Change in Structural Connectivity Following Stereotactic Thermocoagulation in Mesial Temporal Lobe Epilepsy Patients
Funding: This study was supported by grants from Joint Funds for the Innovation of Science and Technology of Fujian Province (grant number: 2018Y9059), Fujian Provincial Natural Science Foundation Program (grant number: 2021J01788) and Fujian Provincial Key Clinical Specialty Construction Project (Official document no. 884 [2022]).
ABSTRACT
Aims
To examine the association between postoperative lesions in distinct ROIs of the brain and the impact that their ablation would have on the structural and functional brain connectivity relative to outcomes.
Methods
We retrospectively reviewed 21 patients with refractory unilateral MTLE. The percentage of each ablated gray matter region of interest (ROIs) was calculated, using a voxel-by-voxel comparison. The percentage of the affected fibers was calculated by assessing the neuronal change reflected by a decrease in anisotropy in the repeat scans (i.e., pre and postoperative). Graph theory analysis was used to investigate the change in the pre and postoperative structural and functional networks between the seizure-free and non-seizure-free groups.
Results
Fifteen patients (71.42%) were seizure-free and six (28.57%) were non-seizure-free at a 12 to 48 months (23.80 ± 8.93) follow-up. Four patients (19.04%) reported memory decline following RFTC. The seizure-free group showed a larger ablation volume of both the amygdala (p = 0.024) and rhinal cortex (p = 0.035), and an alteration in structural connectivity networks metrics (p < 0.05) compared to the non-seizure-free group.
Conclusions
Our study shows that a higher ablation of both the amygdala and rhinal cortex led to improved structural connectivity and was associated with better outcomes. Our results provide insight into some essential elements of brain connectivity networks in MTLE and might contribute to the generation of novel evidence that could improve SEEG-guided RFTC interventions in MTLE patients.
1 Introduction
Treatment of mesial temporal lobe epilepsy (MTLE) is predominantly surgical, with the goal of completely removing or disconnecting the epileptogenic zone (EZ) [1]. Anterior temporal lobectomy (ATL) and selective amygdalohippocampectomy (SAH) are proven effective therapies for medically refractory MTLE, with 60%–80% of patients experiencing seizure freedom [2, 3], but they can result in possible side effects such as cognitive impairment, visual field defects (VFDs), intracranial hemorrhage, and neurological damage [4]. Thus, minimally invasive techniques such as stereoelectroencephalography-guided radiofrequency thermocoagulation (SEEG-guided RFTC) and magnetic resonance-guided laser interstitial thermal treatment (MRgLITT) provide promising alternatives to conventional surgery, resulting in less patient discomfort, shorter hospitalizations, better brain function preservation, and fewer surgical complications [5, 6].
Despite the curative nature of MTLE surgery, 30%–40% of patients continue to experience recurrent seizures with loss of awareness [7]. And those who achieve seizure freedom may see a gradual decline in their seizure freedom rate over time following either resective surgery [8, 9], or SEEG-guided RFTC [10]. The reasons for suboptimal surgical outcomes are complicated, and several studies have examined presurgical parameters that may be linked to seizure freedom. Magnetic resonance imaging (MRIs) showing unilateral hippocampal sclerosis (HS) frequently show excellent postoperative outcomes [11, 12], anatomical resection site and extent have been found to be important predictors of good outcomes, with seizure freedom requiring hippocampus removal. [13, 14] However, more recent research has questioned this idea [15, 16] and has shown that additional mesial structures, such as the entorhinal [17] and piriform cortices [18], which may independently cause or sustain TLE seizures [19], may also affect outcomes. Finally, many recent whole-brain neuroimaging investigations have shown that the structural connectivity (SC) and functional connectivity (FC) of the mesial temporal lobe can also promote TLE seizures when compared to a healthy control group [20-23]. However, these aforementioned investigations only included patients who underwent resective surgery and could therefore only study the postoperative brain connectivity of the regions spared during surgery, or the contralateral brain side of the affected regions of interest (ROIs).
During SEEG-guided RFTC, the ablation of the different ROIs is often only partial, leaving certain sections of these regions intact. In the case of MTLE, the analysis of the alterations in SC and FC between the preoperative ROIs and their respective postoperative remaining portions might be clinically relevant in explaining the reasons for suboptimal surgical outcomes in patients undergoing SEEG-guided RFTC. To date, no studies have assessed the association between the FC and SC alterations and postoperative outcome in MTLE patients who underwent SEEG-guided RFTC, using their preoperative and postoperative remaining ROIs.
This retrospective study aims to examine the association between postoperative lesions in distinct ROIs of the brain and the impact that their ablation would have on the structural and functional brain connectivity relative to outcomes. Based on the evidence supporting the importance of various mesial temporal cortical structures in seizure onset in MTLE [13-19], we hypothesized that a larger surgical ablation of specific ROIs within the temporal lobe could have led to the positive postoperative outcome seen in those experiencing seizure freedom. We further hypothesized that the ablation of these ROIs could have altered their brain connectivity, potentially leading to improved surgical outcomes. We employed a pre and postoperative voxel-by-voxel ablation mapping comparison approach, along with a structural and functional connectivity alterations analysis of the ROIs relative to the postoperative outcomes to test these hypotheses.
2 Methods
2.1 Patients Selection
We performed a retrospective study on patients with MTLE who met the International League Against Epilepsy's definition of drug-resistant epilepsy (DRE) [24]. The study was conducted following the ethical standards of the Declaration of Helsinki and was approved by the Ethics Committee Board of Fujian Medical University Union Hospital (No. 2024KY152), and all participants and the parents or guardians provided informed consent. From October 2019 to January 2023, we reviewed the medical records and video-SEEG recordings of 21 patients (12 males and 9 females) who underwent SEEG-guided RFTC. Patients were selected based on the following criteria: 1- Suspected unilateral MTLE based on ictal temporal lobe epileptiform discharges on video-EEG (VEEG) monitoring later confirmed by the presence of ictal and interictal discharges captured by SEEG; 2- Inadequate response to two anti-seizure medications (ASMs); 3- History of focal seizures with MTLE semiology; 4- Minimum 1-year follow-up; and 5- Declined resective surgery. Patients with tumors, cavernous malformations, arteriovenous malformations, temporal plus epilepsy, infarctions, and dual pathology were excluded. Every patient underwent a thorough preoperative evaluation, which included semiology analysis, scalp VEEG, neuropsychology testing, MRIs, and positron emission tomography (PET).
2.2 MRI Acquisition
All image acquisitions were conducted on a 3 T MR scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) equipped with a 64-channel head coil (Appendix S1 for more details).
2.3 SEEG Implantation and RFTC Procedure
SEEG-guided RFTC was recommended as a surgical option to the included patients after their refusal to undergo resective surgery. The electrode placement was based on preoperative hypotheses derived from semiology, scalp EEG, MRI, and PET findings regarding the localization of the EZs. Avascular electrode trajectories were planned using MRI and magnetic resonance angiography (Sinovation Medical, Beijing, China). Since all patients were suspected of MTLE, electrode implantation focused on the temporal lobe, including the hippocampus, amygdala, rhinal cortex, parahippocampal cortex, and temporal gyri (superior, middle, inferior) (Figure 1) (Appendix S1 for more in-depth details).
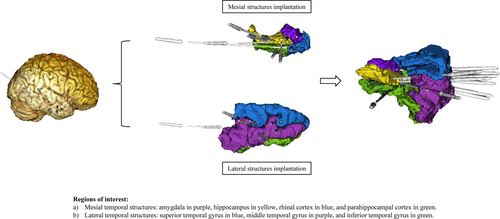
2.4 RFTC Procedure
RFTC was performed without anesthesia using a radiofrequency generator (R2000B-M1, BNS, Beijing). A 7.5 W output was used for 40 s to generate lesions between two adjacent contacts of the selected electrodes, or between two adjacent contacts of different electrodes which exhibited notable patterns of ictal onset, interictal discharges, or electrically elicited seizures during SEEG recordings. A distance of ≥ 3.5 mm away from the optic radiation (OR) on the targeted electrodes contacts was required as our selection criterion prior to performing RFTC, enough to prevent VFDs [25]. Electrodes were removed under anesthesia 1–2 days post-procedure, and the patients were subsequently discharged.
2.5 Post-RFTC Outcome and Data Analysis
Outcomes were assessed at 1, 3, 6 months, and annually. Patients were categorized into seizure-free (SF) (Engel IA) and non-seizure-free (NSF) (Engel ≠ IA) at least 1 year after the procedure. The anatomical location of the thermolesions was confirmed using a 1-year post-RFTC brain MRI.
2.6 Gray and White Matter Volumetric Analysis
2.6.1 Preoperative Image Processing and Parcellation
Each patient's preoperative T1-weighted (T1W) MRI underwent automatic parcellation with standard anatomic definitions using FreeSurfer version 7.4.0, which is documented and freely available for download online (https://surfer.nmr.mgh.harvard.edu/) [26, 27]. The postoperative MRIs were co-registered to their preoperative MRIs using SPM12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/). (Appendix S1 for more in-depth details).
2.6.2 Manual Segmentation of Post-RFTC Cavities
Using each patient's 1-year postoperative T1W MRI, two investigators independently performed the manual segmentation of the post-RFTC cavities using 3D Slicer version 5.2.2 (http://www.slicer.org) (Figure 2) (Appendix S1 for more in-depth details).
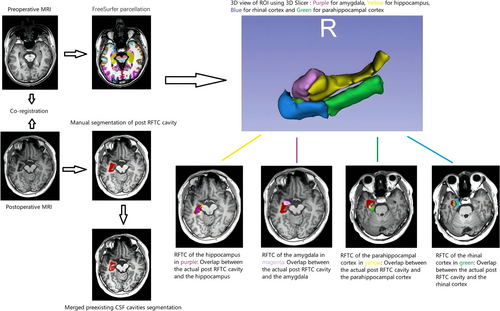
2.6.3 Choice of ROIs and RFTC Volumetric Analysis
All of the seven ROIs that were implanted were selected: the left or right (according to the RFTC side) hippocampus, amygdala, rhinal cortex, parahippocampal cortex, superior, middle, and inferior temporal gyrus. The “Subtract Scalar Volumes” module from 3D Slicer was used in order to obtain the postoperative ROIs. This module substrates each ablated portions structures from their respective preoperative structures, outputting the postoperative remaining portions of the different ROIs. (Appendix S1 for more in-depth details).
2.6.4 White Matter Ablation Analysis
Differential tractography [28] using the February 8th, 2023, build of DSI Studio was used for the volumetric analysis of the different affected fibers associated with the ROIs (https://dsi-studio.labsolver.org/doc/gui_t3_dt.html) (Appendix S1 for more in-depth details).
2.7 Brain Connectivity Analysis
2.7.1 Structural Connectivity Workflow
Each patient's preoperative and 1-year postoperative diffusion weighted imaging (DWI) data were used for the structural connectivity (SC) analysis. The connectivity matrix and network measures (clustering coefficient average, characteristic/average path length, small-worldness and global efficiency) using DSI Studio (https://dsi-studio.labsolver.org/doc/gui_t3_whole_brain.html) (Appendix S1 for more in-depth details). The weighted version of the network measures was employed due to its capacity to encompass a greater amount of information compared to its unweighted (binary) equivalent [29, 30].
2.7.2 Functional Connectivity Workflow
Using the CONN toolbox v22a (Appendix S1), running in a MATLAB R2022a environment (MathWorks, MA, USA) [31], each patient's preoperative and 1-year postoperative BOLD data were used for the functional connectivity (FC) analysis (Appendix S1 for more in-depth details). The FC analysis incorporates both first- and second-level analyses. In the second-level analyses, we used graph theory analysis (network edges were thresholded at a cost of 0.15 one-sided, positive and assessed at a corrected analysis threshold p-FDR < 0.05, two-sided) to measure the network of all the ROIs in each patient following similar studies [31, 32].
3 Statistical Analysis
Ablation volumes of different ROIs were calculated using 3D Slicer. Statistical analyses were performed using SPSS (version 27); two-sided p < 0.05 was considered significant (95% CIs). Mann–Whitney tests and Spearman's rank correlation were used to examine associations between ablation volumes and outcomes. Fisher exact test was used for categorical data, and Wilcoxon Signed-Ranks tests for the analysis of SC and FC alterations in SF and NSF patients.
4 Results
4.1 Patients Demographics
There were 12 males and 9 females, mean age 28.61 ± 14.34 years. A total of 14 patients (66.66%) underwent ablation on both the mesial temporal lobe and the lateral temporal lobe. The remaining 7 patients (33.33%) only underwent ablation on the mesial temporal lobe. A total of 12 patients experienced secondary generalization. Secondary generalization had a negative correlation (r = −0.548) with seizure outcome (p < 0.05) (Table 1). The seizure frequency varied considerably across all individuals, from several per month to many per day. MRI findings were negative (absence of HS during preoperative visual inspection) in 11 patients. The presence of HS was associated with outcomes (p = 0.003) and was negatively correlated (r = −0.663. p = 0.001) (Tables 1 and 2).
| Parameters/(M ± SD) | r | p |
|---|---|---|
| Hippocampus | 0.191 | 0.405 |
| Amygdala | 0.505 | 0.020 |
| Rhinal cortex | 0.471 | 0.030 |
| Parahippocampal cortex | 0.034 | 0.881 |
| Lateral temporal lobe (%) | 0.027 | 0.909 |
| Anterior commissure | 0.139 | 0.547 |
| Arcuate fasciculus | 0.070 | 0.763 |
| Cingulum Parahippocampal Parietal | −0.263 | 0.249 |
| Corpus callosum body | −0.035 | 0.880 |
| Corpus callosum forceps major | −0.335 | 0.138 |
| Corpus callosum tapetum | −0.279 | 0.221 |
| Fornix | 0.367 | 0.102 |
| Inferior fronto-occipital fasciculus | 0.279 | 0.220 |
| Inferior longitudinal fasciculus | 0.035 | 0.881 |
| Middle cerebellar peduncle | 0.090 | 0.700 |
| Middle longitudinal fasciculus | 0.109 | 0.639 |
| Superior longitudinal fasciculus | −0.378 | 0.091 |
| Thalamic radiation anterior | −0.056 | 0.808 |
| Thalamic radiation superior | −0.177 | 0.442 |
| Uncinate fasciculus | 0.238 | 0.299 |
| Epilepsy duration | 0.052 | 0.822 |
| Lateral surface of the temporal lobe ablation | 0.224 | 0.330 |
| Sex | −0.122 | 0.599 |
| Surgical side | 0.030 | 0.897 |
| Follow-up | −0.044 | 0.851 |
| Secondary generalization | −0.548 | 0.010 |
| Hippocampal sclerosis | −0.663 | 0.001 |
| Parameters/(M ± SD) | Outcome | p | |
|---|---|---|---|
| NSF | SF | ||
| Hippocampus (%) | 19.61 ± 8.58 | 24.90 ± 9.40 | 0.392 |
| Amygdala (%) | 11.71 ± 11.29 | 26.22 ± 10.83 | 0.024 |
| Rhinal cortex (%) | 1.55 ± 2.21 | 9.85 ± 9.85 | 0.035 |
| Parahippocampal cortex (%) | 5.25 ± 4.62 | 5.46 ± 5.40 | 0.876 |
| Lateral temporal lobe (%) | 1.45 ± 1.60 | 1.58 ± 1.82 | 0.905 |
| Anterior commissure (%) | 6.96 ± 8.92 | 8.23 ± 9.16 | 0.533 |
| Arcuate fasciculus (%) | 1.60 ± 1.57 | 2.49 ± 3.13 | 0.754 |
| Cingulum parahippocampal parietal (%) | 2.29 ± 2.48 | 0.95 ± 1.37 | 0.240 |
| Corpus callosum body (%) | 7.11 ± 7.84 | 7.42 ± 10.17 | 0.875 |
| Corpus callosum forceps major (%) | 15.26 ± 15.95 | 4.82 ± 4.84 | 0.135 |
| Corpus callosum tapetum (%) | 12.13 ± 7.42 | 7.90 ± 6.77 | 0.213 |
| Fornix (%) | 2.81 ± 3.96 | 7.81 ± 8.93 | 0.101 |
| Inferior fronto-occipital fasciculus (%) | 1.55 ± 1.74 | 5.12 ± 6.11 | 0.211 |
| Inferior longitudinal fasciculus (%) | 12.88 ± 12.08 | 14.69 ± 12.50 | 0.876 |
| Middle cerebellar peduncle (%) | 1.50 ± 2.03 | 3.70 ± 4.99 | 0.689 |
| Middle longitudinal fasciculus (%) | 2.22 ± 4.18 | 2.80 ± 3.68 | 0.627 |
| Superior longitudinal fasciculus (%) | 3.26 ± 5.65 | 0.87 ± 1.79 | 0.091 |
| Thalamic radiation anterior (%) | 1.54 ± 1.76 | 1.56 ± 2.38 | 0.801 |
| Thalamic radiation superior (%) | 1.93 ± 1.89 | 1.04 ± 1.34 | 0.428 |
| Uncinate fasciculus (%) | 5.00 ± 9.42 | 5.57 ± 7.25 | 0.288 |
| Epilepsy duration (months) | 12.50 ± 11.74 | 12.33 ± 9.01 | 0.815 |
| Follow-up (months) | 25.50 ± 11.70 | 23.13 ± 7.95 | 0.846 |
| Parameters/n (%) | Outcome | p | |
|---|---|---|---|
| NSF | SF | ||
| Lateral surface of the temporal lobe ablation | |||
| Yes | 3 (50.00) | 11 (73.33) | 0.354 |
| No | 3 (50.00) | 4 (26.66) | |
| Sex | |||
| Male | 4 (66.66) | 8 (53.33) | 0.659 |
| Female | 2 (33.33) | 7 (46.66) | |
| Surgical side | |||
| Right | 3 (50.00) | 8 (53.33) | 1 |
| Left | 3 (50.00) | 7 (46.66) | |
| Secondary generalization | |||
| With | 5 (83.33) | 7 (46.66) | 0.177 |
| Without | 1 (16.66) | 8 (53.33) | |
| Hippocampal sclerosis | |||
| With | 6 (100) | 4 (26.66) | 0.003 |
| Without | 0 (0) | 11 (73.33) | |
- Note: Bold values indicates statistical significance p > 0.05.
4.2 Outcome and Morbidity Following RFTC
Post-RFTC follow-up ranged from 12 to 48 months (23.80 ± 8.93). A total of 271 electrodes (12.90 ± 2.25 per patient) and 3257 electrode contacts (155.09 ± 27.14 per patient) were implanted in 11 patients in the right hemisphere and 10 patients in the left hemisphere. After coagulation, MRI revealed confluent or isolated regions of necrosis that were easily visible among contacts coagulated along the electrode trajectories. At the last follow-up visit, 15 patients (71.42%) were seizure-free and 6 (28.57%) were non-seizure-free. Four patients (19.04%) reported memory decline following RFTC (Table 3).
| Patient no. | Seizure duration (years) | Seizure frequency | MRI | RFTC side | Number of electrodes | Responder to RFTC | Transient complications | Follow-up (months) | RFTC outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 11 | Monthly | HS | Right | 11 | NSF | Memory decline | 26 | Engel IIA |
| 2 | 7 | Monthly | Negative | Right | 16 | SF | No | 14 | Engel IA |
| 3 | 11 | Monthly | Negative | Left | 13 | SF | No | 17 | Engel IA |
| 4 | 36 | Weekly | HS | Left | 12 | NSF | No | 14 | Engel IIA |
| 5 | 1 | Monthly | Negative | Right | 10 | SF | No | 31 | Engel IA |
| 6 | 10 | Monthly | HS | Right | 14 | NSF | No | 21 | Engel IIA |
| 7 | 5 | Daily | HS | Right | 13 | NSF | No | 23 | Engel IIA |
| 8 | 23 | Monthly | Negative | Right | 15 | SF | Memory decline | 28 | Engel IA |
| 9 | 1 | Daily | HS | Left | 19 | SF | No | 25 | Engel IA |
| 10 | 6 | Weekly | Negative | Right | 10 | SF | No | 34 | Engel IA |
| 11 | 24 | Monthly | Negative | Right | 14 | SF | No | 12 | Engel IA |
| 12 | 28 | Monthly | Negative | Left | 14 | SF | No | 38 | Engel IA |
| 13 | 6 | Monthly | HS | Left | 10 | NSF | No | 21 | Engel IB |
| 14 | 24 | Monthly | HS | Right | 10 | SF | No | 28 | Engel IA |
| 15 | 3 | Monthly | Negative | Right | 13 | SF | Memory decline | 22 | Engel IA |
| 16 | 13 | Monthly | Negative | Left | 12 | SF | No | 16 | Engel IA |
| 17 | 12 | Monthly | HS | Left | 14 | SF | No | 29 | Engel IA |
| 18 | 6 | Monthly | HS | Left | 12 | SF | No | 20 | Engel IA |
| 19 | 7 | Monthly | HS | Left | 14 | NSF | Memory decline | 48 | Engel IIA |
| 20 | 8 | Monthly | Negative | Left | 14 | SF | No | 15 | Engel IA |
| 21 | 18 | Monthly | Negative | Right | 11 | SF | No | 18 | Engel IA |
- Note: Bold values indicates statistical significance p > 0.05.
- Abbreviations: F, female; HS, hippocampal sclerosis; M, male; NSF, non-seizure-free; SF, seizure-free.
4.3 Association Between Seizure Outcome and Ablation Volume
A larger ablation volume of both the amygdala and rhinal cortex was associated with a better post-RFTC outcome (p < 0.05). There was no association between seizure outcome and ablated fibers (p > 0.05) (Table 3). The ablation of the amygdala and rhinal cortex was statistically correlated with postoperative outcome (p < 0.05). There was no correlation between the seizure outcome and the ablated fibers (p > 0.05) (Table 2). (Scatter plots in Appendix S1).
4.4 Structural and Functional Connectivity Alterations
In terms of structural connectivity findings, postoperatively, the SF group showed a significant decrease in clustering coefficient average, small-worldness, and global efficiency (p < 0.05), and a significant increase in path length (p = 0.012), whereas the NSF group showed no statistically significant changes (p > 0.05) (Table 4).
| Network metrics/(M ± SD) | NSF | SF | ||
|---|---|---|---|---|
| Pre/post | p | Pre/post | p | |
| Cluttering coefficient average | 0.20 ± 0.05/0.23 ± 0.03 | 0.249 | 0.27 ± 0.05/0.20 ± 0.07 | 0.023 |
| Characteristic path length | 3.56 ± 0.82/3.37 ± 0.64 | 0.753 | 2.89 ± 0.46/4.81 ± 2.53 | 0.012 |
| Small-worldness | 0.06 ± 0.03/0.07 ± 0.02 | 0.600 | 0.09 ± 0.04/0.05 ± 0.04 | 0.027 |
| Global efficiency | 0.36 ± 0.06/0.40 ± 0.05 | 0.345 | 0.44 ± 0.06/0.35 ± 0.09 | 0.012 |
Characteristic path length measures the average shortest path length between all pairs of nodes in a network; an increase indicates a decline in network connectivity. Clustering coefficient measures the extent to which regions cluster, providing a measure of local connectivity (network) and how close a given node's neighbors are to forming a clique, reduction in this coefficient implies diminished interconnectivity among the regions. Global efficiency measures the network's ability for parallel information transfer; a decline in this metric indicates a reduction in the brain's efficacy in interregional communication. Lastly, small-worldness is the ratio of the clustering coefficient to the characteristic path length and is dependent on both the clustering coefficient and the characteristic path length measure; its reduction can be achieved by increasing either the characteristic path length, decreasing the clustering coefficient, or both. There was a significant decrease in connectivity between the ROIs in the SF group.
In the other hand, postoperatively, both the SF and NSF group showed no significant functional network alterations in any of the network measures (p > 0.05) (Table 5). (Scatter plots in Appendix S1).
| Network metrics/(M ± SD) | NSF | SF | ||
|---|---|---|---|---|
| Pre/post | p | Pre/post | p | |
| Clustering coefficient average | 0.17 ± 0.40/0.00 ± 0.00 | 0.317 | 0.27 ± 0.45/0.13 ± 035 | 0.157 |
| Average path length | 1.36 ± 0.33/1.27 ± 0.19 | 0.516 | 1.42 ± 0.31/1.33 ± 0.24 | 0.404 |
| Small-worldness | 0.17 ± 0.40/0.00 ± 0.15 | 0.317 | 0.27 ± 0.45/0.13 ± 0.35 | 0.865 |
| Global efficiency | 0.17 ± 0.03/0.17 ± 0.01 | 0.516 | 0.18 ± 0.02/0.18 ± 0.02 | 0.157 |
5 Discussion
This study employed a voxel-by-voxel ablation mapping comparison, along with the structural and functional connectivity of the selected ROIs, to examine the impact that the ablation of different temporal lobe ROIs would have on the structural and functional brain connectivity relative to outcomes in a consecutive group of refractory MTLE patients who underwent SEEG-guided RFTC. We found that a better outcome was associated with a larger ablation volume of both the amygdala and rhinal cortex and with an alteration in structural connectivity networks. We also found no statistically significant alterations in functional connectivity network measures in either group.
Previous studies have shown a significant association between the extent of entorhinal and parahippocampal cortex resection and the outcome [17, 33]. Keller et al. studied 87 TLE patients (47 SF, 40 NSF) and found that SF patients tended to have a larger resection volume of the entorhinal cortex and amygdala compared to NSF [34]. Oren Sagher et al. reviewed 96 refractory MTLE patients; standard ATL was conducted in 51 nondominant TLE patients, while 45 language-dominant patients underwent transcortical SAH [35]. Two years post-surgery, 86%–90% of patients with ATL or SAH were seizure-free, with up to 90% of the amygdala, hippocampal, and entorhinal cortex removed, regardless of the technique. They pointed out that their study could not determine if successful seizure control required a specific resection extent as their patients had 90% or more of their mesial structures removed. It was impossible to evaluate the correlation between less extensive resection and a poorer seizure outcome. A recent study of 107 patients with unilateral TLE by Marian Galovic et al. found that removal of at least 50% of the piriform cortex was necessary for achieving seizure freedom after temporal lobe resections [18]. Removing other mesial structures wasn't associated with seizure control. In a recent study of 113 patients with TLE, Ezequiel Gleichgerrcht et al. reported that anterior hippocampus-amygdala-piriform or entorhinal-perirhinal resection and disconnection of the frontal, limbic, and temporal regions through white matter tract loss within the fornix, anterior commissure, and uncinate fasciculus improved the seizure outcome [36]. However, they could not ascertain if removing these sites alone or together improved surgical outcomes. Our results showed that increased ablation of the amygdala and rhinal cortex contributed to a positive outcome. However, as in other studies, we couldn't confirm if ablating both the amygdala and rhinal cortex alone provided surgical freedom or if their combined ablation with the other ROIs improved outcomes. Postoperatively, none of the white matter tracts examined using differential tractography demonstrated a significant correlation with seizure outcomes. The absence of correlation is probably due to the analogous and restricted degree of damage sustained by these tracts during RFTC in both cohorts.
Due to the significant importance of the piriform cortex [18, 37, 38], we examined the potential unintentional ablation of some parts during the thermocoagulation of the amygdala and rhinal cortex due to its closeness to these structures. The piriform cortex lies at the junction of the frontal and temporal lobes, medial to the temporal stem, and lines the superior and inferior banks of the rhinal sulcus in humans. It features a U-shaped cross section that curves around the middle cerebral artery in coronal slices. We manually carried out its segmentation using a protocol based on anatomical landmarks and correlation with histology (Appendix S1). We observed that none of its portions were affected by thermocoagulation, likely due to our reluctance to place electrodes close to the middle cerebral artery during preoperative planning.
Most studies have reported on the structural brain networks connectivity of temporal lobe epilepsy patients relative to healthy control groups using graph theory analysis, MRI data, and machine-learning algorithm [20, 23, 39-42]. But few have analyzed the SC alterations relative to outcomes [20, 23, 42, 43]. Taylor et al. reported on 53 TLE patients who underwent temporal lobe epilepsy surgery, and using graph theory and machine learning, they investigated the properties of change between the preoperative and predicted postoperative networks [20]. They found that seizure-free patients had greater reductions of connectivity. Bernhardt et al. examined 122 patients with drug-resistant TLE and 47 healthy controls, using MRI-based cortical thickness measurements [42]. Their study showed that an increase in clustering coefficient and path length was associated with decreased postoperative seizure freedom. Bonilha et al. reviewed 20 patients with refractory MTLE and a matched 18 healthy control group [23]. Their findings revealed that when compared to controls, patients had a decrease in ipsilateral thalamocortical connection and a pathological increase in medial temporal, insular, and frontal connectivity. Ko et al. reported on the seizure outcomes of 24 MTLE patients who underwent LITT and whether structural connectomes could predict surgical outcomes [43]. They employed preoperative diffusion tensor imaging (DTI) to simulate changes in structural connectivity following laser ablation. They found that favorable SC changes in connection strength were associated with an ablation volume involving relatively mesial, anterior, and inferior locations.
Our results are in line with these findings despite our differences in methodology. Both Taylor and Ko et al. used machine learning and preoperative DWI to generate the structural white matter network, along with the postoperative T1W to infer the impact of surgical resection on this network. In this study, we used both the pre and postoperative DWI along with their respective pre and post T1W to more accurately analyze the SC alterations following surgery; we also employed diffusion spectrum imaging (DSI) tractography instead of DTI due to its reported generation of fewer false connections compared with DTI [44]. We found a significant decrease in SC in the SF group, whereas the NSF showed no alterations. We theorize that despite the similar extent of white matter injury incurred by the fiber tracts in both groups after RFTC, the ablation of the different gray matter ROIs in the SF patients, notably the amygdala and rhinal cortex, which showed higher ablation volume in the SF compared to the NSF group, might have been significantly sufficient to positively disrupt the SC to achieve seizure freedom, as opposed to the NSF patients. The smaller ablation volume of the amygdala and rhinal cortex might have been the major contributor to the non-significant alteration in SC seen in the NSF group.
As for SC analysis, few studies have investigated the FC in patients with TLE both before and after surgery, specifically focusing on the differences in connectivity in SF and NSF patients, with most conducting comparative analyses of patients relative to healthy control groups [45-49]. Maccotta et al. reported on the FC of 17 patients with refractory TLE who underwent resective surgery with an Engel I outcome and a matching group of 17 healthy controls [45]. Their findings revealed that, following epilepsy surgery, the seizure-free state of their patients did not result in substantial alterations in functional network connectivity. The functional network anomalies observed after the surgery closely resembled the preoperative condition. They theorized that epilepsy has a chronic effect on brain connectivity that is “burned in” by the time a patient with intractable TLE has surgery, which usually occurs years after diagnosis. Morgan et al. reported on the FC of 20 patients (9 SF, 11 NSF) with refractory TLE who underwent resective surgery and a matching 44 healthy controls [46]. Their findings revealed that, when examining the impact of FC at the regional level, no significant differences in FC were seen postoperatively in the individual regional connections among the groups. The SF group showed no significant difference in both the pre and postoperative FC when compared to the control group, whereas the NSF group, which preoperatively exhibited no discernible distinction from the control group, demonstrated a lower FC compared to the control group postoperatively. Conversely, a study by Simula et al. has shown that thermocoagulation by LITT elicited FC alterations in the electrical brain activity of individuals with DRE for a duration of at least 15 min [49]. Functional connectivity decreased in responders (delta band only) and increased for the majority of frequency bands in nonresponders following RFTC.
Our findings align with those of Maccotta and Morgan et al., although they differ from the conclusions presented by Simula et al. In their analysis, Maccotta et al. employed graph theory analysis and network measures using both the pre- and postoperative T1W and BOLD from patients with a follow-up of 2 years. However, Simula et al. employed local power spectral density (PSD) to assess FC alterations in a 3-min segments recorded immediately prior to (baseline), immediately following, and 15 min after RFTC. Their analysis only assessed short-term alterations in FC in different types of DRE patients (not specifically in TLE patients). These different findings at a first glance seem to be contradictory; however, they may in fact suggest that while a short-term alteration in FC after thermocoagulation might be observed in DRE patients, in the case of TLE patients specifically, this alteration seems to revert back to the prior preoperative brain network state in the long term, probably due to the “burned in” effect theorized by Maccotta et al. (Appendix S1 for more in-depth discussion).
Ablation of most of the entire mesial temporal lobe complex may not be needed to positively alter the SC in MTLE. Minimally invasive treatments on key therapeutic ROIs through surgical destruction may interrupt the occurrence or propagation of epilepsy, thereby achieving cessation of epileptic seizures [50].
6 Limitations
The current findings should be taken in light of the study's limitations. We didn't include a healthy control group, which could have been of significant interest to describe a graph theory measure's baseline. Our sample size was relatively small, resulting in the combination of left and right MTLE patients into one group. Other limitations may include the potential biases of DSI, such as challenges related to spatial resolution, partial volume effects, crossing fibers that may not be fully resolved, and the reliability of tracts that are very small. Finally, the generalizability of these findings may not be applicable to other epilepsy populations due to our sole inclusion of MTLE patients.
7 Conclusion
Our study provides insight into some essential elements of brain connectivity networks in MTLE and might contribute to the generation of novel evidence that could potentially facilitate progress in surgical procedures in the future and improve SEEG-guided RFTC interventions in MTLE patients who choose this treatment option. Further research is required to substantiate the validity of our findings via a more extensive set of data.
Author Contributions
Stéphane Jean: conceptualization, methodology, software, formal analysis, writing – original draft, writing – review and editing, investigation, data curation, validation. Rifeng Jiang: data curation, supervision, software, formal analysis. Yihai Dai: project administration, resources, supervision. Weitao Chen: project administration, resources, data curation. Weihong Liu: supervision, data curation, project administration. Donghuo Deng: data curation, resources. Panashe Tevin Tagu: writing – review and editing. Xiaoqiang Wei: data curation. Shan Chen: data curation. Xinrong Fang: data curation. Shiwei Song: conceptualization, funding acquisition, validation, visualization, supervision, resources, data curation, project administration, investigation.
Acknowledgements
This work was supported by grants from Joint Funds for the Innovation of Science and Technology of Fujian Province (grant number: 2018Y9059), Fujian Provincial Natural Science Foundation Program (grant number: 2021J01788) and Fujian Provincial Key Clinical Specialty Construction Project (Official document no. 884 [2022]). Declaration of generative AI and AI-assisted technologies in the writing process: We confirm that no artificial intelligence (AI) and AI-assisted technologies were used in the writing process.
Ethics Statement
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Consent
This study was conducted under the ethical standards of the Declaration of Helsinki. It was approved by the Ethics Committee Board of Fujian Medical University Union Hospital (2024KY152), and informed consent was obtained from all participants and the parents or guardians.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




