Male secondary sexual traits do not predict female preference in Caribbean livebearing fishes (Limia)
[Correction added on 12 August 2021, after initial online publication: An error in Wiley content management processes resulted in publication of an earlier version of this article prior to completion of peer review. Some final minor wording changes have now been implemented, improving the clarity of the text. The publisher offers sincere apologies to everyone involved, particularly the authors and readers, but also the journal editor and peer reviewers.]
Abstract
Female preference is widely described in various taxa, and the underlying mechanisms shaping preferences remain a major focus of sexual selection studies, particularly in species where males contribute minimally to offspring. Female preference is associated with maintaining male secondary sexual traits (SST). However, how male SST impact female preference is less-understood. We hypothesized the strength of female preference should scale with the expression of male SST. To test this prediction, we compared female preference for male body size (an easily quantifiable trait that scales with other SST) in three species of Limia (Poeciliidae) varying in secondary sexual traits: L. perugiae, L. dominicensis and L. zonata. The degree of SST was assessed based on the amount of ornamentation and the presence of courtship in the species. Limia perugiae, L. dominicensis and L. zonata were designated as possessing high, intermediate and low male SST, respectively. Female preference was quantified as the relative amount of time females associated with males of various size classes: small, intermediate and large. Therefore, we predicted that because L. perugiae males have the most SST, females would associate more strongly with large males. Limia perugiae females were the only species to display female preference in relation to male body size, but they preferred small males. Although preference was observed, the direction of preference was unexpected. Moreover, the lack of preference for large male size and thereby other SST in the species suggests pre-copulatory female preference is unimpacted by male SST in these species. We suggest cryptic female choice (i.e. preference enacted during or after copulation) may maintain costly male traits. However, future work remains necessary. The present study provides foundational behavioural work on Limia and examines the ubiquity of the evolution of female preference in poeciliids.
1 INTRODUCTION
Sexual selection is a mechanism of evolution in which fitness is determined by an individual's ability to secure matings. Mate choice is one of the primary drivers of sexual selection (Andersson, 1994; Rosenthal, 2017) and is typically defined as any behaviour or trait that leads to non-random mating (Edward, 2015). Pre-copulatory female preference is amongst one of the most well-studied and prevalent aspects of mate choice (Ryan & Keddy-Hector, 1992; Varela et al., 2018; Wilson et al., 2017). Although female preference is widely described, the underlying mechanisms that shape preferences are not fully understood, hence these are still a major focus of sexual selection studies (Candolin et al., 2007; Kirkpatrick & Ryan, 1991; Mitoyen et al., 2019). Our lack of knowledge is most apparent when examining species where males contribute little or nothing to the offspring beyond sperm to fertilize eggs (Achorn & Rosenthal, 2020).
In species where males contribute minimally to offspring, males more readily re-enter the breeding population after mating than females causing a male-biased operational sex ratio (OSR) (Ahnesjö, 1996). The abundance of males allows females to mate selectively, with female preferences selecting for elaborate male secondary sexual traits (Clutton-Brock, 2007). Often secondary sexual traits take the form of ornaments such as fluorescent colours, movement patterns and body size (Maynard-Smith and Harper, 2003). Although it is well-established male secondary sexual traits are commonly a product of female preference (Fisher et al., 2009; Mitoyen et al., 2019), the impact of male secondary sexual traits on female preference is less well-understood (Ptacek & Travis, 1997). Given that the intensity of male secondary sexual traits is often attributed to Fisherian runaway selection, whereby male SST become more exaggerated due to directional female choice, we hypothesized that female preference should scale with the intensity of male secondary sexual traits as well (Fisher, 1915).
We determined if female preferences scaled with male secondary sexual traits by comparing female preferences between three species of Limia. Limia is a genus of livebearing fishes in the family Poeciliidae, endemic to the Greater Antilles (Weaver et al., 2016). The species of Limia are closely related and share similar life histories (Cohen et al., 2015); however, they vary considerably in male sexual strategies (Farr, 1984). Specifically, species differ in their degree of ornamentation and the use of courtship displays. Here, we compared female preference between L. perugiae, L. dominicensis and L. zonata. The species serve as treatment groups: high, intermediate and low degrees of secondary sexual traits, respectively. Species degree of secondary sexual traits was based on ornamentation indices using dorsal fin size as described by Goldberg et al. (2019) (L. perugiae 0.36, L. dominicensis 0.09 and L. zonata −0.11) as well as the presence of courtship, which is only present in L. perugiae (Erbelding-Denk et al., 1994). These differences in mating strategies make them ideal for our comparative study.
However, comparing mating preferences between species presents challenges. The primary challenge being mate preference data gathered via mate choice designs (i.e. binary choice tests) cannot be directly compared between species (Wagner, 1998). We circumvented this problem by using absolute preference functions to measure preferences in lieu of binary choice tests. Preference functions describe the correlation between a phenotypic character expressed by males and the resource(s) expended by females (Lande, 1981). Here we elected to measure female preference for male body size in Limia because preference for large males is widely described in poeciliids and correlated with other male secondary sexual traits. For example, female guppies (Poecilia reticulata) prefer highly ornamented males (Houde & Endler, 1990), and swordtail (Xiphophorus pygmaeus) females show strong preferences for large male body size (Hankison & Morris, 2002; Morris, 1998). Moreover, other male secondary sexual traits, such as ornamentation and courtship, are known to scale with male size in poeciliids, including Limia (Farr, 1984). Therefore, by using male body size as an SST as well as a proxy for ornamentation, we were able to easily ascertain female preference in Limia.
Because we hypothesize female preference should scale with male secondary sexual characters, we predicted 1) L. perugiae females possessed the most pronounced preferences for male body size of the three species. 2) Limia zonata to display the least exaggerated preference, 3) whilst L. dominicensis showed an intermediate preference. However, we expected females of all species to show a preference for large males because of the purported indirect genetic benefits associated with mating with large males (Hankison & Morris, 2002; Morris, 1998). Support for the null hypotheses was determined as female preference being indistinguishable between species and a lack of preference for larger males. In addition to testing our hypothesis, our results provide some of the first descriptions of female preference in the Limia.
2 MATERIALS AND METHODS
2.1 Collection and size classification
For this study, we used three species of closely related Caribbean livebearing fishes: Limia perugiae, L. dominicensis and L. zonata (Weaver et al., 2016). The fishes we used were descendants of wild-caught populations from the Dominican Republic on Hispaniola. Limia perugiae were collected in 2014 in a small ditch near the south shore of Lake Enriquillo (18°24'4.61"N, 71°34'16.61"W). Limia dominicensis were also caught in 2014 in a ditch east of Polo, Barahona Province (18°19'6.93"N, 71°34'14.24"W). Limia zonata were caught in 2012 in the shallows of the Río Yuna near Bonao (18°57'33.5"N, 70°24'32.1"W). All specimens were transported to a greenhouse (now the International Stock Center for Livebearing Fishes) at the Aquatic Research Facility at the University of Oklahoma, where they were kept under common garden conditions. The L. perugiae and L. dominicensis populations were kept in 1000-l flow-through stock tanks. Limia zonata were housed in a similar 500-l tank due to their smaller population size compared to L. perugiae and L. dominicensis.
We haphazardly collected 40 individuals (20 females and 20 males) of each species (N = 120) from these stock tanks, moved them into an indoor fish room, divided them by sex, and placed them in 37-l holding tanks. All individuals were acclimated to the laboratory environment for 14 days prior to any subsequent handling. All individuals were kept in a climate-controlled room with 26°C (±3°) on a 12-h day–night cycle. The fishes were fed ad libitum, a mixture of frozen brine shrimp nauplii, Daphnia, bloodworms (mosquito larvae) and Tetra Min flakes twice daily before and after behavioural assays.
A laminated grid was used to measure the standard length of each individual (to the nearest 0.1 mm). Fishes were then photographed (Nikon D5200 camera with a Nikon AF-S DX NIKKOR 18–200 mm f/3.5–5.6G ED VR II Standard Zoom Lens). Fish were gradually cooled to anaesthetize them for photographs; they were held in ice water until they became still enough to be photographed (Collymore et al., 2014; Klontz & Smith, 1968). We elected to cool fishes because preliminary studies of L. dominicensis found that mortality increased when alternative methods of anaesthesia, such as tricaine methanesulfonate (MS-222), were used (Spikes Obs). Immediately afterwards, individuals were placed in a recovery tank and were given 2 days to recover before beginning the behavioural tests. No mortality was associated with this procedure. Females of each species were randomly assigned an ID number and then placed in individual 5-l tanks under identical conditions in a climate-controlled fish room. To ensure individuals were reproductively mature, we used only fish with a standard length (measured from the tip of the snout to the end of the spinal column) greater than 16 mm (Arriaga & Schlupp, 2013). All females less than 16 mm were classified as juveniles and returned to their respective stock populations. Males with fully developed gonopodia, the intromitting organ typical for the family, were determined to be reproductively mature.
We used male size as a likely female-preferred trait in Limia because female preference for large males is well-documented in other Poeciliid species (Ríos-Cardenas and Morris, 2011). Within each species, we sorted males into discrete size categories based on the measured population distribution of male standard length. Using median standard length as an anchor, we divided the size distribution into equal quartiles. We classified males as small if they were in the lowest quartile (five males), intermediate if they were within the two middle quartiles (10 males total), and large if they fell into the upper quartile (five males). Limia perugiae size classes were defined as small (14–20 mm), medium (21–26 mm) and large (27 mm<). Similarly, L. dominicensis size classes were defined as small (14–23 mm), medium (24–26 mm) and large (27 mm<). Limia zonata size classes were defined as small (14–20 mm), medium (21–24 mm) and large (25 mm<). The males of each species were then placed into three 37-l aquaria (nine aquaria total), according to size classifications.
2.2 Experimental setup
We used an absolute preference function assay to measure female preference in all three species. Assays were conducted in a 76-l tank that was divided lengthwise into three equal zones (Figure 1). Two vertical lines were drawn on the front pane of the tank to designate zones. The centre zone was deemed the neutral zone, with the two outer zones designated preference and non-preference zones. In each zone, we placed a clear Plexiglas tube (8.5 cm × 8.5 cm rectangular prism), which was used to restrict a male within its assigned zone and to reduce the transmission of mechanosensory and chemical signals (Figure 1). In the preference zone, one male fish was placed in a Plexiglas tube, and in the non-preference zone, there was no male present (i.e. an empty clear Plexiglas tube to control for any bias the female might show toward the tube). The preference and non-preference zones were randomized to control for side bias.
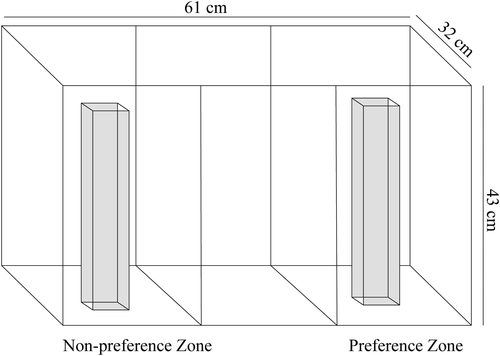
During absolute preference function assays, females of each species (N = 20 per species) were sequentially presented with three conspecific males of varying size (small, medium and large) in a randomized order. Females were randomly selected by ID number and placed in a clear Plexiglas tube in the centre of the neutral zone after her assigned male was first placed into the preference zone. Both individuals were then given 300 s to acclimate to the environment. To begin the trial, we released the female from the tube, and after she was swimming freely around the tank (i.e. showing no signs of stress-like behaviours), we recorded for 300 s the amount of time (s) the female spent in the preference or non-preference zone. Females were defined to be in the preference or non-preference zone if their entire head passed the vertical line on the front pane of the tank. After the 300 s passed, we gently placed the female back in the tube in the centre of the neutral zone, and the male was removed. Then a male of another size class was presented to the focal female. The process was repeated until the female was exposed to one male from each of the three size classes. Once a female was exposed to three males, she was returned to her home tank, and males were placed in size-specific recovery tanks labelled “used.” Males remained in recovery tanks for 3 days before haphazardly being used in another trial with a different conspecific female. After a trial, the testing tank received a 50% partial water change to reduce any lingering chemical signals from affecting future trials. All trials for any given species were conducted over the course of 30 days. Trials began in November 2017 and were concluded in January 2018.
2.3 Data analysis
All analyses were conducted in R using the nlme and BayesFactor packages (R Core Team, 2017; Pinheiro et al., 2020; Morey & Rouder, 2018). A linear mixed-effects model was used to determine if the intensity of male secondary sexual traits influenced female preference. Specifically, we loaded species and male size class as explanatory variables and association time as the response variable. Presentation order, female size and female identity were considered as random factors to control for order effects and prevent pseudo-replication in the analyses, respectively. However, to preserve non-singularity, both presentation and order female size was removed from the model. If a significant effect was observed, we conducted a post hoc Tukey test to determine the direction of preference. After running the full model, including all species, we used independent species-specific linear mixed-effects models on each species to compare female association time as it relates to male size within species, loading the same factors excluding species.
Because Limia has previously demonstrated atypical behaviour as compared to other poeciliids (Spikes & Schlupp, 2021), we elected to conduct a Bayes factor post hoc analysis. The Bayes factor analysis uses Bayesian inference to compute an integer known as a Bayes factor (BF), which can be interpreted using the cut-offs posited by Morey et al. (2016). The Bayes factor—like the p-value—is then indicative of how much support one has for the hypothesis. Unlike the p-value, however, the Bayes factor allows for interpretation of the support for the null hypothesis. Hence, the analysis is particularly advantageous when interpreting insignificant results. Particularly if a study yields negative data that requires the rejection of the hypothesis, Bayes factors will indicate if the results are inconclusive or if the null hypothesis should be accepted.
3 RESULTS
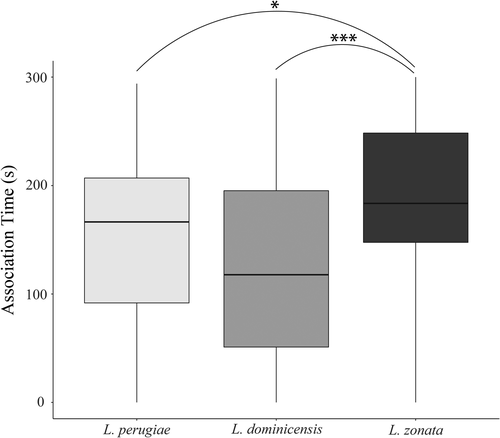
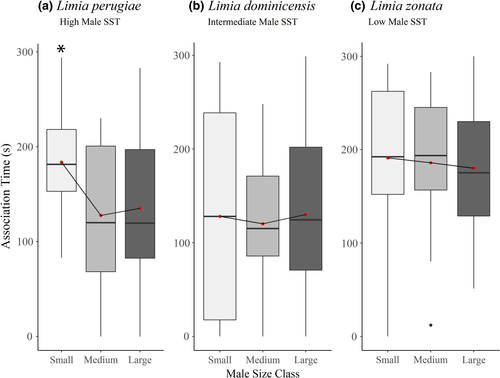
The variance between species had the largest impact on the amount of time females associated with males (Table 1). Limia zonata, the low male SST species, associated with males more than both L. perugiae, the high male SST species (p = .03), and L. dominicensis, the intermediate male SST species (p = .0002; Figure 2). Species-specific linear mixed-effects models revealed L. perugiae females possess preference. Using a post hoc Tukey's test, we observed L. perugiae females prefer small males over both intermediates and large males (p = .02; p = .04; Figure 3a). Neither L. zonata nor L. dominicensis displayed a preference for male body size (p = .94; Figure 3b; p = .90; Figure 3c). In all three species, neither presentation order, female identity, nor female size significantly influenced female preference. The Bayes factor analysis revealed substantial evidence for L. perugiae's preference for small males (BF = 4.81), as well as anecdotal evidence for no relationship between female preference and male size in both L. dominicensis (BF = 0.51) and L. zonata (BF = 0.35).
| npar | AIC | BIC | logLik | deviance | Chisq | Df | Pr(>Chisq) | |
|---|---|---|---|---|---|---|---|---|
| Male size | 4 | 2109.833 | 2122.605 | −1050.917 | 2101.833 | NA | NA | NA |
| Species | 4 | 2096.207 | 2108.978 | −1044.103 | 2088.207 | 13.626730 | 0 | NA |
| Male size + ID | 5 | 2103.842 | 2119.807 | −1046.921 | 2093.842 | 0.000000 | 1 | 1.00000000 |
| Species + ID | 5 | 2095.121 | 2111.086 | −1042.560 | 2085.121 | 8.721572 | 0 | NA |
| Male size * Species | 6 | 2097.309 | 2116.467 | −1042.655 | 2085.309 | 0.000000 | 1 | 1.00000000 |
| Male size * Species + ID | 7 | 2095.749 | 2118.100 | −1040.875 | 2081.749 | 3.560017 | 1 | 0.05918715 |
Note
- Explanatory variables were male size and species. Female identity was included as a random factor. The inclusion of female size and male presentation order were considered as random factors but were removed a priori to ensure non-singularity.
4 DISCUSSION
When comparing female preference for male size, we expected female preference to scale with male secondary sexual traits. Female L. zonata associated with males more than the other two species; however, they did not demonstrate a preference for male size. Limia perugiae, the species with the most secondary sexual traits, was the only species to display female preference, providing support to our hypothesis that female preference should scale with male SST. However, L. perugiae females preferred small males, which tend to be minimally ornamented and rely on coercion to secure matings (Farr, 1984). Together with our results, this suggests that pre-copulatory female preferences are not maintaining male ornaments in these species (Figure 3).
The results of the present study may have been influenced by the design of our experiment. Absolute preference function assays introduce males sequentially to females and remove social information females may use to evaluate mates. However, we aimed to understand the preferences underlying female mating decisions, and by controlling for social information via sequential exposure of males, we removed the element of choice present in other methodologies. Our experiment would have benefited from allowing sensory cues other than only visual. Poeciliiids such as Poecilia chica and P. sphenops use chemical and tactile feedback, respectively, when assessing mates (Brett & Grosse, 1982; Schlupp et al., 2010). Although, the bulk of poeciliid studies have found very strong responses using only visual cues. Indeed, one study found that visual information was the strongest when comparing multiple sensory channels (Makowicz et al., 2016). Finally, live males introduce variation in individual stimuli which is difficult to control. For example, L. perugiae females' preference for small males may be indicative of an increase of reproductive effort on behalf of males via courtship in an environment where predation risk is minimal. Despite this possibility, studies using live individuals in preference assays have demonstrated live individuals do not compromise the integrity of preference studies (Fisher et al., 2009).
In the bigger picture, it is well-documented in livebearing fishes and beyond that females tend to prefer large male body size and heavily ornamented males (Bisazza & Marin, 1991; Fernandez & Bowser, 2010; Hughes, 1985; Plath et al., 2004). Moreover, females across many taxa show a consistent pattern of preference for larger mates (Ryan & Keddy-Hector, 1992). Females of a closely related species, also from Hispaniola, Limia nigrofasciata, show a clear preference for large males (Holz, 2015), which makes our result stand out as very unusual. The lack of pre-copulatory female preference for larger males within the Limia species studied here raises the important question: How are male ornaments seemingly uninvolved in female preference evolved and maintained? We offer two hypotheses for the maintenance of ornamentation in the absence of female pre-copulatory preference. First, females may exhibit cryptic preferences, as opposed to pre-copulatory choice, wherein females seemingly mate indiscriminately and bias male paternity towards preferred males via selecting sperm from preferred males. Secondly, male secondary sexual characters, like body size, could be the product of alternative selective forces or may not even be adaptive.
Cryptic female choice in livebearing fishes tends to be expressed as a bias towards the sperm of the preferred male being retained in the female reproductive tract (Firman et al., 2017). The higher retention of preferred male sperm leads to biases in offspring paternity toward preferred mates. This mating strategy is particularly effective in species where female pre-copulatory preferences are suppressed due to male harassment and forced copulations, like in many livebearing fishes (Plath et al., 2007). We hypothesize that cryptic female choice is a mechanism maintaining male secondary sexual traits in Limia. Initial work by Schartl et al. (1993) found that L. perugiae non-dominant males are more reproductively successful than dominants. However, it is inconclusive whether this is a product of cryptic female preference or male behaviour. Therefore, we suggest an experimental design that tests if male secondary sexual traits are positively correlated with a bias in sperm retention in the female reproductive tract in Limia.
Given the relatively recent diversification of Limia found on Hispaniola (Weaver et al., 2016), we would be remiss in not acknowledging that there are a variety of other evolutionary alternatives we are unable to mention here, which could result in sexual dimorphism in Limia (Bisazza, 1993). Our study assumed, as with many other taxa, that females were evaluating male secondary sexual characters. Therefore, male secondary sexual characters are maintained through mate choice. Our results suggest this may not be the case. Instead, the sexual dimorphism found in Limia could be a means of hybrid avoidance, male–male competition, or even a vestigial trait (Berglund et al., 1996; Liou & Price, 1994). Further study of female L. perugiae’s preference for small males, in particular, may shed light on the unusual preferences we find. It is worth-noting that the consideration of these alternative research opportunities would not be possible without the use of an animal system where there is limited knowledge. Future work in Limia provides an excellent opportunity to assess the ubiquity of the phenomena described in well-researched taxa.
ACKNOWLEDGEMENTS
We thank Rodet Rodriguez-Silva, Amber Makowicz, Romy Fawaz, Margaret Zwick, and Zeeshawn Beg, for assistance with fish care and feedback on previous versions of this manuscript. George Martin kindly built the Plexiglas cylinders. This work was approved by the University of Oklahoma IACUC (R17-014). This is contribution #1 of the International Stock Center for Livebearing Fishes at the University of Oklahoma.




