BRAF V600E Mutation in Ganglioglioma: Impact on Epileptogenicity and Implications for Surgical Strategy
Funding: This study was supported by Medical University of Vienna Comprehensive Center for Pediatrics Starter Grant.
ABSTRACT
Objective
Gangliogliomas are commonly found pathologies in patients undergoing epilepsy surgery. While resections can be curative, seizure relapses occur. Expression of CD34 and the BRAF V600E mutation are the most common molecular biomarkers found in gangliogliomas, but their influence on seizure outcomes is unclear. We therefore reviewed our experience over two decades to better describe prognostic factors.
Methods
We performed a retrospective chart review of all patients operated on for ganglioglioma at our institution since the year 2000. We included patients with preoperative epilepsy and a minimum follow-up of 1 year. Available tumor specimens were immunohistochemically stained for CD34 and BRAF V600E.
Results
We included 62 patients with epilepsy operated for ganglioglioma. Lesionectomies were performed in 32 (51.6%), extended resections in 21 (33.9%), and partial resections in 9 cases (14.5%). Residual tumor mass on postoperative MRI was diagnosed in 21 patients (33.9%). CD34 reactivity was found in 57 patients (91.9%) and the BRAF V600E mutation was detected in 30 patients (48.4%). Patients with a BRAF V600E mutation were younger at the time of epilepsy onset (9.1 years vs. 15.2 years) and surgery (14.5 years vs. 23.7 years). Residual tumor was the largest risk factor for seizure relapses (hazard ratio 8.45) and the BRAF V600E mutation also increased this risk (hazard ratio 3.94).
Conclusions
BRAF V600E status in patients with ganglioglioma-associated epilepsy is a potential biomarker to stratify the risk for seizure relapse after surgery. BRAF V600E-positive patients might benefit from a more aggressive surgical strategy.
1 Introduction
Gangliogliomas (GG) are glioneural, slowly growing tumors that often occur early in childhood or adolescence [1, 2]. Around 80%–90% of these tumors in supratentorial locations are associated with epilepsy, which often becomes refractory to anti-seizure medication [3]. Malignant transformation of these tumors is rare, so a major part of the associated high morbidity is explained through epileptic seizures and their sequelae [4-6]. Fortunately, surgical resection often provides a curative option for these patients. Indeed, GG is the most common brain tumor in pediatric patients undergoing surgery for intractable epilepsy [7]. However, even after presumed complete resection, seizure relapses and tumor recurrences can occur. Histological studies have shown that the borders of these tumors are less well defined than initially believed [8, 9]. Therefore, sparse resections, strictly limited to the visible lesion, which aim to preserve unaffected normal brain tissue, might miss microscopic extensions into the surrounding areas. Indiscriminate, large resections can, however, increase the risk for neurologic impairment [10, 11]. Balancing the need for a complete resection with the reluctance to cause a neurological or neuropsychological deficit is the dilemma in epilepsy surgery for ganglioglioma, and in many cases, clinical equipoise exists. To improve the decision-making process in these patients, prognostic markers are direly needed.
The last decade has seen growing interest in the stratification of tumors based on molecular markers as they sometimes describe tumor biology better than the histological description alone [12]. While the possible molecular alterations are vast and multifaceted, two biomarkers already well associated with GG are CD34 expression and BRAF V600E mutation [1, 13-16]. While there is evidence that the BRAF V600E mutation influences the oncological outcome [17], no study to date has been able to clearly show an influence of the molecular profile of GG on seizure outcome. Only a small number of studies exist, often with a small sample size or a heterogenous cohort of a mixture of glioneural tumors.
Therefore, we aimed to review our experience with GG and to correlate clinical data and molecular markers with the surgical outcome after GG resection in patients with epilepsy.
2 Methods
This study was approved by the Ethics Committee of the Medical University of Vienna (EK 1761/2020) and was performed in accordance with the Declaration of Helsinki. The need for informed consent was waived due to the retrospective nature.
We reviewed all histologic reports from 2000 to 2020 with a neuropathological diagnosis of ganglioglioma from a database of histological findings kept at the Department of Neurosurgery. Patients' charts were reviewed retrospectively for demographic and surgical details. We included patients with supratentorial, histologically verified ganglioglioma, who suffered from at least one epileptic seizure preoperatively, operated between 2000 and 2020 at our tertiary care center. For inclusion, the seizure frequency had to be clearly documented pre- and postoperatively. Previous surgery at our or an external center was acceptable for inclusion if surgical and clinical history since then was available. Follow-up periods are calculated from the index surgery. We grouped the extent of surgeries into partial resection, complete lesionectomy, and extended resection (e.g., partial lobe resection). The minimum follow-up after surgery was 1 year, and patients were followed until December 2023. Reported seizure frequency was averaged into a monthly frequency per patient, and surgical outcome was classified according to the ILAE surgical outcome scale [18], as we feel it is less ambiguous than the older Engel seizure outcome scale [19]. Early postoperative seizures in the first 14 days after surgery were regarded as neighborhood seizures and not counted as recurrences when patients were seizure-free thereafter. We reviewed MRI findings and performed volumetric measurements of tumor size as well as resection size using tools from the Brainlab Elements software (Brainlab AG, Munich, Germany). The extent of tumor and resection were measured on high-resolution T2-weighted images. Progression of residual tumor tissue was defined in line with the RANO criteria as the development of a new contrast-enhancing lesion or a size increase on T2-weighted images greater than 25% [20]. Development of a new lesion with or without contrast enhancement in proximity to the resection area where no residual tumor had been seen postoperatively was diagnosed as a tumor recurrence. The standard neuropathological workup of tumor samples was reviewed using pathology reports. Because tumors were resected and described until 2020, our manuscript still uses roman numerals when reporting the WHO grade. In older samples without BRAF status, immunohistochemistry with an antibody against mutant BRAF V600E protein was performed. To this end, formalin-fixed, paraffin-embedded blocks of tumor tissue were cut into 3 μm sections. Subsequently, the specimens were deparaffinized following a standard protocol with xylene and alcohol, followed by a treatment in sodium citrate solution with a pH of 6. The slides were incubated with an anti-CD34 antibody (NCL-L-END, Novocastra, Newcastle upon Tyne, UK) for 30 min at room temperature, and the Dako detection system (FLEX + Mouse, K8002) was used to perceive the antibody signal. For the staining with an antibody directed at the BRAF V600E mutation (VE1, 760-5095 Roche Holding AG, Basel, Switzerland), the automated Ventana BenchMark staining system was used with the UltraView DAB detection kit (760-500) according to the manufacturer's specifications. Counterstaining of all tissue specimens was performed manually with hematoxylin. We performed a visual qualitative analysis on the specimens categorizing CD34 and BRAF V600E mutation status as present or absent.
2.1 Statistics
Continuous variables are given with their median and interquartile range (IQR). Differences between groups were analyzed using Fisher's exact test for categorical and Mann–Whitney U or Kruskal–Wallis test for continuous variables. A Kaplan–Meier analysis and a log-rank test were used to find differences between subgroups' time until seizure relapse or tumor recurrence. The influence of variables on relapse-free survival was analyzed using univariate and multivariate Cox proportional hazard regression models.
Two-sided p-values < 0.05 were considered significant. Statistical analysis and plotting of data were performed using R version 4.1.2.
3 Results
From a database with 105 gangliogliomas operated since the year 2000, we identified 62 patients, who fulfilled our inclusion criteria. Twenty patients had inadequate pre- or postoperative documentation, nine patients had an infratentorial or spinal ganglioglioma, 11 patients were excluded for not having preoperative seizures, no specimen was available for two patients, and one patient with a complex genetic syndrome was excluded as the cause of his epilepsy could not be unequivocally tied to the GG. Median age at surgery was 16.1 years (total range: 4 months to 64.5 years) and our cohort consisted of 63.5% male patients. At the time of surgery, patients already had seizures for a median of 1.6 years (IQR 0.4–3.9 years). The median postoperative follow-up was 6.7 years (IQR 4.3–11.1 years). Preoperative MRI data in digital form were available for 53 patients in our cohort, and the median preoperative tumor size was 3.8 cm3 (IQR 1.7–9.5 cm3) on T2-weighted imaging. In this cohort of supratentorial GG, 73.0% were located in the temporal lobe, and 56.5% of tumors were on the right side. Further clinical and radiological data can be found in Table 1.
| BRAF V600E negative, n = 32 | BRAF V600E positive, n = 30 | p | |
|---|---|---|---|
| Median age at surgery in years (IQR) | 23.7 (13.2–32.4) | 14.1 (7.9–20.1) | 0.019 |
| Median age at epilepsy onset in years (IQR) | 15.2 (8.4–25.9) | 9.5 (3.9–15.1) | 0.032 |
| Median duration of epilepsy in years (IQR) | 1.2 (0.4–4.8) | 1.8 (0.4–3.8) | 0.854 |
| Side | |||
| Left | 12 (37.5%) | 15 (50.0%) | 0.443 |
| Right | 20 (62.5%) | 15 (50.0%) | |
| Localization | |||
| Frontal | 5 (15.6%) | 2 (6.67%) | 0.332 |
| Insular | 0 | 2 (6.67%) | |
| Occipital | 2 (6.25%) | 0 | |
| Parietal | 3 (9.38%) | 3 (10.0%) | |
| Temporal | 22 (68.8%) | 23 (76.7%) | |
| CD34 reactivity | 27 (84.4%) | 30 (100%) | 0.053 |
| Monthly preoperative seizure frequency | 1.8 (0.6–10.5) | 5.3 (1.0–32.4) | 0.125 |
| Preoperative tumor size (cm3) | 4.0 (2.3–10.0) | 3.0 (1.7–5.8) | 0.439 |
| Intended surgical approach | |||
| Extended resection | 11 (34.4%) | 10 (33.3%) | 0.885 |
| Lesionectomy | 15 (46.9%) | 17 (56.7%) | |
| Partial resection | 6 (18.8%) | 3 (10.0%) | |
- Note: Significant p-values are highlighted in bold print.
- Abbreviation: IQR, interquartile range.
3.1 Surgical Details
A concise lesionectomy was performed in 32 cases (51.6%), an extended resection in 21 (33.9%), and a partial resection in 9 cases (14.5%). Patients with a partial resection had a larger tumor volume, median 12.0 cm3 versus 2.9 cm3 and 3.4 cm3 in lesionectomies and extended resections, respectively (p = 0.025). The resection was intentionally subtotal in the latter nine patients because it showed spread into the sublenticular area and thalamus in four cases, widespread involvement of the medial temporal lobe from the level of the amygdala to behind the level of the splenium in two cases, and involvement of the lower calcarine lip in one case. Another tumor was growing around the pericallosal arteries and was only amenable to partial resection due to the risk of vascular damage. Finally, one larger tumor was suspected to be a diffuse glioma, and the goal of the surgery was limited to the removal of the PET hypermetabolic region. In the remaining 12 cases, residual tumor was present on postoperative MRI after an intended gross total resection.
Progression of the residual tumor occurred in seven of 21 patients (33.3%) and recurrences were seen in five of 41 patients without visible tumor remnants on postoperative MRI (12.2%) who had undergone a concise lesionectomy. No recurrences were seen in patients after extended lesionectomies. We performed a second resective surgery in 14 cases and one patient received Gamma knife radiosurgery. Seven of these patients were operated on because of ongoing seizures and a residual tumor. A further five patients had increased seizure frequency in the context of a tumor recurrence or progression of residual tumor. One reoperation was performed for uncontrolled seizures without radiological evidence of tumor. The final two patients had no seizures and were operated on because of a radiological progression of residual tumor and a tumor recurrence. Patients with a residual tumor were more likely to require a second surgery (p < 0.001) and patients after an extended operation had lower rates of reoperations than patients after concise lesionectomies or partial resections: 4.8%, 31.3%, and 44.4%, respectively (p = 0.018). Four patients—including the patient treated with gamma knife—required a third surgery because of further tumor recurrences.
3.2 Pathohistological Details and Association With Clinical Phenotype
Histologically, 60 tumors were classified as a WHO grade I ganglioglioma, one of which was a desmoplastic infantile ganglioglioma. The two other tumors were classified as atypical GG, WHO grade II. Fifty-seven gangliogliomas (91.9%) showed expression of CD34, while 30 had an immunohistochemically detected BRAF V600E mutation (48.4%). BRAF V600E mutation status showed no association with localization, side, or size of the radiologically defined tumor volume. Patients with a BRAF V600E mutated ganglioglioma were younger at the age of seizure onset and surgery.
While the median preoperative seizure frequency was higher in cases with a BRAF V600E mutation, this finding was not statistically significant using a non-parametric test due to the high variation (Table 1). We found no association between the BRAF V600E mutation and recurrent tumor growth after surgery. All patients with a BRAF V600E mutation were also CD34 positive. No difference was found between tumors with or without CD34 reactivity regarding size, preoperative seizure frequency, patient age, or incidence of recurrence. In all cases with reoperations, the resected tumors retained their diagnosis of WHO grade I ganglioglioma. Analysis of the one reoperation performed without radiologically visible residual tumor also revealed tumor remnants reaching into neighboring gyri. We found no case of histologically verified malignant transformation in this study cohort.
3.3 Seizure Outcome
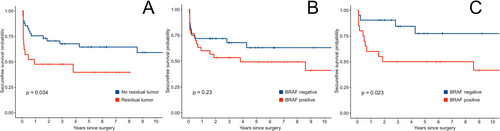
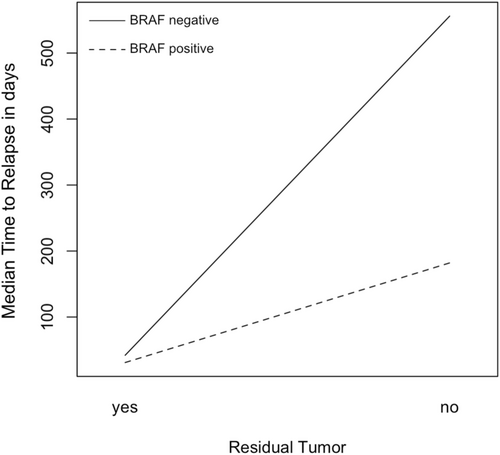
One year after surgery, 42 patients (67.7%) had a seizure outcome of ILAE class 1 or 1a. Outcomes after 1 year and at the last follow-up did not differ significantly between surgical approaches. We found no association with BRAF V600E or CD34 status and the postsurgical outcome. During the follow-up period, seven patients, who were initially seizure free, suffered from seizure relapses after a median of 1.8 years, while another 20 patients had recurring seizures within the first year. We further analyzed the time from surgery to seizure relapse using Kaplan–Meier analysis. Residual tumor was the strongest predictor for early seizure relapse (Figure 1A). While the BRAF V600E mutation status did appear to have a significant impact in the entire patient population, its effect was obscured by early relapses in patients with residual tumor irrespective of mutation status (Figure 1B). We found that the BRAF V600E mutation is a significant factor for seizure-free survival in patients with radiologically complete resection (Figure 1C). CD34 reactivity was not associated with differences in age, tumor size, seizure relapses, or final seizure outcome, but the small number of CD34 negative tumors limits the statistical strength of this statement. The effects of these immunohistochemical biomarkers were also analyzed in a multivariate Cox regression model, which included an interaction term for the interplay of residual tumor and BRAF V600E mutation. Younger age was associated with earlier relapses. The regression also revealed that BRAF V600E mutation is a risk factor for shorter relapse-free survival and that the presence of mutation and residual tumor influence each other (Table 2 and Figure 2). All five patients, without visible residual tumors, had a seizure relapse much earlier than the tumor recurrence. The seizures reoccurred after a median of 5.1 months [IQR: 2.0–22.7 months], while visible tumor was diagnosed after 86.2 months [IQR: 62.4–93.0 months].
| Hazard ratio [95% CI] | p | |
|---|---|---|
| Age at surgery | 1.03 [1.00–1.06] | 0.021 |
| Residual tumor | 10.4 [2.63–40.94] | < 0.001 |
| CD34 reactivity | 3.70 [0.44–30.88] | 0.227 |
| BRAF V600E mutation | 4.00 [1.23–13.00] | 0.022 |
| Interaction: Residual tumor × BRAF V600E | 0.15 [0.03–0.78] | 0.024 |
- Note: Significant p-values are highlighted in bold print.
- Abbreviation: 95% CI, 95% confidence interval.
At the last follow-up, 52 patients reported being seizure free (83.9%). Of patients still experiencing seizures, the ILAE outcome was class 3 in four, class 4 in four, and class 5 in two patients. Seven of these ten patients (70.0%) had a BRAF V600E positive GG, but this effect was not significant in the cohort as a whole, only in the subset without residual tumor after the first surgery. Seizure outcome at the last follow-up was not associated with residual tumor, CD34 reactivity status, or surgical technique in the initial operation.
4 Discussion
We present the first clinical study to indicate that BRAF V600E mutational status has an influence on the likelihood of seizure relapse after surgery. Our study confirms that complete resection is the mainstay of surgical treatment. Furthermore, it suggests that in cases with a BRAF V600E mutation, microscopically small tumor residuals increase the risk of seizure relapse, and a more radical resection should be pursued when long-lasting seizure freedom is the goal.
So far, the BRAF V600E mutational status in GG patients was largely investigated only from an oncological perspective addressing questions like the time to tumor recurrence and the value of additional therapy. Some smaller studies reported shorter recurrence-free survival in BRAF V600E mutated tumors [21-23], but another study did not corroborate this finding [24]. One other study also described how patients with the mutation had seizures at an earlier age but did not find an association with outcome [25].
The influence of BRAF V600E status on epileptogenicity has mainly been investigated in preclinical studies showing that BRAF V600E mutations lead to activation of the mTOR pathway, and this pathway has also been implicated in the development of focal cortical dysplasias causing intractable epilepsy [26, 27]. Indeed, histopathological analysis of ganglioglioma samples has described cortical malformations associated with glioneural tumors [13, 25, 28, 29]. These findings may suggest a common origin, but their validity, significance, and influence on clinical outcomes need to be further evaluated. Another study investigated the effects of BRAF V600E in vitro and in a murine model and showed that the mutation increases the potential for epileptogenic discharges in neurons. They also demonstrated that its effect can be reversed using BRAF-targeted therapies [30].
On the other hand, a large meta-analysis investigating multiple glioneural tumor types did not find an association between BRAF mutation and seizure outcome [31]. However, this study did not differentiate between tumor types when investigating clinical symptoms. Therefore, it is possible that the epileptogenicity of the BRAF V600E mutation is more pronounced in GG but is concealed in glioneural tumors as a whole.
Furthermore, no study has investigated the interplay with a residual tumor. Our results suggest that a residual tumor alone is so strongly epileptogenic that the effects of the BRAF V600E mutation are obscured by it if not properly considered. Yet, when the bulk of the tumor is removed, and only cell nests remain, the mutation possibly increases the risk of ongoing seizures.
The other investigated biomarker, CD34, is a stem-cell marker expressed during early neurulation but not in the adult brain [32]. Its presence in GG has already been described 25 years ago and now serves to characterize GG during histopathological diagnosis [32, 33]. Due to this specific marker, pathologists were able to show how GG cells can be spread outside the tumor, which is visible on hematoxylin and eosin stains. Extensions across sulcal borders and satellite lesions have been described [8, 9, 33].
As in many other subfields of neurosurgery, a complete or even supramarginal resection has clear benefits in pediatric patients from an epileptological and an oncological viewpoint regarding the outcome [34-36]. In our cohort, patients after extended resections showed no tumor recurrences. However, the risk of damaging important structures in the brain and causing irreversible injury constantly accompanies each surgery and can increase in larger resections. The lead pediatric neurosurgeon during the study period was eager to pursue a concise resection and more willing to risk having to perform a second operation than to risk causing unnecessary neurological damage. Our rate of residual tumor tissue after lesionectomies was certainly influenced by this mentality.
The histopathological evidence of widespread gangliogliomas and the benefits of removing all tumor cells has tipped the scale weighing between pure lesionectomy versus extended resection toward larger resections in our and many other centers. Perhaps the advent of surgical adjuncts like high-resolution intraoperative MRI or in situ tissue imaging of resection borders might help to better tailor the extent of resection. Until then, it is of utmost importance to recognize the negative effects of residual tumor tissue on seizure outcome. We therefore add data to the literature suggesting that confined resections are especially risky from a seizure perspective when BRAF mutations are present. Due to this mutation, even smaller remaining cell islands are a risk factor for seizure relapses.
Thus, our data implies that early detection of a BRAF V600E mutation could impact the surgical and treatment strategy of GG. Liquid biopsies, where trace amounts of tumor specific markers can be detected in blood or cerebrospinal fluid, have become widely used in neurooncology and will further shape the management of neurooncological patients in the future [37]. In specific, the BRAF V600E mutation has been detected as a biomarker in multiple studies involving patients with melanomas but also other tumor entities [38-41]. This development in neurooncology should not be neglected in the field of epilepsy surgery. The knowledge of whether or not a BRAF V600E mutation is present in the presurgical evaluation could influence the decision-making process when discussing surgical strategies. The combination with liquid biopsy opens an avenue for continuously monitoring treatment success and potential recurrences, as the mutation can be quantitatively detected using digital droplet PCR [42]. Furthermore, the detection of a BRAF V600E mutation also provides a possible treatment target for patients with surgically not accessible GG [43-45].
4.1 Limitations
This study carries all the associated limitations of a retrospective study. The long study period allows for a long follow-up time but also introduces a bias as diagnostic modalities, for example, MRI and surgical techniques, have been continuously developed. This limits the comparability of patients operated on recently to patients operated 20 years ago. It was not always evident whether the decision for reoperation was made due to epileptological or oncological reasons. Because of the large overlap of these issues in patients with GG, we would argue that most centers also pursue an integrated, multidisciplinary approach similar to ours.
Over the duration of the study period, the histological classification of low-grade glioneural tumors and GG has also changed. For example, GG can no longer be classified as WHO grade II/CNS WHO grade 2. Furthermore, immunohistochemical staining for CD34 and BRAF V600E was not originally performed in older specimens. We tried to remedy this by repeating the staining and only including patients where tissue specimens were available for analysis. Additionally, as in all studies involving neurosurgical specimens, a degree of sampling bias can occur as some of the tissue might be lost in the preparation through use of the suction device. The presence of CD34 and BRAF V600E mutations, however, was in line with previously published studies, which makes our results comparable to the published literature.
Medical decisions and surgical aggressiveness were at the discretion of the treating physicians. This is, however, in line with the daily experience of most pediatric and neurosurgical centers and thus provides “real-life” data. Unfortunately, we cannot prove that remaining GG cell nests were the cause of seizure relapses in BRAF V600E positive cases with radiologically complete resection. However, it is striking that four patients who were radiologically tumor-free, had a subsequent recurrence of their GG and thus proven remnant cells.
5 Conclusion
The BRAF V600E status in patients with ganglioglioma associated epilepsy may be an important biomarker with the potential of shaping the surgical and treatment strategy in the future. Especially young patients diagnosed with GG, who have a high seizure frequency, should be suspected of harboring a BRAF V600E mutation. Knowing the mutation is present could help to indicate an extended resection because tumor cells with the mutation may harbor higher epileptogenicity. Efforts to establish a preoperative pipeline to define the BRAF status in blood or cerebrospinal fluid need to be expedited, and the significance of this mutation needs to be investigated in prospective clinical studies.
Author Contributions
Matthias Tomschik: conceptualization, investigation, funding acquisition, writing – original draft, visualization, methodology, formal analysis. Eva Horner: investigation, data curation, writing – review and editing. Alexandra Lang: investigation, methodology, data curation, resources. Florian Mayer: investigation, resources. Thomas Czech: investigation, validation, writing – review and editing. Gregor Kasprian: investigation, writing – review and editing. Ekaterina Pataraia: investigation, validation. Amedeo A. Azizi: investigation, validation, writing – review and editing. Martha Feucht: investigation, validation, writing – review and editing. Karl Rössler: investigation, writing – review and editing, resources, supervision. Christine Haberler: investigation, methodology, validation, writing – review and editing. Christian Dorfer: conceptualization, investigation, funding acquisition, writing – review and editing, supervision.
Acknowledgments
We thank the Comprehensive Center for Pediatrics (CCP) of the Medical University of Vienna, which has funded this research with money from the CCP Starter Grant in 2020. Open access funding provided by Medizinische Universitat Wien/KEMÖ.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




