153] | |
| Secondary to other diseases | Inflammatory dermatosis | [154] |
| Coffin-Siris syndrome | [155] | |
| Churg-Strauss syndrome | [156] | |
| Cryoglobulinemia | [157] | |
| Leukocytoclastic vasculitis | [158] | |
| Drug reaction (Labetalol, Clindamycin, Sorafenib) | [159-162] | |
| Virus infection (Hand-foot-and-mouth disease, Virus varicella zoster) | [163, 164] |
2.3 Incubation period
KP tends to be present in the progressive stage. However, the incubation period from skin injury to the onset of KP has not been determined. Studies on vitiligo patients suggested that KP mainly occurs within 1–2 months after injury. In psoriasis, KP usually appears within 10–14 days but can also occur as early as 3 days or as late as two years.16 Some lichen planus patients with malignant tumours exhibit KP locally around 1 month after radiotherapy.17, 18 KP may develop at different time intervals after multiple injuries to the same patient. However, delayed or relatively immediate onset of KP depends on the location, type, depth, width and timing of skin trauma, as well as individual differences. This specific process and mechanisms need to be explored further.
2.4 Predilection sites
KP usually occurs at sites with prior trauma or inflammation. In vitiligo patients, it is more likely to occur in regions subjected to repeated friction or pressure (such as on the waist and wrist), whereas the protected regions (such as the scalp and palm) seem less susceptible.19 It is currently accepted that psoriatic lesions at the sites with high mechanical tension (such as elbows and knees) may indicate KP.20 For instance, the flexor tendon pulleys are subjected to very high physical stress, which makes them thickened and contributes to psoriatic arthritis (PsA)-related tenosynovitis and dactylitis. The excessive repair responses in the pulleys support the idea of “deep Koebner” phenomenon in PsA.21, 22 However, the thicker pulleys in rheumatoid arthritis might point towards a non-specific effect of a chronic autoimmune tenosynovitis on the adjacent pulleys, not an intrinsic pathological change in the pulley. The ultrasonographic evaluation of the pulleys may help to differentiate between RA and PsA, especially in the early phase of the disease.23, 24 The location of KP in lichen planus is not specific. A few reports have shown that oral lichen planus may be related to KP, but the sample sizes are too small to draw definite conclusions.25
2.5 Characteristics of skin lesions
KP is clinically and histologically identical to the pre-existing lesions. The depigmented lesions along the site of skin injury in vitiligo are indistinguishable from primary lesions. KP can not only be consistent with or extend from the trauma site but can also appear as trichromatic vitiligo or confetti depigmentation similar to advanced vitiligo.19 In lichen planus, new lesions with linear or beaded KP could be along the scratch. The dermatoscopic features of linear KP in lichen planus differ significantly based on the skin pigmentation.26 However, the clinical manifestations of psoriasis KP are generally the same as the primary lesions, and there is a lack of specific descriptions of the lesions.
2.6 Evaluation
KP is usually indicated by the appearance of primary lesion-like lesions after local trauma or other stimuli. However, there is a lack of consensus in the current evaluation methods of KP. Based on the response intensity, KP can be divided into four grades: (1) maximum KP that develops throughout the trauma site, (2) minimum KP that appears as small foci in the trauma area, (3) abortive KP wherein the secondary lesions initially appear as primary lesions but subside naturally within 12–20 days and (4) lack of response indicating normalization of the trauma site. Individual differences in response intensity of KP may be linked to the overall or local immune status of the patients.2
According to the correlation between depigmentation and skin trauma, the Vitiligo European Taskforce (VETF) has proposed a new classification system to evaluate KP in clinical practice or in experimental studies.19 In addition, Diallo et al. have developed and validated a simple clinical score for KP in patients with vitiligo (K-VSCOR), although it remains to be validated in large clinical studies.27 Recently, Van Geel developed the Vitiligo Signs of Activity Score (VSAS) to quantify the visible clinical signs linked to disease activity such as KP (K-VSAS).28 These criteria can improve understanding of vitiligo KP, determine disease progression and guide treatment. Nevertheless, standard diagnostic approaches for psoriasis and lichen planus are still scarce, and more research is needed in the future.
2.7 Treatment
Since KP is the outcome of re-activation of an underlying skin disease, the associated lesions are treated in the same way as the primary lesions. The presence of KP appears to be associated with rapid disease progression and a lower response to treatment. Therefore, we need to evaluate the disease stage, select appropriate treatment for KP, and control the development of lesions at the earliest. In addition, invasive treatments such as surgical transplantation for vitiligo should be avoided during KP to prevent disease progression.4-6
2.8 Other subtypes of KP
Reverse KP: refers to the disappearance of existing lesions after local trauma. A few cases have been reported in psoriasis and oral lichen planus. Psoriatic lesions resolved after radiation therapy in patients who underwent mastectomy for breast cancer, suggesting involvement of reverse KP.29-31
Wolf's Isotopic Response: was described as an unrelated disease affecting the area of the healed skin lesions.32 Herpes zoster is the most common primary disease preceding Wolf's isotopic response, while circular granuloma, pseudo-lymphoma and Bowen's disease are less common. It is likely associated with viral infection and dysfunctional immune system, blood vessels and nerves. The interval between the two diseases is highly variable (from a few days to several years) and there are no predictors so far.32, 33
Renbök Phenomenon: refers to the disappearance of a primary lesion after the onset of a new lesion at the same location. Bon et al. observed that a patient with alopecia areata began to show hair growth locally after psoriatic lesions on the scalp and proposed Renbök phenomenon to describe this phenomenon.34 This concept has been applied to illustrate different clinical settings.35, 36 It has been suggested that the different T-helper (Th) inflammatory subsets may play a key role in the competition between these two immune-related diseases. Psoriatic inflammation may antagonize the inflammatory reactions in alopecia areata.37
3 POSSIBLE PATHOGENESIS OF KP
3.1 Vitiligo
Immunological disorders
The innate immune cells are persistently activated throughout the surface of vitiligo lesions. Tulic et al. found that type 1 innate lymphocytes (ILC1) and NK cells were significantly increased in non-lesioned skin of vitiligo compared to healthy skin. These cells produce IFN-γ in response to external trauma and induce keratinocytes to release CXCL10, which then binds to the CXCR3B on the surface of melanocytes and triggers apoptosis and antigen release.38, 39 The residual melanocytes stimulated by IFN-γ express co-stimulatory molecules (CD40, CD80, MHC-II, ICAM-1) that present autoantigens to CD8+ T cells, thereby initiating adaptive immune responses and may eventually trigger vitiligo KP.40-42 These findings support the central role of the IFN-γ-CXCL10 pathway in driving autoimmune responses in vitiligo (Figure 2).
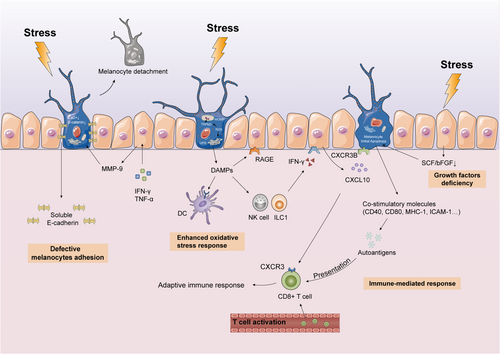
Enhanced oxidative stress response
The oxidative stress response is characterized by excessive production of reactive oxygen species (ROS) that cannot be neutralized by the antioxidant system. Overproduction of ROS in melanocytes due to ultraviolet (UV) radiation, cytotoxic chemicals, trauma, or vaccination triggers the oxidative stress response in vitiligo, leading to KP.43
Redox disorders affect melanocyte integrity and function and induce melanocyte death and melanocyte-specific antigen exposure. Melanocytes chronically exposed to ROS release damage-associated molecular patterns (DAMPs), such as high-mobility group box 1 (HMGB1), in the form of cell debris and exosomes.44 It has been suggested that extracellular stress proteins elicit an immune response by acting as autoantigens that facilitate presentation of other antigens to dendritic cells (DCs) and enhance phagocytosis.45, 46 Additionally, HMGB1 promotes the secretion of CXCL16 and IL-8 from keratinocytes and induces CD8+ T-cell migration through binding to receptors for advanced glycosylation end products (RAGE) and activating NF-κB and ERK pathways.47
Oxidative stress also contributes to dysfunctional mitochondria and endoplasmic reticulum (ER). Mitochondria control cell energy supply, senescence and apoptosis, thus mitochondrial damage inevitably affects the survival of melanocytes.48 Kang et al. found that oxidative stress promotes the expression of transient receptor potential cation channel subfamily M member 2 (TRPM2) and mediates Ca2+ influx, which aggravates mitochondrial-dependent apoptosis of melanocytes.49 Mitochondria are also involved in ROS-associated melanocyte apoptosis through the formation of mitochondrial outer membrane permeability (MOMP).48 Furthermore, ER is the site of protein biosynthesis and is therefore critical for survival. Excessive ROS activates ER's unfolded protein response (UPR) to trigger melanocyte apoptosis.43 These findings implicate that enhanced oxidative stress may affect melanocytes through multiple pathways, thus contributing to the development of vitiligo KP (Figure 2).
Defective melanocytes adhesion
Keratinocytes and melanocytes constitute the “epiderm-melanin unit” and maintain epidermal homeostasis through paracrine signalling of growth factors.50 The stability of melanocytes in the basal layer of the epidermis depends on E-cadherin, the primary adhesion molecule that mediates melanocyte-keratinocyte interactions in a Ca2+-dependent manner. E-cadherin levels and distribution are significantly altered in the non-lesional skin of vitiligo.51 Mechanical trauma such as repeated friction decreases E-cadherin levels in the melanocytes, which alters their resistance to the stress. This leads to melanocyte detachment and trans-epidermal elimination, which is probably the cause of depigmentation in vitiligo KP (Figure 2).52
Using a 3D model of reconstructed human pigment epidermis (RHPE), Boukhedouni et al. demonstrated that IFN-γ and TNF-α regulate matrix MMP-9 production and E-cadherin disruption, thereby promoting melanocyte detachment in vitiligo.53 In conclusion, defective melanocyte adhesion is a causative factor and a potential therapeutic target in vitiligo KP. To stabilize melanocytes in the basal layer by preventing E-cadherin destruction shows promising therapeutic prospects.
Growth factors deficiency
Keratinocytes control melanin metabolism by producing and secreting several cytokines. The stem cell factor (SCF) binds to KIT receptors on melanocytes and regulates melanocyte growth and survival. Down regulation of SCF, basic fibroblast growth factor (bFGF) and other melanocyte growth factors due to trauma, UV exposure or elevated H2O2 may cause melanocyte apoptosis and induce KP (Figure 2).54
3.2 psoriasis
3.2.1 Inflammatory infiltration
The immune barrier function of the epidermis is maintained by a rapid and precise innate immune response of the keratinocytes to external trauma. Mechanical scratch of the epidermis activates the keratinocytes to produce and release CCL20 and CXCL8 via the EGFR-ERK/JNK pathway, which recruits the positive CCR6+ Th17 cells and neutrophils to the lesions. High concentrations of IL-17A strongly stimulate keratinocyte proliferation and inhibit differentiation in vitro, which may be the pathological basis of the induction of KP due to scratching.55-57 Furthermore, skin injury induces the release of dsRNAs from the keratinocytes, which triggers IL-36γ production through TLR3 signalling. This process can be synergistically enhanced by IL-17A, resulting in the intraepidermal accumulation of neutrophils.58 Moreover, nerve growth factor (NGF), secreted by the keratinocytes, is upregulated markedly in KP-positive psoriasis after skin trauma.59
In addition to keratinocytes, plasmacytoid DCs (pDCs) also play an indispensable role in psoriatic KP. During skin injury, stressed keratinocytes release nucleic acids and antimicrobial peptides (e.g., LL37). The LL37-DNA complexes activate dermal pDCs through TLR signalling to produce type 1 interferons that promote myeloid DC (mDC) maturation. Activated mDCs trigger the adaptive immune system by expressing TNF-α, IL-12 and IL-23, which further activate Th1 and Th17 autoimmune cells, contributing to the development of psoriatic KP (Figure 3).60-62 Thus, pDCs sense tissue damage and promote inflammatory responses in skin wounds. Although dermal pDC activation in wounded skin may partially explain KP, it is still unclear why even superficial tattoos cause psoriasis.60
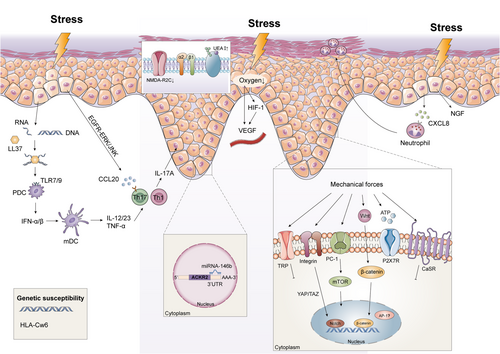
Inflammatory regulators are also frequently disrupted during KP in psoriasis. Atypical chemokine receptor 2 (ACKR2) limits the spread of psoriatic skin inflammation to other sites by scavenging, internalizing and degrading chemokines.63, 64 Reports have proposed that tensile cell stress downregulated ACKR2 and upregulated miR-146b in psoriatic lesions. Furthermore, miR-146b directly binds to the ACKR2 3′-UTR, which decreases ACKR2 transcript and protein levels. Overall, tissue trauma and miR-146b exacerbate inflammatory immune responses by downregulating ACKR2, which is in part responsible for psoriatic KP.65
3.2.2 Proliferation and differentiation
Cutaneous wound healing is usually divided into three phases: inflammation, proliferation and remodelling. Following trauma to the epidermis, the keratinocytes are rapidly activated and begin to proliferate vigorously and migrate to the wound center. This helps repair the damaged skin by rapid re-epithelialization of the wound.66 However, in psoriasis patients, epidermal trauma may promote excessive keratinocyte proliferation and terminal differentiation disorders leading to KP. N-methyl-D-aspartic acid receptor 2C subunit (NMDA-R2C) is activated during wound re-epithelialization and inhibits the process. Whereas NMDA-R2C is significantly down-regulated expression in the basal layer of the psoriatic skin. Trauma leads to keratinocytes hyperplasia by further down-regulating NMDA-R2C, which promotes the development of KP.67-69
Keratinocytes in psoriatic lesions also exhibit severe terminal differentiation disorders.70 The density of ulex europaeus agglutinin-I (UEA-I) binding sites on cell-surface glycoproteins is often used as a marker of terminal differentiation. Koebner-positive lesions exhibit a marked increase of UEA-I binding sites on keratinocytes, compared to KP-negative and healthy individuals. This change in the behaviour of keratinocytes increases the likelihood of developing secondary psoriatic lesions.71
The proliferative capacity of keratinocytes depends not only on their differentiation but also on matrix adhesion. Loss of matrix adhesion leads to a sudden cessation of keratinocyte proliferation.72 Studies have demonstrated that a mild epidermal wound in α2β1 integrin transgenic mice stimulates keratinocyte hyperproliferation and chronic inflammation, a process very similar to that of psoriasis KP.73
3.2.3 Angiogenesis
The psoriatic lesions are characterized by increased microvascular permeability and angiogenesis as opposed to the normal histological, morphological and proliferative features of non-lesional skin. In fact, significantly transcriptomic differences have been detected in the genes related to vascular function between the non-lesional skin of psoriasis patients and healthy skin.74, 75
In psoriasis, persistent skin barrier disruption due to repeated injury increases epidermal metabolic demands and further reduce oxygen levels in local tissues. Reduced oxygen content in the epidermis activates a key transcription factor called hypoxia-inducible factor-1α (HIF-1α), which induces sustained VEGF production in response to hypoxia stress (Figure 3).76, 77 In a repeated tape stripping psoriasiform hyperplasia model, acute barrier disruption rapidly stimulated VEGF-A mRNA and protein expression in normal hairless mice.78 The high VEGF levels in turn lead to superficial dermal angiogenesis and epidermal proliferation at the trauma site. However, whether VEGF stimulates epidermal proliferation directly or through other downstream paracrine mechanisms has not been understood. To summarize, sustained permeability barrier disruption upregulates epidermal VEGF, involved in the aberrant angiogenesis and psoriatic KP.
3.2.4 Mechano-transduction
Psoriasis usually occurs in skin areas with stronger mechanical tension, such as the elbows, knees and scalp. Keratinocytes sense this mechanical signal and convert it into an intracellular biochemical signal that leads to a cellular response. Mechano-transduction regulates the proliferation, differentiation, morphology and migration of keratinocytes and maintains cellular homeostasis.79 However, mechanical stress (scratches, abrasion and stretch) in apparently healthy skin tends to aggravate or induce new psoriatic plaques that can be explained as KP. (Figure 3).
Mechanical stretch has been known to impair skin barrier function and upregulate mRNA expression of psoriasis-associated cytokines and chemokines both in human epidermal keratinocytes and animal models.80, 81 Furthermore, mechanical stretch-induced ATP released from keratinocytes is considered as the potential candidate for KP in psoriasis.15 ATP has recently been shown to activate CD70highCD11clow cells, followed by the production of IL-1β, IL-6 and IL-23 as well as TGF-b activation, thereby facilitating TH17 cells differentiation in the intestinal lamina propria.82 Additionally, ATP signalling through purinergic receptors, particularly P2X7R, induces innate and adaptive Th17 inflammatory immune responses in human skin. Mechanistic studies revealed a P2X7R-dependent miR-21 angiogenesis pathway that is involved in the conversion of non-lesional skin to psoriatic lesions.83, 84 Thus, a possible trigger of KP is the release of extracellular ATP from trauma, which initiates and perpetuates psoriatic lesion from non-lesional skin.
Polycystin (PC) is a key regulator of cellular mechano-transduction.85 Downregulation of PC1 expression may be associated with the development of psoriatic lesions. PC1 knockdown in HaCaT cells upregulated psoriasis-related biomarkers through the ERK-mTOR pathway, resulting in increased cell proliferation and migration.86 Additionally, extracellular mechanical stimuli activate the Wnt and Notch pathways, transport β-catenin into the nucleus and reduce nuclear activator protein-1 (AP-1) levels in psoriasis lesions.87-89 Furthermore, calcium-sensing receptors (CaSR) and transient receptor potential (TRP) channels are dysfunctional or abnormally expressed in lesional psoriatic skin,90-92 which disrupt tight junctions and promote over-proliferation of keratinocytes and inflammatory responses. Thus, these findings define a potential role for mechano-transduction in the induction of KP in patients with psoriasis.
3.2.5 Genetic susceptibility
Genetic susceptibility is a major risk determinant for psoriasis. More than 80 risk loci of psoriasis have been identified so far by genome-wide association studies (GWAS), which explains the inherited nature of 30% of the cases.93 Furthermore, environmental triggers increase the risk of inflammation in the non-lesional psoriatic skin. Therefore, it is essential to explore how environmental triggers and genetic predisposition affect disease susceptibility.94
HLA-Cw6 is one of the risk alleles of psoriasis and is associated with disease severity. The autoantigens LL37 and ADAMTSL5 bind to HLA-CW6 with high affinity and are significantly increased in injured skin. Thus, psoriasis patients harbouring the HLA-Cw6 allele are at a higher risk of developing psoriatic lesions following minor trauma.95-97 However, given the heterogenous nature of the disease and the differences in the positive rate of HLA-Cw6 across different ethnic populations, more studies are needed to clarify the role of HLA-Cw6 in psoriatic KP.98
3.3 Lichen planus
3.3.1 Mechano-transduction
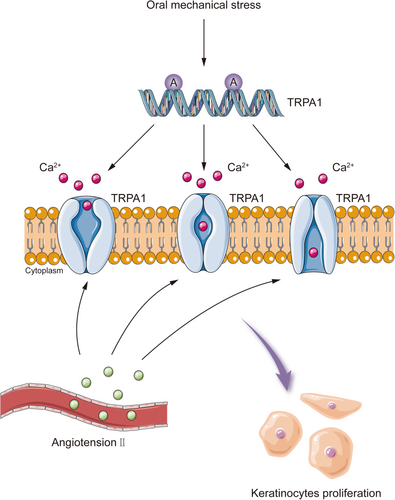
Lichen planus is a chronic inflammatory disease that usually involves the skin and mucous membranes. Mechanical stress can trigger KP in oral lichen planus. Circulating angiotensin II is elevated in patients with oral lichen planus, which activates and upregulates the mechanosensory receptor TRPA1 in both the lesions and non-lesional skin.99 TRPA1 is usually induced by mechanical stress and promotes keratinocytes proliferation and local immune responses (Figure 4). Repeated and prolonged mechanical stress in the oral cavity, for instance, due to mechanical trauma from dental surgery, friction from sharp cusps and bad oral habits, activate TRPA1 and exacerbate oral lichen planus through KP.25, 100 Angiotensin-converting enzyme inhibitors (ACEI) may prevent oral lichen planus from deteriorating by down-regulating the expression of angiotensin II and TRPA1.99, 101 TRPA1-targeted therapy may help to stabilize oral lichen planus, although it remains to be verified whether TRPA1 mediates the onset of KP in lichen planus at other sites.
3.3.2 Autoimmunity and actinic damage theory
In some patients with lichen planus, lesions are confined to the vitiligo macules and aggravated on the sun-exposed areas of the skin. Thus, it is supposed that actinic damage releases inflammatory mediators in genetically susceptible individuals, which promotes the accumulation of effector T cells and induces KP.102, 103 Another hypothesis is that vitiligo may alter the expression of antigens identified by the effector T cells or inactivate suppressor T-cells, leading to disease occurrence.104 However, these hypotheses are derived from case reports of lichen planus with KP, and the real pathological basis needs to be further explored.
4 POSSIBLE PATHOGENESIS OF KP IN OTHER SKIN DISEASES
KP is relatively rare in other dermatoses, and studies on the role of trauma as a predisposing factor are limited to case reports. The pathogenesis of KP in these diseases, which is not clear, may involve in many factors such as environment, genetic and immunity dysregulation.
Trauma-induced sporadic KP has been observed in lupus erythematosus, a classic autoimmune disease that includes both cutaneous and systemic forms.105, 106 Based on studies on lupus erythematosus patients with KP, Ueki et al. proposed that the pathogenesis can be divided into the non-specific inflammatory phase and disease-specific phase. Inflammatory factors such as TNF-α, IL-1, IL-6, HSP70, HSP72, HSP90 and ICAM-1 are triggered by environmental stimuli during the initial phase, which then induce corresponding autoantibodies or receptors in the second phase, resulting in new lesions identical to the primary disease.107
Lichen sclerosis is a chronic inflammatory disease common in women and occurs around anogenital and damaged skin areas. Skin trauma may lead to an increase in CD4+ lymphocytes, cytokines and adhesion molecules, resulting in the appearance of new lesions due to KP.108
Furthermore, KP has also been observed in pemphigus and bullous pemphigoid, which has led to the hypothesis of epitope diffusion. The disintegrated epidermis express antigens or expose new epitopes, which react with circulating antibodies and lead to the development of KP. However, epidermolysis does not occur in the absence of injury due to low antigen expression.109-111 In addition, the healing process itself may also affect the normal differentiation of keratinocytes and increase their susceptibility to pemphigus autoantibodies.112
5 CONCLUSIONS AND PERSPECTIVES
KP was initially described in psoriasis and later observed in vitiligo, lichen planus and other dermatoses. In the context of genetic susceptibility, various environmental stimuli induce KP, particularly in the advanced stage of diseases. However, it is unclear whether KP differs depending on the location, degree, mode, time of injury or individual differences. In vitiligo, external trauma contributes to KP from many aspects, such as immunity dysregulation, enhanced oxidative stress response, defective melanocyte adhesion and growth factors deficiency. Besides, external injury exacerbates psoriatic phenotypes through different signalling pathways. Importantly, mechano-transduction is a potential mechanism of the initiation of KP in psoriasis. Stretch-induced ATP released from keratinocytes may provide new insights for the prevention and treatment of psoriasis. Based on current evidence, TRPA1-targeted therapy may help to prevent oral lichen planus deterioration and maintain its stability.
Stressed skin cells induced by environmental triggers initiate an inflammatory cascade, which involves antigen presentation, production of inflammatory factors and T cell activation, leading to a self-amplifying mechanism that causes tissue damage and ultimately trigger KP. However, it remains to be ascertained whether a common mechanism links KP in these different diseases. Therefore, further studies need to be fulfilled to elucidate the mechanisms underlying the development of KP, and thus improve the therapeutic outcomes and prevent recurrence.
ACKNOWLEDGEMENTS
The authors would like to thank C.J. and Q.Z. for his involvement in the conception and content development of this review.
AUTHOR CONTRIBUTIONS
C.J. and Q.Z. conceived of the paper. X.Z. wrote the initial draft of the paper and generated the figures and tables. C.J. and Q.Z. edited and revised the paper. All authors approved the final paper.
FUNDING INFORMATION
This work is supported by the National Natural Science Foundation of China (No. 82073420, No. 82073421, No. 82003326, No. 82103704), Natural Science Foundation of Hunan Province (No. 2021JJ40924), the Wisdom Accumulation and Talent Cultivation Project of the Third Xiangya Hospital of Central South University (No. YX202007) and the science and technology innovation Program of Hunan Province (2021RC3035).
CONFLICT OF INTEREST
The authors declare that there are no competing interests associated with the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.




